Abstract
Objective
Our study objective was to determine feasibility and mapping rates using indocyanine green (ICG) for sentinel lymph node (SLN) mapping in early-stage cervical cancer.
Methods
We performed a retrospective review of all women who underwent SLN mapping with ICG during primary surgical management of early-stage cervical cancer by robotic-assisted radical hysterectomy (RA-RH) or fertility-sparing surgery. Patients were treated at two high-volume centers from 10/2012 to 02/2016. Completion pelvic lymphadenectomy was performed after SLN biopsy; additionally, removal of clinically enlarged/suspicious nodes was part of the SLN treatment algorithm.
Results
Thirty women with a median age of 42.5 and BMI of 26.5 were included. Most (90%) had stage IB disease, and 67% had squamous histology. RA-RH was performed in 86.7% of cases. One patient underwent fertility-sparing surgery. Median cervical tumor size was 2.0 cm. At least one SLN was detected in all cases (100%), with bilateral mapping achieved in 87%. SLN detection was not impacted by tumor size and was most commonly identified in the hypogastric (40.3%), obturator (26.0%), and external iliac (20.8%) regions. Five cases of lymphatic metastasis were identified (16.7%): three in clinically enlarged SLNs, one in a clinically enlarged non-SLN, and one case with cytokeratin positive cells in an SLN. All metastatic disease would have been detected even if full lymphadenectomy had been omitted from our treatment algorithm,
Conclusions
SLN mapping with ICG is feasible and results in high detection rates in women with early-stage cervical cancer. Prospective studies are needed to determine if SLN mapping can replace lymphadenectomy in this setting.
Keywords: Cervical cancer, Fluorescence imaging, Indocyanine green, Robotic hysterectomy, Sentinel lymph node
1. Introduction
In the United States, nearly half of the 13,000 women diagnosed with cervical cancer this year will have disease confined to the cervix, and therefore, be eligible for surgical management [1,2]. Given that lymphatic involvement is the most important prognostic factor in this disease, a comprehensive assessment of the lymph nodes that drain the cervix is paramount [3,4]. However, the majority of patients undergoing a pelvic and/or para-aortic lymphadenectomy in this setting will ultimately have negative lymph nodes, yet are subjected to the morbidity of lymphadenectomy. Potential lymphadenectomy-associated complications include bleeding, neurovascular injury, infection, lymphocyst development, and debilitating lymphedema [5,6]. Thus, there is great interest in developing novel nodal assessment techniques that minimize morbidity and maximize detection of lymphatic metastases.
The sentinel lymph node (SLN) mapping technique is based on the principle that the first nodal group receiving lymphatic drainage from a primary tumor can be identified. Hence, if the lymphatic drainage from an organ or tumor is predictable, and a SLN is detected and negative for metastasis, the remainder of the lymph nodes in that basin should theoretically be negative as well [7]. SLN mapping is widely used in melanoma, breast, and vulvar cancers and is well-studied in cervical cancer [8,9].
Despite growing evidence, the use of SLN mapping in cervical cancer has not become widespread in the United States as the technique has met with skepticism due to relatively low sensitivity and detection rates, as well as high false negative rates, particularly in women with larger cervical tumors [7,8,10,11]. As such, while National Comprehensive Cancer Network (NCCN) cervical cancer guidelines include SLN mapping as an alternative in the surgical management of early-stage tumors, it cautions against its use in tumors ≥2 cm [7,8,10,12]. These recommendations were based on the cumulative body of evidence demonstrating worse detection rates as well as higher false negative rates in those with larger tumors [8,13]. Therefore, support for the use of SLN mapping as a substitute for complete lymphadenectomy in cervical cancer has remained limited, and only advisable in select patients with small primary tumors [7,8,10,11]. Of note, most of these studies utilized a radiocolloid tracer, typically 99Technetium (99Tc), alone, or in combination with blue dye [8].
Recent data suggests that the use of indocyanine green (ICG), a dye which fluoresces when excited by near infra-red light, may be superior to blue dye alone for SLN detection in endometrial cancer [14,15]. There is little data on the use of ICG in the SLN mapping of cervical cancer. We sought to determine if ICG is a feasible option for SLN mapping in this setting and determine the detection rates with this technique.
