Abstract
Objectives
To describe the US national trends and factors associated with cytoreductive surgical radicality in women with advanced ovarian cancer (OC).
Methods
An analysis of the National Inpatient Sample database was performed. All admissions from 1993 to 2011 for advanced OC cytoreductive surgery (CRS) were identified and categorized as simple pelvic (SP), extensive pelvic (EP), and extensive upper abdominal (EUA) surgery. Annual trends in CRS were analyzed. Associations between patient- and hospital-specific factors, with CRS radicality as well as perioperative complications were explored between 2007 and 2011.
Results
In total, 28,677 un-weighted admissions were analyzed. The rate of EP and EUA resections increased over time (8% to 18.1% and 1.3% to 5.4%, P < 0.01, respectively). On multivariate analysis, patients were more likely to undergo EUA resections in the Northeast (OR 1.44) or West Coast (OR 1.47) at urban (OR 2.3), or large hospitals (OR 1.4), or if they had private insurance (OR 1.45). EUA surgeries were performed more frequently at high-volume ovarian cancer centers (OR 2.65); additionally, fewer complications were observed after EUA at high compared with low and medium volume hospitals (10.2%, 21.2%, and 21.7%, respectively; P = 0.01). Specifically, patients treated at high volume hospitals experienced lower rates of hemorrhage, vascular/nerve injury, prolonged hospitalization, and non-routine discharge than at lower (P < 0.05).
Conclusions
The US rate of radical cytoreductive surgery for advanced ovarian cancer is increasing. At high-volume hospitals, patients receive more radical surgery with fewer complications, supporting further study of a centralized ovarian cancer care model.
Keywords: Ovarian cancer, Cytoreductive surgery, Centralized care, Debulking, Upper abdominal procedures, Disparities
1. Introduction
The mainstay of treatment for advanced epithelial ovarian cancer consists of a combination of cytoreductive surgery and platinum/taxane based chemotherapy [1]. It is well established that residual disease volume following cytoreductive surgery is a major determinant of survival for women with ovarian cancer [1,2]. As the majority of women will be diagnosed with advanced stage disease, surgical cytoreduction often requires radical multi-organ resections to achieve the goal of “optimal” or complete removal of disease [3]. In 2009, Chi et al. described the improvement in both progression-free and overall survival resulting from a change in surgical paradigm occurring around the year 2000 [4]. The incorporation of radical upper abdominal surgery led to increased rates of optimal and more importantly complete gross resection of disease. While these procedures carry an increased risk of peri-operative morbidity [5], the National Comprehensive Cancer Network (NCCN) Guidelines for Ovarian Cancer and the Society for Gynecologic Oncology continue to recommend maximal surgical effort in the upfront setting when feasible due to the survival benefit associated with complete cytoreduction [1,4].
Unfortunately, access to cancer specialists such as gynecologic oncologists, and treatment with standard of care surgery and chemotherapeutic regimens vary significantly across the country. Disparities in ovarian cancer clearly still exist [6,7], with many studies demonstrating significant racial, socioeconomic and geographic disparities in the quality of ovarian cancer care in the United States [7–13]. Approximately 50% of women with ovarian cancer receive suboptimal surgical staging with factors such as hospital and surgeon ovarian cancer volume playing a significant role in the receipt of adequate care [14]. Furthermore, patients treated at hospitals with high ovarian cancer volumes are more likely to be offered NCCN adherent and experience better survival outcomes than those treated at lower volume facilities [11].
Determining the optimal allocation of maximal cytoreductive surgical efforts is complex and challenging. Patient and physician specific factors, such as a patient’s age, performance status, disease burden and personal preferences, as well as surgeon philosophy and expertise, all contribute to the decision making process. Other factors, such as race, socioeconomic status, insurance status, geographic location, and hospital type inevitably impact these decisions as well. Without a better understanding of these factors, the goal of achieving equal and optimal care to all patients with ovarian cancer becomes exceedingly difficult. Therefore, the objective of this study is to describe the trends in radical cytoreductive procedures in the United States over the past 20 years and to examine the association of patient and systems based factors on the rate and outcomes of radical cytoreductive surgery in a contemporary time period.
2. Materials and methods
2.1. Database and population
This was an institutional review board exempt, retrospective cohort analysis of the National Inpatient Sample Database of the Agency for Healthcare Research and Quality’s Healthcare Cost and Utilization Project (NIS-HCUP) [15,16]. The Nationwide Inpatient Sample is the largest publicly available database in the U.S. and includes patient-level hospital discharge data from a representative stratified, weighted sample of 1051 U.S. hospitals in 45 states. Sampling weights were applied to provide national estimates.