2. Methods
Institutional Review Board approval was obtained from Johns Hopkins University, Baltimore, MD and Texas Oncology, San Antonio, TX to perform a retrospective review of all early-stage cervical cancer cases treated surgically with SLN mapping from October 2012 to February 2016. Patients were eligible for surgical management with lymphadenectomy if they had preoperative FIGO stage IA1 with evidence of lymph vascular space invasion (LVSI) or stage IA2–IB2 disease, and a biopsy-confirmed diagnosis of squamous, adenosquamous, or adenocarcinoma of the cervix. At the surgeon’s discretion, pre-operative imaging with positron emission tomography/computed tomography (PET-CT) or computed tomography (CT) was performed prior to surgery to rule out distant disease. The treating physician determined tumor size preoperatively by clinical exam. Patient demographics, tumor and surgical variables were recorded and analyzed.
2.1. Injection and evaluation of SLNs; completion lymphadenectomy
Both institutions had adopted a treatment protocol for ICG injection and SLN evaluation shown in Fig. 1 [14]. This treatment algorithm differs from that previously published and presented in the NCCN guidelines for SLN mapping in cervical cancer [12] because we performed lymphadenectomy regardless of mapping success given the unknown predictive value of this technique using ICG dye. One 25 mg single use vial of ICG was reconstituted in 10 mL of aqueous solvent and then diluted in 10 mL of normal saline. Of note, ICG dye is not approved by the Federal Drug Administration (FDA) for SLN mapping and is used off-label. Just prior to uterine manipulator placement, the cervix was injected with a total of 2 mL of indocyanine green (ICG) at 3 and 9 o’clock, both superficially and deep into the cervical stroma. Select patients also underwent superficial cervical injection at 6 and 12 o’clock (0.5 mL each) depending on tumor size and location. During robotic-assisted laparoscopy, the retroperitoneum was assessed with near-infrared light with the aid of the Firefly Fluorescence Vision Imaging System (Intuitive Surgical, Sunnyvale, CA), which excited the ICG, allowing identification of the lymphatic channels and SLNs, which were then removed. All patients then underwent a complete bilateral pelvic lymphadenectomy according to GOG staging criteria. Para-aortic lymph nodes were assessed in all patients, but dissection was performed at the surgeon’s discretion. As part of the surgical algorithm, all suspicious or enlarged lymph nodes were removed prior to lymphadenectomy, regardless of their sentinel status, and sent separately to pathology. Additionally, at the surgeon’s discretion, if suspicious or clinically enlarged nodes were identified, they could be sent for frozen section at the time of procedure. Fig. 1 demonstrates the treatment algorithm of these patients.
Fig. 1.
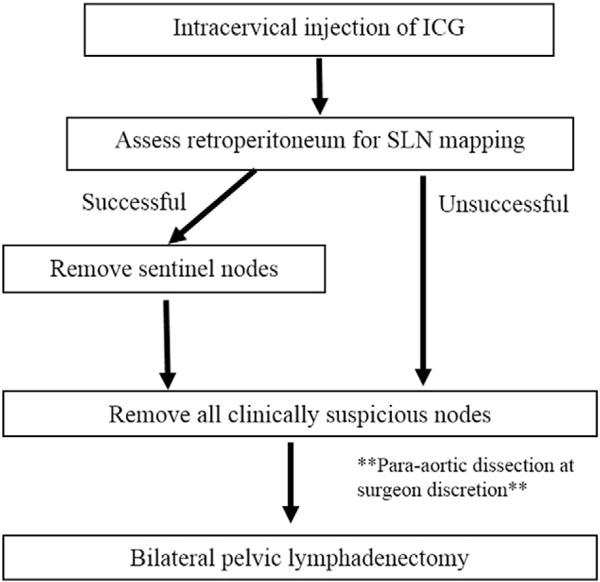
Sentinel Lymph Node Mapping Algorithm.
2.2. Pathologic evaluation of the SLNs
At the onset of SLN mapping at our two institutions, histopathology assessment of SLNs was performed in a standard fashion similar to that used for the non-sentinel lymph nodes: after being embedded in paraffin, the node was sectioned once along the longitudinal axis, and hematoxylin and eosin (H&E) stain was applied, after which the node was evaluated for metastatic tumor cells. Ultrastaging was first described in breast cancer as a technique to improve the sensitivity of SLN evaluation for detecting micro-metastasis; its use has now been widely adopted for lymphatic assessment of cervical cancer as well [16,17]. In our ultrastaging protocol, the SLN is sectioned once along the longitudinal axis, and then two additional adjacent 5-μm sections are cut at two levels and stained with H&E and immunohistochemistry (IHC) for pancytokeratin (clone AE1: AE3) similar to others’ published methods for evaluation of SLNs in cervical cancer [17]. Macrometastases were defined as tumors >2.0 mm in diameter, micrometastases were defined as clusters or single tumor cells which measure 0.2 mm to 2.0 mm in diameter. Isolated tumor cells were defined as microscopic clusters or single cells <0.2 mm in diameter. Finally, cytokeratin positive cells are those that are not clearly defined as carcinoma cells, but which stain positive for cytokeratin [16].