All admissions from 1993 to 2011 for women undergoing cytoreductive surgery for advanced ovarian cancer were included in the analysis. Due to the lag in reporting of national databases, at the time of data analysis only 2011 data was available. Furthermore, data after 2011 was abstracted using a different sampling mechanism which includes self-weighted systematic data. As such decision was made not to use data after 2011 to ensure homogeneity in data collection and applied weights and eliminate any bias in the trend analysis that could be introduced by comparing datasets with different sampling mechanisms.
Similar to prior studies [5,17] the definition for primary surgical treatment for ovarian cancer was defined as a laparotomy with oophorectomy (ICD9 65.3, 65.39, 65.5, 65.51, 65.52, 65.4, 65.49, 65.61, 65, 62) in combination with excision or destruction of a peritoneal lesion (ICD9 54.4) in patients with ovarian cancer (ICD9 183.0). Subsequently, analysis was limited to patients with advanced metastatic ovarian carcinoma by using the “late/advanced” variable as defined by the NIS-HCUP. Age was analyzed as a categorical variable of greater or <65 years, race as White, Black, Hispanic, Asian, or Native American (as per the HCUP classification) and comorbidities were assessed utilizing the Elixhauser Comorbidity Index as 0, 1–2, or >3. The Elixhauser Comorbidity Index is based on the presence or absence of 31 individual conditions. The index score is based on the cumulative number of conditions present [18]. The Elixhauser Comorbidity Software was developed as part of the Healthcare Cost and Utilization Project was used for calculation of this score. Patient income was stratified by quartile. Hospitals were then assessed for size, rural or urban setting, teaching status, and for geographic location (West, Midwest, Northeast, and South). Hospitals were further classified based upon annual ovarian cancer surgical volume into equal tertiles (low:1–6 cases annually, medium: 6–28 cases annually, high: >28 cases annually) by dividing the total number of ovarian cancer procedures performed by the number of years in which the individual hospital had at least one ovarian cancer surgery recorded.
Surgical procedures were classified into three categories based on the complexity of the surgical procedure and the utilization of radical procedures: simple pelvic (SP), extensive pelvic (EP), or extensive upper abdominal (EUA). Patients were classified into the EUA category if they underwent a splenectomy, pancreatic resection, cholecystectomy, liver resection, or diaphragm resection. Patients were categorized into the EP category if they received procedures that include small bowel resection, recto-sigmoid resection, colectomy, or a bladder resection. All other patients were classified as SP. Postoperative complications that occurred during the primary hospitalization were grouped based on their nature into medical complications (myocardial infarction, cardiopulmonary arrest, respiratory failure, renal failure, stroke, shock, and venous thromboembolism), infectious complications (wound infections, pneumonia, bacteremia, postoperative abscess), hemorrhage, nerve or vessel injury, prolonged hospitalization (index hospitalization >7 days) and non-routine discharge (discharge to skilled nursing facility, rehab, short term hospital, intermediate care facility, or hospice). Perioperative mortality was defined as death during the index hospitalization.
2.2. Statistics
Trends in cytoreductive effort were abstracted from 1993 to 2011 and stratified across the three surgical categories (SP, EP, EUA). The Joint-point Regression Program, (Version 4.2.0.2. June 2015; Statistical Research and Applications Branch, National Cancer Institute) was used for trend analysis with significance set at 0.05% [19]. The average annual percent change (AAPC) was calculated for each surgical category between 1993 and 2011. Specific trends were identified and more detailed trend analysis was undertaken for a better understanding of the temporal changes using 2 joint points. The specific annual percent change (APC) was then calculated for the time periods between each joint point. The empirical quantile method and the parametric method were used to construct 95% CI for AAPC and APC.
To evaluate factors associated with surgical radicality in a contemporary time period, data from patients with advanced ovarian cancer between 2007 and 2011 was weighted and grouped and then compared utilizing the χ2 test. Multivariable logistic regression analysis was then performed to identify factors that were independently associated with the performance of EUA procedures. Finally, Fischer’s Exact test was used to identify the impact of hospital ovarian cancer volume on the rate complications, specifically in patients who received EUA procedures. STATA 13 and SAS 9.2 were used for statistical analysis with statistical significance set at 0.05.
3. Results
3.1. Trend analysis 1993–2011 (Figs. 1, 2)
Fig. 1.
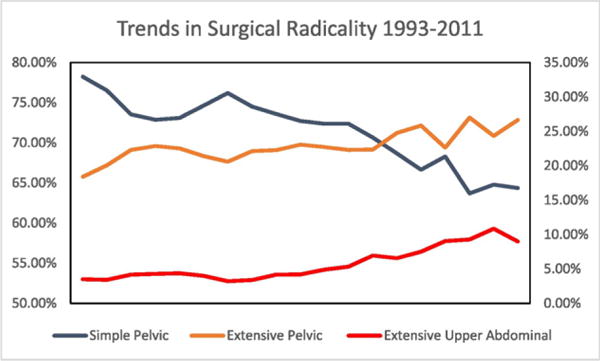
Trends in Surgical Radicality in Ovarian Cancer 1993–2011. Simple pelvic (left axis): procedures not classified as extensive pelvic or extensive upper abdominal. Extensive Pelvic (right axis): procedures that include small bowel resection, recto-sigmoid resection, colectomy, bladder resection. Extensive upper abdominal (right axis): procedures that include splenectomy, cholecystectomy, liver resection, diaphragm resection.