2.3. Statistics
Overall, SLN detection rate was defined as the proportion of cases with any successful SLN mapping. Bilateral detection rate was defined as the proportion of cases in which SLNs were identified intraoperatively in both hemi-pelvises. Side-specific detection rates were calculated as the proportion of hemi-pelvises in which at least one SLN was identified intraoperatively. Mapping rates were compared between tumors <2 cm in size and those ≥2 cm using a two-tailed Fisher’s exact test for differences of proportions. All statistical calculations were performed using Stata (version 13) and a p-value <0.05 was considered significant.
3. Results
3.1. Clinical and pathologic data
Thirty patients underwent surgical management with SLN mapping during the study period (Table 1). The median patient age was 42.5 years (range, 28–77), and median body mass index (BMI) was 26.5 kg/m2 (range, 18.9–52.0). Over half (n = 19, 63.3%) had pre-operative PET/CT scans. The majority of patients (n = 20, 66.7%) had squamous cell carcinoma (SCC), followed by adenocarcinoma (n = 7, 23.3%), and adenosquamous carcinoma (n = 3, 10.0%). Most patients (n = 26, 86.7%) underwent a robotic-assisted radical hysterectomy. In 3 cases, robotic-assisted radical hysterectomy was aborted due to evidence of extra-cervical disease: one case was found to be stage IB2 on exam under anesthesia and the patient had been counseled and preferred primary chemoradiation if the stage was >IB1; lymph nodes were still surgically assessed as the pre-operative PET-CT noted several suspicious retroperitoneal nodes; in the two other cases, positive lymph nodes were encountered in enlarged nodes sent for frozen section (described in detail below). One patient underwent cold knife conization for fertility preservation; lymph nodes were assessed with robotic-assisted SLN mapping and pelvic lymphadenectomy.
Table 1.
Demographic and clinicopathologic information.
| Demographic and clinical information (n = 30) | ||
|---|---|---|
| Median age, years (range) | 42.5 | (28–77) |
| Median BMI, kg/m2 (range) | 26.5 | (18.9–52) |
| N | (%) | |
| Clinical stage | 3 | (10.0) |
| ≤IA2 | 25 | (83.3) |
| IB1 | 2 | (6.7) |
| IB2 | ||
| Procedure | ||
| RA-RH or MRH | 26 | (86.7) |
| Cold knife cone | 1 | (3.3) |
| Aborted RA-RH or RA-MRH | 3 | (10.0) |
| Location of procedure | ||
| Johns Hopkins Hospital | 18 | (60.0) |
| Texas Oncology | 12 | (40.0) |
| History of conization prior to procedure | 16 | (55.7) |
| Median clinical tumor size, cm (range) | 2.0 | (0–4.0) |
| Microscopic | 7 | (23.3) |
| <1 cm | 3 | (10.0) |
| ≥1 & <2 cm | 3 | (10.0) |
| ≥2 & <3 cm | 7 | (23.3) |
| ≥3 & <4 cm | 6 | (20.0) |
| ≥4 cm | 4 | (13.3) |
| Pathologic and surgical information | ||
|
| ||
| Median pathologic size, cm (range) | 2.1 | (0.1–4.5) |
| Histology | ||
| Squamous cell carcinoma | 20 | (66.7) |
| Adenocarcinoma | 7 | (23.3) |
| Adenosquamous carcinoma | 3 | (10.0) |
| Gradea | ||
| Well differentiated | 5 | (17.9) |
| Moderately differentiated | 15 | (53.6) |
| Poorly differentiated | 8 | (28.6) |
| Lymphovascular space invasion presentb | 9 | (33.3) |
| Ultrastaging performed | 13 | (43.3) |
| Median (mean) number of SLN retrieved per hemipelvis (range) | 2 (2.4) | (0–11) |
| Median (mean) number of non-SLN pelvic nodes per hemi-pelvis (range) | 7 (6.5) | (0–20) |
| Cases with evidence of lymphatic metastasis | ||
| Frank metastasis | 4 | (13.3) |
| Cytokeratin positive cells only | 1 | (3.3) |
RA-RH = Robotic assisted radical hysterectomy.