Fig. 2.
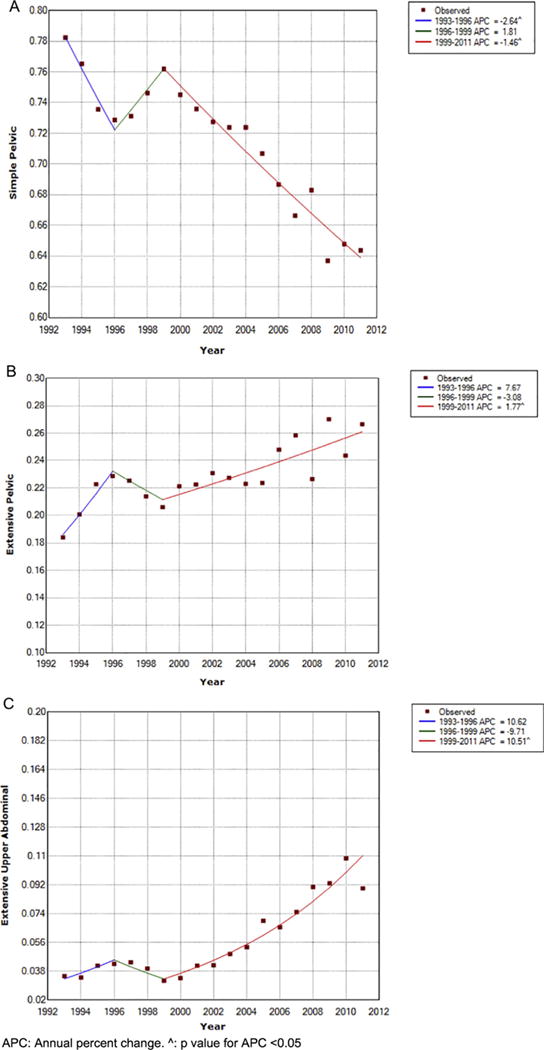
Trend Analysis for the Proportions of Simple Pelvic (2A), Extensive Pelvic (2B), and Extensive Upper Abdominal Procedures (2C). APC: annual percent change. ˆ: p value for APC <0.05.
Between 1993 and 2011, 28,677 primary un-weighted surgical admissions for advanced ovarian cancer were identified with in the NIS database. Simple pelvic resections were performed in 71.4%, extensive pelvic in 22.9%, and extensive upper abdominal in 5.7% of patients. The rate of SP resection decreased significantly over the time period with an average annual percentage change (AAPC) of −1.1%, 95% CI −1.7, −1.2). The rate of decline was sharpest between 1993 and 1996 (APC: −2.6%, 95% CI −5.0%, −0.2%), was stable between 1996 and 1999 (APC: 1.85, 95% CI −3.1%, 6.9%) and resumed its decline between 1999 and 2011 (APC −1.5%, 95% CI −1.7%, −1.2%). The rate of extensive pelvic procedures increased over the study period with an AAPC of 1.8%, 95% CI 0.0%–3.5%. The proportion of patients receiving EP remained stable between 1993 and 1996 and between 1996 and 1999 (APC 7.7% and −3.1% p value > 0.05) but significantly increased after 1999 with an APC of 1.8%, 95% CI 0.9%–2.7%. Similarly, the proportion of patients undergoing extensive upper abdominal procedures remained stable between 1993 and 1996 and 1996–1999 (APC 10.6 and −9.7 p value > 0.05) but exhibited a sharp and continuous increase after 1999 with an APC of 10.5%, 95% CI 5.6%–12.5%. The AAPC for EUA from 1993 to 2011 was 6.9%, 95% CI 1.6%–12.4%.
3.2. Factors associated with extensive upper abdominal procedures (2007–2011)
To identify factors associated with extensive upper abdominal procedures during a contemporary time period, the second part of our analysis was limited to the last five years of available data (2007–2011). During this time period, and after applying the appropriate statistical weights, 34,387 primary weighted cytoreductive procedures were identified (Table 1). Most patients were younger than 65 (58.3%), white (79.3%), had private insurance (45.9%) and had an Elixhauser score >3 (50.5%). Ovarian cancer surgery was performed mostly at large hospitals (73.8%), assigned as teaching institutions (76.4%). Most procedures were performed at medium and high ovarian cancer volume hospitals (43.7% and 43.3% respectively).