MRH = modified radical hysterectomy.
Data on grade was not available for 2 patients.
Data on LVSI not available for 3 patients.
The median clinical tumor size was 2.0 cm (range, 0–4.0), and 60.6% of tumors were ≥2 cm. Approximately one third of tumors were poorly differentiated (n = 8, 28.6%), and one third demonstrated LVSI (n = 9, 33.3%). Nearly half (n = 13, 46.4%) underwent the ultra-staging protocol for SLN evaluation.
3.2. Mapping rates and locations
At least one SLN was detected in all cases, resulting in a 100% overall detection rate (Table 2). The median number of SLNs removed per hemi-pelvis was 2 (range 0–11). Successful bilateral mapping occurred in 26 (86.7%) cases; there was no difference between tumors ≥2 cm vs. <2 cm in diameter (88.2% versus 84.6%, p = 1.0). Considering each hemi-pelvis separately, at least one SLN was detected in 56 out of 60 hemi-pelvises, which reflects a side-specific detection rate of 93.3%. The side-specific detection rate did not differ between tumors ≥2 cm compared with those <2 cm in diameter (94.1% versus 92.3%, p = 1.0).
Table 2.
Sentinel lymph node mapping rates and locations.
| Detection rates | |||
|---|---|---|---|
| Overall detection rate | 100% | (30/30 cases) | |
| Side specific detection rate | |||
| Overall | 93.3% | (56/60 hemipelvises) | p = 1a |
| Tumors <2 cm | 92.3% | (24/26 hemipelvises) | |
| Tumors ≥2 cm | 94.1% | (32/34 hemipelvises) | |
| Bilateral mapping rate | |||
| Overall | 86.7% | (26/30 cases) | p = 1a |
| Tumors <2 cm | 84.6% | (11/13 cases) | |
| Tumors ≥2 cm | 88.2% | (15/17 cases) | |
| Sentinel lymph node locations | |||
|
| |||
| Hypogastric | 40.3% | ||
| Obturator | 26.0% | ||
| External iliac | 20.8% | ||
| Common iliac | 6.5% | ||
| Para-aortic | 5.2% | ||
| Parametrium | 1.3% | ||
Two tailed Fisher’s exact test comparing tumors >2 cm to ≤2 cm.
SLNs were most frequently identified in the hypogastric region (40.3%), followed by the obturator (26.0%), external iliac (20.8%), common iliac (6.5%), para-aortic (5.2%), and parametria (1.3)%. A median of 7 non-SLNs were removed per hemi-pelvis (range, 0–20). Five patients (16.7%) underwent para-aortic lymphadenectomy based on surgeon discretion.
3.3. Metastatic lymph node detection
Positive lymph nodes were identified in five women (16.7%). Preoperative PET/CT scans were negative for suspicion of nodal involvement in all patients. The first patient had a stage IB1, 2 cm moderately differentiated SCC with LVSI. SLNs were identified bilaterally. Upon removal, they appeared slightly enlarged and were sent for frozen section, which confirmed metastatic disease. The second patient also had a stage IB1, 2 cm moderately differentiated SCC. SLN mapping was successful bilaterally. However, there was diffuse shoddy adenopathy present throughout the pelvis; this was felt to have been possibly due to her medical co-morbidities. Intraoperative frozen section of the most enlarged node was negative, but final pathology demonstrated metastatic disease on H&E in another suspicious non-sentinel node removed separately by the surgeon. Ultrastaging was not performed on the SLNs given the positive node identified on H&E. In the third case, the patient had a stage IB1, 4 cm poorly differentiated SCC. SLNs were detected bilaterally, and appeared clinically enlarged. Intraoperative frozen section was performed and confirmed metastatic disease; thus, the hysterectomy was aborted. The fourth case was similar: the patient had a stage IB1, 1.7 cm moderately differentiated SCC tumor. The SLNs mapped bilaterally, and appeared clinically enlarged; intraoperative frozen section confirmed metastatic disease and the hysterectomy was aborted. Finally, a patient with a stage IB1, 3 cm, poorly differentiated adenosquamous carcinoma had isolated cytokeratin positive cells identified on ultrastaging of the bilateral SLNs, but no frank metastases in any other lymph nodes. The patient had other high-risk features identified after radical hysterectomy, including LVSI and parametrial involvement, and therefore, the cytokeratin positive cells in the lymph nodes were felt to reflect metastatic disease. In all five cases, metastatic disease was detected by removal of either the SLNs alone or by removal of clinically enlarged lymph nodes. All patients with metastatic involvement of their lymph nodes were treated with adjuvant cisplatin-based chemoradiation.