Table 1.
Weighted demographic and clinical factors 2007–2011 n = 34,387.
| Total number | Percent | |
|---|---|---|
| Age | ||
| <65 | 20,049 | 58.3 |
| >65 | 14,336 | 41.7 |
| Elixhauser score | ||
| 0 | 15,539 | 45.2 |
| 1–2 | 1508 | 4.3 |
| >3 | 17,337 | 50.5 |
| Race/ethnicity | ||
| White | 22,966 | 79.3 |
| Black | 1732 | 6.0 |
| Hispanic | 2229 | 7.7 |
| Asian | 822 | 2.8 |
| Native American | 1219 | 4.2 |
| Income quartile | ||
| Q1 | 6672 | 19.8 |
| Q2 | 7681 | 22.8 |
| Q3 | 8854 | 26.3 |
| Q4 | 10,462 | 31.1 |
| Hospital size | ||
| Small | 2460 | 7.3 |
| Medium | 6443 | 18.9 |
| Large | 25,139 | 73.8 |
| Hospital type | ||
| Rural | 901 | 2.6 |
| Urban | 22,141 | 97.4 |
| Hospital teaching status | ||
| Teaching | 26,007 | 76.4 |
| Nonteaching | 8035 | 23.6 |
| Hospital location | ||
| Northeast | 7428 | 21.6 |
| Midwest | 7368 | 21.5 |
| South | 11,515 | 33.5 |
| West | 8058 | 23.4 |
| Insurance status | ||
| Medicare | 14,201 | 41.4 |
| Medicaid | 2284 | 6.6 |
| Commercial insurance | 15,741 | 45.9 |
| Other | 2093 | 6.1 |
| Ovarian cancer volume | ||
| Low | 4468 | 13.0 |
| Medium | 15,030 | 43.7 |
| High | 14,888 | 43.3 |
On univariate analysis, patients were more likely to receive EUA procedures if they were younger than 65 years of age (p < 0.001), were treated at urban (p = 0.01) teaching (0.002) hospitals, or were operated on in the Northeast (p < 0.001). Furthermore, patients who underwent EUA procedures were more likely to have a higher Elixhauser comorbidity index on univariate analysis. While race and income quartile were not significantly associated with surgical radicality (p > 0.05), insurance status was, with the highest proportion of patients undergoing EUA procedures found in those who were privately insured (p < 0.001). Hospital ovarian cancer volume was significantly associated with performance of EUA procedures as well, with the highest proportion of patients receiving EUA procedures occurring at high ovarian cancer volume centers (>28 cases annually) (Table 2).
Table 2.
Univariate analysis of factors associated with surgical radicality in advanced ovarian cancer n = 34,387.
| Simple pelvic | Extended pelvic | Extensive upper abdominal | P value | |
|---|---|---|---|---|
| Age | ||||
| <65 | 67.6% | 22.0% | 10.3% | |
| >65 | 62.9% | 29.5% | 7.5% | <0.001 |
| Elixhauser score | ||||
| 0 | 71.5% | 20.6% | 7.9% | |
| 1–2 | 71.0% | 21.9% | 7.2% | |
| >3 | 59.9% | 29.6% | 10.5% | <0.001 |
| Race | ||||
| White | 64.4% | 26.4% | 9.1% | |
| Black | 68.5% | 23.1% | 8.4% | |
| Hispanic | 69.2%% | 21.3% | 9.4% | |
| Asian | 67.2% | 23.0% | 10.1% | |
| Native American | 66.5% | 21.7% | 11.7% | 0.098 |
| Income quartile | ||||
| Q1 | 65.7 | 25.1 | 9.2 | |
| Q2 | 67.8 | 24.7 | 7.5 | |
| Q3 | 66.1 | 25.1 | 8.8 | |
| Q4 | 64.2 | 25.3 | 10.5 | 0.095 |
| Hospital size | ||||
| Small | 63.4 | 25.6 | 11.1 | |
| Middle | 71.3 | 23.3 | 5.4 | |
| Large | 64.3 | 25.7 | 10.0 | <0.001 |
| Hospital type | ||||
| Rural | 75.1 | 20.9 | 3.9 | |
| Urban | 65.3 | 25.3 | 9.4 | 0.01 |
| Teaching | 65.5 | 24.6 | 9.9 | |
| Nonteaching | 65.8 | 27.3 | 7.0 | 0.002 |
| Hospital location | ||||
| Northeast | 66.6 | 22.1 | 11.3 | |
| Midwest | 67.6 | 23.3 | 9.1 | |
| South | 64.8 | 27.8 | 7.4 | |
| West | 64.2 | 26.0 | 9.8 | <0.0001 |
| Insurance status | ||||
| Medicare | 63.7 | 29.3 | 7.0 | |
| Medicaid | 70.3 | 19.2 | 10.5 | |
| Commercial insurance | 66.2 | 23.0 | 10.8 | |
| Other | 69.9 | 19.8 | 10.4 | <0.0001 |
| Ovarian cancer volume | ||||
| Low | 67.7 | 27.6 | 4.8 | |
| Medium | 68.1 | 24.3 | 7.6 | |
| High | 62.6 | 25.4 | 12.1 | <0.0001 |
On multivariate analysis (Table 3) hospital ovarian cancer volume was most predicative of performance of EUA procedures OR 2.65, 95% CI (1.82–3.83). Other factors associated with EUA resections include urban location OR 2.3, 95% CI (1.07–4.97), large hospitals OR 1.4, 95% CI (1.01–2.19), and private insurance OR 1.45, 95% CI (1.09–195). Patients treated in the South had the lowest rate of EUA procedures (referent), where patients treated in the Northeast (OR 1.44, 95% CI 1.16–1.78) and West Coast (OR 1.47, 95% CI 1.19–1.82) had the highest percentage of EUA resections. Age, race, and hospital teaching status were not found to be significant (P < 0.05).