4. Discussion
Despite acceptance by NCCN guidelines as an alternative treatment approach, the role of SLN evaluation in women with apparent early-stage cervical cancer has remained unclear, largely due to historically poor detection rates and low negative predictive value with radiocolloid and blue dye techniques [7,8,10,11]. However, in our dual-institution, retrospective analysis, we observed high bilateral mapping and side-specific SLN detection rates using indocyanine green dye (ICG) and robotic surgery. In the hands of experienced gynecologic oncology surgeons, SLN detection was successful in 100% of cases, with a side-specific detection rate of 93.3%. In contrast, the reported SLN detection rates using the radiocolloid/blue dye have been suboptimal, particularly in tumors >2 cm. For these larger tumors, overall SLN detection rates are 80.1%, with side specific detection rates of only 58.8% [8]. Our study demonstrated higher overall and side-specific detection rates, regardless of primary tumor size. Additionally, our treatment algorithm (Fig. 1), which mandates the removal of all clinically-enlarged non-SLNs in addition to all SLNs, would have correctly identified all patients (n = 5) with metastatic nodal involvement even if the pelvic lymphadenectomy had been omitted. While our study results are preliminary, they demonstrate that SLN mapping with ICG is feasible and results in high detection rates in women with early-stage cervical cancer.
In addition to the potential for improved detection rates, ICG offers several other advantages over the traditional radiocolloid/blue dye mapping technique. Practical advantages include the avoidance of radiation exposure to both patients and staff, and the need for fewer personnel as radiology staff are no longer required. Additionally, ICG dye is injected while the patient is under anesthesia, which avoids the painful administration of radiocolloid performed in the preoperative setting. Lastly, the cost of the ICG dye technique is significantly lower than with the combined technique because additional injection time and lymphoscintigraphy are not required [18]. Given the high rates of morbidity associated with complete lymphadenectomy in this setting and that ICG dye may represent a superior SLN detection technique compared to other mapping methods, further investigation of ICG and other fluorescent dyes is merited.
Our study offers promising results for the use of ICG in SLN mapping, and adds to the body of literature supporting its use in cervical cancer. Initial studies in open surgery reported poor overall detection rates of just over 75% [11]. However, laparoscopic techniques with ICG have proven more successful in gynecologic cancer [14,15,18–20]. Several studies evaluating ICG for SLN mapping in endometrial cancer also included cervical cancer cases in their publications [18,19]. One of the largest included 18 cervical cancer patients, but did not report results separately from the 209 endometrial cancer patients in the study [19]. Thus, conclusions on the efficacy of ICG in cervical SLN detection cannot be drawn from this study. Similarly, Buda et al. reported on SLN mapping techniques in women with early-stage endometrial and cervical cancers and included 9 cervical cancer patients who underwent SLN mapping with ICG; 100% mapped bilaterally [18]. Again, however, the sample size was too small to define the accuracy and false negative rates of the SLN approach in this patient population.
A recent retrospective European study has conducted an analysis similar to ours. Imboden et al. evaluated patients undergoing SLN mapping for cervical cancer using ICG (n = 22) versus 99Tc with blue dye (n = 36) [20]. The authors reported significantly better bilateral detection rates with ICG (95.5% vs. 61%, p < 0.005) as well as a non-significant trend towards better bilateral detection rates in tumors larger than 2 cm [20]. Data from our study also confirms a high side-specific and bilateral mapping rate with the use of ICG, and further demonstrates no difference in detection between small and large cervical tumors. Our pilot data and that of Imboden et al. suggest that SLN mapping using ICG dye may overcome the limitations of blue dye alone or combination blue dye and radiocolloid, irrespective of tumor size [20]. The inherent properties of ICG may lend itself to improved mapping rates: ICG rapidly binds with lipoproteins in the plasma and is quickly taken up by the lymphatics. Additionally, the near-infrared light can penetrate tissues several millimeters, which white light cannot, making it easier to identify even with obstructing tissues such as fat [21]. We hypothesize that these features may contribute to the improved mapping rates seen in our study and that of Imboden et al. when compared to traditional techniques [20].
While maximizing mapping rates may encourage the uptake of SLN mapping in the care of early-stage cervical cancer patients, the issue of low sensitivity must also be addressed [7,8,10]. Achieving maximal sensitivity of the SLN technique depends on several factors. First, with anatomically centralized tumors, bilateral SLN mapping rates must be optimal, as was previously discussed. Data from our study suggest that the use of ICG results in high bilateral mapping rates when experienced surgeons perform the mapping procedure. Second, ultrastaging of the SLNs improves sensitivity and reduces the false negative rate [17]. In our study, ultrastaging identified cytokeratin positive cells in the SLNs of one patient; the clinical significance of cytokeratin positive cells in early-stage cervical cancer remains unclear, but in this particular patient with positive parametrial involvement, this finding is likely not a spurious event. While isolated tumor cells and micrometastatic disease in non-SLNs have been previously associated with a worse prognosis [22–24], a more contemporary multi-center study reviewing cases with isolated tumor cells in the SLNs of cervical cancer patients without other risk factors alone found no difference in survival [25]. However, a major confounding variable in this study was that approximately half the patients received adjuvant therapy, limiting the conclusions regarding recurrence rates and survival [25]. Regardless, ultrastaging of SLNs in cervical cancer appears to improve the sensitivity and negative predictive value and, for now, is an integral component of most SLN algorithms [17].
Lastly, it is important to examine the sensitivity of a SLN algorithm as a whole. In our study, 5 cases (16.7%), had evidence of lymph node metastases. All would have been successfully detected utilizing our treatment algorithm (Fig. 1) even if the completion pelvic lymphadenectomy were hypothetically omitted. This is because the treatment algorithm takes into account removal of any SLN detected, as well as removal of clinically enlarged or suspicious-appearing lymph nodes. In 4 patients, metastatic disease was detected in clinically enlarged lymph nodes, highlighting the fact that removal of suspicious lymph nodes is a critical component of any SLN algorithm. Particularly, in the patient with shoddy lymphadenopathy throughout her pelvis, the SLN were negative while other enlarged nodes were positive, underlining the importance of removing enlarged or suspicious nodes and raising the question if patients with diffuse adenopathy are good candidates for SLN mapping. Notably, none of the pre-operative PET/CT scans of these patients identified any suspicious nodes.
Our study has limitations inherent to the retrospective design. After the adoption of SLN mapping in cervical cancer at our institutions, ICG was used almost exclusively. Therefore, we are unable to provide a direct comparison to different dye techniques. Additionally, early-stage cervical cancer is a rare disease, which hinders the rapid accrual of a large sample size of patients. While we attempted to maximize the number of patients included by gathering data from two institutions, our sample size is still small. However, we report on one of the largest cohorts of cervical cancer patients who underwent SLN mapping with ICG. Lastly, due to the differential uptake of this innovative technique in the two centers involved in its implementation, ultrastaging was not performed on every patient.
In conclusion, our study demonstrates that SLN mapping with ICG is feasible and results in high detection rates in patients with early-stage cervical cancer with both small and large primary tumors, when performed by experienced surgeons. Our data support that a SLN algorithm must include the removal of suspicious or clinically enlarged nodes. Ultrastaging of SLNs is likely an important component of a SLN algorithm, although further studies are needed to understand the prognostic implications of isolated tumor cells and cytokeratin positive cells detected by this technique. Additionally, Larger studies are certainly needed to determine if a SLN algorithm can replace a more complete lymphatic assessment in patients with early-stage cervical cancer.
HIGHLIGHTS.
Sentinel lymph node mapping with indocyanine green in women with early-stage cervical cancer is feasible.
Sentinel lymph node detection rates with this modality do not appear to differ by tumor size.
Sentinel lymph node detection rates with indocyanine green are acceptably high to warrant further study in this setting.