Table 3.
Multivariate analysis of factors associated with extensive upper abdominal procedures.
| Odds Ratio | P Value | 95% CI LL | 95% CI UL | |
|---|---|---|---|---|
| Age > 65 | 1.10 | 0.51 | 0.83 | 1.47 |
| Elixhauser score | ||||
| 0 | (Ref) | |||
| 1,2 | 0.93 | 0.75 | 0.61 | 1.42 |
| >3 | 2.01 | 0.00 | 1.70 | 2.37 |
| Race | ||||
| White | (Ref) | |||
| Black | 0.94 | 0.72 | 0.66 | 1.34 |
| Hispanic | 1.08 | 0.63 | 0.80 | 1.46 |
| Asian | 1.00 | 0.99 | 0.65 | 1.56 |
| Native American | 1.23 | 0.29 | 0.84 | 1.80 |
| Hospital Size | ||||
| Small | (Ref) | |||
| Middle | 0.91 | 0.67 | 0.59 | 1.41 |
| Large | 1.57 | 0.02 | 1.07 | 2.32 |
| Hospital type | ||||
| Rural | Ref | |||
| Urban | 2.53 | 0.02 | 1.18 | 5.43 |
| Teaching status | ||||
| Non-teaching | Ref | |||
| Teaching | 0.92 | 0.45 | 0.74 | 1.14 |
| Hospital location | ||||
| South | Ref | |||
| Northeast | 1.44 | 0.00 | 1.16 | 1.78 |
| Midwest | 1.10 | 0.48 | 0.84 | 1.44 |
| West | 1.47 | 0.00 | 1.19 | 1.82 |
| Insurance status | ||||
| Medicare | Ref | |||
| Medicaid | 1.34 | 0.16 | 0.89 | 2.02 |
| Commercial insurance | 1.45 | 0.01 | 1.09 | 1.95 |
| Other | 1.30 | 0.21 | 0.86 | 1.97 |
| Ovarian cancer volume | ||||
| Low | Ref | |||
| Medium | 1.78 | 0.00 | 1.27 | 2.48 |
| High | 2.42 | 0.00 | 1.74 | 3.38 |
3.3. Complications in patients undergoing extensive upper abdominal procedures (2007–2011)
During the 2007–2011 time period, 3156 patients underwent extensive upper abdominal procedures. Within this subgroup of patients, complications were stratified by hospital ovarian cancer volume (Table 4). The rate of any complication occurring was significantly lower in high volume hospitals (18%) when compared with low and medium volume hospitals (23.8% and 21.7% respectively p = 0.01). The OR for any complication occurring when comparing high volume hospitals versus medium and low ovarian cancer volume hospitals was 0.77, 95% CI (0.65–0.92). Similarly, performance of EUA procedures at high ovarian cancer volume hospitals (>28 cases annually) resulted in less patients experiencing prolonged hospitalization OR 0.79, 95% CI (0.64–0.98) and less vascular and nerve injury at the time of surgery OR 0.48 95% CI (0.27–0.85). Patients treated at low ovarian cancer volume hospitals (<6 cases annually) were more likely to experience an intraoperative hemorrhage OR 3.02 95% CI (1.05–8.64).
Table 4.