Footnotes
Conflict of interest
All authors confirm they have no conflict of interest to disclose.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. http://dx.doi.org/10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N FE, Noone AM, Krapcho M, Garshell J, Neyman N, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Lewis DR, Chen HS. SEER Cancer Statistics Review, 1975–2010. National Cancer Institute; Bethesda, MD: http://seer.cancer.gov/csr/1975_2010/, based on November 2012 SEER data submission, posted to the SEER web site, April 2013., n.d. [Google Scholar]
- 3.Fuller AF, Elliott N, Kosloff C, Hoskins WJ, Lewis JL. Determinants of increased risk for recurrence in patients undergoing radical hysterectomy for stage IB and IIA carcinoma of the cervix. Gynecol Oncol. 1989;33:34–39. doi: 10.1016/0090-8258(89)90598-2. http://www.ncbi.nlm.nih.gov/pubmed/2703164 (accessed February 10, 2016) [DOI] [PubMed] [Google Scholar]
- 4.Kato T, Watari H, Takeda M, Hosaka M, Mitamura T, Kobayashi N, et al. Multivariate prognostic analysis of adenocarcinoma of the uterine cervix treated with radical hysterectomy and systematic lymphadenectomy. J Gynecol Oncol. 2013;24:222–228. doi: 10.3802/jgo.2013.24.3.222. http://dx.doi.org/10.3802/jgo.2013.24.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Havrilesky LJ, Leath CA, Huh W, Calingaert B, Bentley RC, Soper JT, et al. Radical hysterectomy and pelvic lymphadenectomy for stage IB2 cervical cancer. Gynecol Oncol. 2004;93:429–434. doi: 10.1016/j.ygyno.2004.01.038. http://dx.doi.org/10.1016/j.ygyno.2004.01.038. [DOI] [PubMed] [Google Scholar]
- 6.Benito V, Romeu S, Esparza M, Carballo S, Arencibia O, Medina N, et al. Safety and feasibility analysis of laparoscopic lymphadenectomy in pelvic gynecologic malignancies: a prospective study. Int J Gynecol Cancer. 2015;25:1704–1710. doi: 10.1097/IGC.0000000000000555. http://dx.doi.org/10.1097/IGC.0000000000000555. [DOI] [PubMed] [Google Scholar]
- 7.Holman LL, Levenback CF, Frumovitz M. Sentinel lymph node evaluation in women with cervical cancer. J Minim Invasive Gynecol. 2013;21:540–545. doi: 10.1016/j.jmig.2013.12.095. http://dx.doi.org/10.1016/j.jmig.2013.12.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rob L, Lukas R, Robova H, Helena R, Halaska MJ, Jiri HM, et al. Current status of sentinel lymph node mapping in the management of cervical cancer. Expert Rev Anticancer Ther. 2013;13:861–870. doi: 10.1586/14737140.2013.811147. http://dx.doi.org/10.1586/14737140.2013.811147. [DOI] [PubMed] [Google Scholar]
- 9.Van der Zee AGJ, Oonk MH, De Hullu JA, Ansink AC, Vergote I, Verheijen RH, et al. Sentinel node dissection is safe in the treatment of early-stage vulvar cancer. J Clin Oncol. 2008;26:884–889. doi: 10.1200/JCO.2007.14.0566. http://dx.doi.org/10.1200/JCO.2007.14.0566. [DOI] [PubMed] [Google Scholar]
- 10.Fader AN, Edwards RP, Cost M, Kanbour-Shakir A, Kelley JL, Schwartz B, et al. Sentinel lymph node biopsy in early-stage cervical cancer: utility of intra-operative versus postoperative assessment. Gynecol Oncol. 2008;111:13–17. doi: 10.1016/j.ygyno.2008.06.009. http://dx.doi.org/10.1016/j.ygyno.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 11.Kadkhodayan S, Hasanzadeh M, Treglia G, Azad A, Yousefi Z, Zarifmahmoudi L, et al. Sentinel node biopsy for lymph nodal staging of uterine cervix cancer: a systematic review and meta-analysis of the pertinent literature. Eur J Surg Oncol. 2015;41:1–20. doi: 10.1016/j.ejso.2014.09.010. http://dx.doi.org/10.1016/j.ejso.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network. Cervical Cancer (Version 1.2016) n.d. http://www.nccn.org/professionals/physician_gls/pdf/cervical.pdf (accessed February 11, 2016)
- 13.Wuntakal R, Papadopoulos AJ, Montalto SA, Perovic M, Coutts M, Devaja O. Location of sentinel lymph node in cervical carcinoma and factors associated with unilateral detection. Int J Gynecol Cancer. 2015 doi: 10.1097/IGC.0000000000000539. http://dx.doi.org/10.1097/IGC.0000000000000539. [DOI] [PubMed]