Complications in extensive upper abdominal group by hospital ovarian cancer volume.
| Hospital ovarian cancer volume
|
||||
|---|---|---|---|---|
| Complications in patients undergoing EUA n = 3156 | Low | Medium | High | p value |
| Any complication | 23.8 | 21.7 | 18.0 | 0.01 |
| Medical complication | 0.9 | 1.2 | 1.0 | 0.2 |
| Infectious complication | 3.3 | 3.1 | 2.8 | 0.5 |
| Hemorrhage | 1.9 | 1.4 | 0.8 | 0.09 |
| Nerve or vessel injury | 1.4 | 2.1 | 1.1 | <0.01 |
| Prolonged Hospitalization > 7 days | 15.0 | 12.7 | 10.9 | 0.04 |
| Non-routine discharge | 0.9 | 0.8 | 1.3 | 0.2 |
| Mortality | 0.5 | 0.3 | 0.1 | 0.11 |
4. Discussion
Our data suggest that over the last two decades, there has been a nationwide trend in the U.S. towards more radical cytoreductive surgery for women with advanced ovarian cancer. Traditional “simple” cytoreduction (categorized as “simple pelvic” resections) occur less frequently and the incorporation of extensive upper abdominal procedures in cytoreductive surgical practice has increased. While these data demonstrate that surgical efforts have become increasingly radical over time, they also highlight significant disparities in access to optimal surgical care in this country. Whereas previous studies have shown racial, socioeconomic, and geographic disparities exist with regard to adherence to minimal NCCN guidelines in ovarian cancer care [14,17,20,21], our study results suggest that there may also be significant disparities with regard to access to optimal surgical care, and specifically, access to radical surgical procedures that lead to better survival outcomes for patients [4,18,19]. Patients who underwent the most radical procedures (EUA) at the time of ovarian cancer surgery were more likely to have private insurance and receive treatment at large, high volume and urban hospitals. These factors suggest that the socioeconomic and geographic inequalities in our society may translate to a disparity in the care that women with ovarian cancer receive, with insured women living in urban areas having potentially greater access to high quality subspecialty oncology care.
Notably, our findings suggest that race is not an independent predictor of access to radical surgery. Prior studies have demonstrated the negative association between black race and NCCN guideline adherent care [22], and a shorter overall survival in black women with ovarian cancer [23]. On the other hand, the Health Disparities Taskforce of the Society of Gynecologic Oncology reported in 2014 that once adjustments are made for disease characteristics and socioeconomic status, racial disparities are reduced and/or eliminated in many studies [24]. It is difficult to isolate race as an independent variable given the intertwined nature of race and socioeconomic status in the United States and it also is plausible that unevaluated confounders still exist that could impact access to radical surgical care.
As the radicality of ovarian cancer surgery increases, so does the risk of perioperative morbidity. Previous analysis of the same HCUP-NIS database reported a higher rate of complications with the use of radical cytoreductive procedures at the time of ovarian cancer surgery [5]. Other studies have shown that despite the increased morbidity of these surgical procedures, there is still a survival benefit to optimal cytoreduction, especially when the outcome is no gross residual disease. Therefore, the goal of these procedures should be to maximize oncologic outcomes while minimizing surgical morbidity. The current study examined the rates of complications amongst patients who undergo a similar cytoreductive effort, specifically, the extensive upper abdominal procedures group. When these procedures are performed at high volume centers (performing >28 cases annually), the overall complication rate was significantly lower than when these procedures are performed at low and medium volume centers (centers that perform < 28 cases annually). A recent Nationwide Inpatient Sample study showed that despite having a slightly higher rate of postoperative complications, high volume ovarian cancer hospitals were able to “rescue” patients at a significantly higher rate after complications arise [25], whereas patients treated at low volume hospitals were 50% more likely to die after experiencing a complication. Currently, 65–80% of initial surgery for advanced ovarian cancer in the United States is performed at low to medium volume centers [26,27].
Our results contribute to the growing body of literature supporting a centralized care model for women with advanced ovarian cancer. Such model would result in a larger proportion of women receiving care at high volume ovarian cancer centers which is associated with higher rates of complete cytoreduction [28], longer overall survival [29,30], and as described above, a lower rate of perioperative complications. Bristow et al., further showed that while initial cost of referring patients to high volume centralized ovarian cancer centers may be higher, the improvement in quality of life adjusted years results in significant cost effectiveness [31].
A centralized cancer care model has been implemented in smaller countries with more homogeneously spread out population centers [32]. In the US, specialized centers have been award NCI designation as when a critical mass of cancer related expertise is available. To gain a comprehensive cancer center designation a center must meet criteria that include programs in clinical, basic science and translational science research. Furthermore it must contribute to the training and education of healthcare professionals, perform community outreach and education, include cancer prevention and control programs, and provide cancer information services. Most importantly, the center must be involved in innovative cancer treatments and clinical trials. For other cancer disease sites, surgical outcomes have been shown to be improved for patients treated at NCI-designated Cancer Centers [33]. Cancer specific mortality has additionally been shown to be decreased when patients are treated as NCI-designated sites [34]. Our data showed a similar effect of treatment at centers where disease specific expertise is available, and although race was not correlated to receipt of more radical surgery in our model, our results mirror other studies which have shown insurance and socio-economic status to be significant barriers receipt of high quality care at high volume centers.