- 14.Sinno AK, Fader AN, Roche KL, Giuntoli RL, Tanner EJ. A comparison of colorimetric versus fluorometric sentinel lymph node mapping during robotic surgery for endometrial cancer. Gynecol Oncol. 2014;134:281–286. doi: 10.1016/j.ygyno.2014.05.022. http://dx.doi.org/10.1016/j.ygyno.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 15.Tanner EJ, Sinno AK, Stone RL, Levinson KL, Long KC, Fader AN. Factors associated with successful bilateral sentinel lymph node mapping in endometrial cancer. Gynecol Oncol. 2015;138:542–547. doi: 10.1016/j.ygyno.2015.06.024. http://dx.doi.org/10.1016/j.ygyno.2015.06.024. [DOI] [PubMed] [Google Scholar]
- 16.Schwartz GF, Giuliano AE, Veronesi U, Schwartz GF, Giuliano AE, Cady B, et al. Proceedings of the consensus conference on the role of sentinel lymph node biopsy in carcinoma of the breast, April 19–22, 2001, Philadelphia, Pennsylvania. Cancer. 2002;94:2542–2551. doi: 10.1002/cncr.10539. http://dx.doi.org/10.1002/cncr.10539. [DOI] [PubMed] [Google Scholar]
- 17.Cibula D, Abu-Rustum NR, Dusek L, Slama J, Zikán M, Zaal A, et al. Bilateral ultrastaging of sentinel lymph node in cervical cancer: lowering the false-negative rate and improving the detection of micrometastasis. Gynecol Oncol. 2012;127:462–466. doi: 10.1016/j.ygyno.2012.08.035. http://dx.doi.org/10.1016/j.ygyno.2012.08.035. [DOI] [PubMed] [Google Scholar]
- 18.Buda A, Crivellaro C, Elisei F, Di Martino G, Guerra L, De Ponti E, et al. Impact of indocyanine green for sentinel lymph node mapping in early stage endometrial and cervical cancer: comparison with conventional radiotracer (99 m)Tc and/or blue dye. Ann Surg Oncol. 2015 doi: 10.1245/s10434-015-5022-1. http://dx.doi.org/10.1245/s10434-015-5022-1. [DOI] [PMC free article] [PubMed]
- 19.Jewell EL, Huang JJ, Abu-Rustum NR, Gardner GJ, Brown CL, Sonoda Y, et al. Detection of sentinel lymph nodes in minimally invasive surgery using indocyanine green and near-infrared fluorescence imaging for uterine and cervical malignancies. Gynecol Oncol. 2014;133:274–277. doi: 10.1016/j.ygyno.2014.02.028. http://dx.doi.org/10.1016/j.ygyno.2014.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Imboden S, Papadia A, Nauwerk M, McKinnon B, Kollmann Z, Mohr S, et al. A comparison of radiocolloid and indocyanine green fluorescence imaging, sentinel lymph node mapping in patients with cervical cancer undergoing laparoscopic surgery. Ann Surg Oncol. 2015 doi: 10.1245/s10434-015-4701-2. http://dx.doi.org/10.1245/s10434-015-4701-2. [DOI] [PMC free article] [PubMed]
- 21.Alander JT, Kaartinen I, Laakso A, Pätilä T, Spillmann T, Tuchin VV, et al. A review of indocyanine green fluorescent imaging in surgery. Int J Biomed Imaging 2012. 2012 doi: 10.1155/2012/940585. http://dx.doi.org/10.1155/2012/940585. [DOI] [PMC free article] [PubMed]
- 22.Juretzka MM, Jensen KC, Longacre TA, Teng NN, Husain A. Detection of pelvic lymph node micrometastasis in stage IA2–IB2 cervical cancer by immunohistochemical analysis. Gynecol Oncol. 2004;93:107–111. doi: 10.1016/j.ygyno.2003.11.033. http://dx.doi.org/10.1016/j.ygyno.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 23.Fregnani JHTG, Latorre MRDO, Novik PR, Lopes A, Soares FA. Assessment of pelvic lymph node micrometastatic disease in stages IB and IIA of carcinoma of the uterine cervix. Int J Gynecol Cancer. 2006;16:1188–1194. doi: 10.1111/j.1525-1438.2006.00519.x. http://dx.doi.org/10.1111/j.1525-1438.2006.00519.x. [DOI] [PubMed] [Google Scholar]
- 24.Girardi F, Haas J. The importance of the histologic processing of pelvic lymph nodes in the treatment of cervical cancer. Int J Gynecol Cancer. 1993;3:12–17. doi: 10.1046/j.1525-1438.1993.03010012.x. http://dx.doi.org/10.1046/j.1525-1438.1993.03010012.x. [DOI] [PubMed] [Google Scholar]
- 25.Cibula D, Abu-Rustum NR, Dusek L, Zikán M, Zaal A, Sevcik L, et al. Prognostic significance of low volume sentinel lymph node disease in early-stage cervical cancer. Gynecol Oncol. 2012;124:496–501. doi: 10.1016/j.ygyno.2011.11.037. http://dx.doi.org/10.1016/j.ygyno.2011.11.037. [DOI] [PubMed] [Google Scholar]