While decreasing the number of centers that provide cancer care will undoubtedly be associated with the benefits described above, the distance to these centralized centers should not exceed a yet to be determined threshold distance to avoid creating another barrier to care. Furthermore, third party payers must be willing to accept the increase initial cost that may result from referral to centers of excellence and must be incentivized to do so. These high volume centers should be equipped with the ability to accept the increased patient volume associated with new referral practices to avoid treatment delays that can negatively impact patient outcomes. These, and other barriers to centralized cancer care should be addressed by our evolving health care system to improve quality of care and the quality of life of cancer patients.
Given this growing body of evidence, it is difficult to justify performance of complex, high morbidity surgery for ovarian cancer by hospitals and surgeons without the minimal volume, and training to develop the expertise required to manage all aspects of perioperative care in ovarian cancer surgery. Fewer than 50% of patients with ovarian cancer in the United States are treated by gynecologic oncologists [35] and this has been shown to negatively impact survival [36] and increase postoperative complications and death [25]. As such, local health care leadership should identify situations where referral to higher levels of care is logistically feasible and encourage these referrals from lower volume surgeons and non-gynecologic oncologists, and from lower ovarian cancer volume hospitals. Adherence to responsible referral patterns should be considered a quality measure to further encourage best practices.
Limitations of this study include those inherent to administrative claims databases especially with regard to incomplete coding and missing data. This study is also limited by the lack of data on residual disease, chemotherapeutic regimens, and survival outcomes, and therefore, the assumption of benefit from radical cytoreductive surgery must be extrapolated from prior work. Furthermore, due to the inevitable time lag associated with administrative claims data, there could theoretically be change in the trend of radical cytoreductive surgery after 2011. Furthermore, the etiology of the low rate of EUA resections (5.4%) is unclear but probably multifactorial. Surgeon philosophy, skill level, comfort with radical upper abdominal resections, and having the infrastructure to postoperatively manage patients who receive these radical procedures could potentially all play a role in this lower rate. As with any administrative claims database, failure to capture is another potential factor. This may be less likely given the extensive validation of the HCUP-NIS database.
In conclusion, this study describes an increase in radicality of ovarian cancer surgery in recent times. Surgical care is not uniform, however, differences in geographic location, hospital type, and insurance status are seen in the application of optimal surgical management. In order to provide the optimal surgical care to patients to maximize oncologic outcomes and minimize complications, systems-related disparities must be examined and addressed. Centralization of care, or development of more formal hybrid, systems-based care models, is one potential mechanism to optimize the care of all women with ovarian cancer.
HIGHLIGHTS.
Ovarian cancer surgical radicality has increased in the United States.
A disparity exists in the receipt of maximal cytoreductive effort in the US.
Both patient and healthcare system factors contribute to this disparity.
Ovarian cancer volume correlates with surgical radicality and complication rates.
A centralized ovarian cancer care model may improve patient outcomes.
Footnotes
Financial disclosure
The authors did not report any potential conflicts of interest.
References
- 1.Bristow RE, Tomacruz RS, Armstrong DK, Trimble EL, Montz FJ. Survival effect of maximal cytoreductive surgery for advanced ovarian carcinoma during the platinum era: a meta-analysis. J Clin Oncol. 2002;20(5):1248–1259. doi: 10.1200/JCO.2002.20.5.1248. [DOI] [PubMed] [Google Scholar]
- 2.Hoskins WJ. Epithelial ovarian carcinoma: principles of primary surgery. Gynecol Oncol. 1994;55(3 Pt 2):S91–S96. doi: 10.1006/gyno.1994.1346. [DOI] [PubMed] [Google Scholar]
- 3.Griffiths CT. Surgical resection of tumor bulk in the primary treatment of ovarian carcinoma. Natl Cancer Inst Monogr. 1975;42:101–104. [PubMed] [Google Scholar]
- 4.Chi DS, Eisenhauer EL, Zivanovic O, Sonoda Y, Abu-Rustum NR, Levine DA, et al. Improved progression-free and overall survival in advanced ovarian cancer as a result of a change in surgical paradigm. Gynecol Oncol. 2009;114(1):26–31. doi: 10.1016/j.ygyno.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Wright JD, Lewin SN, Deutsch I, Burke WM, Sun X, Neugut AI, et al. Defining the limits of radical cytoreductive surgery for ovarian cancer. Gynecol Oncol. 2011;123(3):467–473. doi: 10.1016/j.ygyno.2011.08.027. [DOI] [PubMed] [Google Scholar]
- 6.Thrall MM, Gray HJ, Symons RG, Weiss NS, Flum DR, Goff BA. Trends in treatment of advanced epithelial ovarian cancer in the Medicare population. Gynecol Oncol. 2011;122(1):100–106. doi: 10.1016/j.ygyno.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zeng C, Wen W, Morgans AK, Pao W, Shu XO, Zheng W. Disparities by race, age, and sex in the improvement of survival for major cancers: results from the National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) program in the United States, 1990 to 2010. JAMA Oncol. 2015;1(1):88–96. doi: 10.1001/jamaoncol.2014.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Howard J, Hankey BF, Greenberg RS, Austin DF, Correa P, Chen VW, et al. A collaborative study of differences in the survival rates of black patients and white patients with cancer. Cancer. 1992;69(9):2349–2360. doi: 10.1002/1097-0142(19920501)69:9<2349::aid-cncr2820690925>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 9.Chan JK, Zhang M, Hu JM, Shin JY, Osann K, Kapp DS. Racial disparities in surgical treatment and survival of epithelial ovarian cancer in United States. J Surg Oncol. 2008;97(2):103–107. doi: 10.1002/jso.20932. [DOI] [PubMed] [Google Scholar]
- 10.Dottino JA, Cliby WA, Myers ER, Bristow RE, Havrilesky LJ. Improving NCCN guideline-adherent care for ovarian cancer: value of an intervention. Gynecol Oncol. 2015;138(3):694–699. doi: 10.1016/j.ygyno.2015.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Bristow RE, Palis BE, Chi DS, Cliby WA. The National Cancer Database report on advanced-stage epithelial ovarian cancer: impact of hospital surgical case volume on overall survival and surgical treatment paradigm. Gynecol Oncol. 2010;118(3):262–267. doi: 10.1016/j.ygyno.2010.05.025. [DOI] [PubMed] [Google Scholar]
- 12.Terplan M, Schluterman N, McNamara EJ, Tracy JK, Temkin SM. Have racial disparities in ovarian cancer increased over time? An analysis of SEER data. Gynecol Oncol. 2012;125(1):19–24. doi: 10.1016/j.ygyno.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 13.Temkin SM, Fleming SA, Amrane S, Schluterman N, Terplan M. Geographic disparities amongst patients with gynecologic malignancies at an urban NCI-designated cancer center. Gynecol Oncol. 2015;137(3):497–502. doi: 10.1016/j.ygyno.2015.03.010. [DOI] [PubMed] [Google Scholar]
- 14.Goff BA, Matthews BJ, Wynn M, Muntz HG, Lishner DM, Baldwin LM. Ovarian cancer: patterns of surgical care across the United States. Gynecol Oncol. 2006;103(2):383–390. doi: 10.1016/j.ygyno.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Overview of the Nationwide Inpatient Sample (NIS), Healthcare Cost and Utilization Project (HCUP) 2013 Available at: www.hcup-us.ahrq.gov/nisoverview.jsp.
- 16.Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality; Rockville, MD: 2011. www.hcup-us.ahrq.gov/nisoverview.jsp. [PubMed] [Google Scholar]
- 17.Goff BA, Matthews BJ, Larson EH, Andrilla CH, Wynn M, Lishner DM, et al. Predictors of comprehensive surgical treatment in patients with ovarian cancer. Cancer. 2007;109(10):2031–2042. doi: 10.1002/cncr.22604. [DOI] [PubMed] [Google Scholar]
- 18.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36(1):8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19(3):335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 20.Galvan-Turner VB, Chang J, Ziogas A, Bristow RE. Observed-to-expected ratio for adherence to treatment guidelines as a quality of care indicator for ovarian cancer. Gynecol Oncol. 2015;139(3):495–499. doi: 10.1016/j.ygyno.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bristow RE, Chang J, Ziogas A, Anton-Culver H. Adherence to treatment guidelines for ovarian cancer as a measure of quality care. Obstet Gynecol. 2013;121(6):1226–1234. doi: 10.1097/AOG.0b013e3182922a17. [DOI] [PubMed] [Google Scholar]
- 22.Bristow RE, Chang J, Ziogas A, Campos B, Chavez LR, Anton-Culver H. Sociodemographic disparities in advanced ovarian cancer survival and adherence to treatment guidelines. Obstet Gynecol. 2015;125(4):833–842. doi: 10.1097/AOG.0000000000000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Howell EA, Egorova N, Hayes MP, Wisnivesky J, Franco R, Bickell N. Racial disparities in the treatment of advanced epithelial ovarian cancer. Obstet Gynecol. 2013;122(5):1025–1032. doi: 10.1097/AOG.0b013e3182a92011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins Y, Holcomb K, Chapman-Davis E, Khabele D, Farley JH. Gynecologic cancer disparities: a report from the health disparities taskforce of the society of gynecologic oncology. Gynecol Oncol. 2014;133(2):353–361. doi: 10.1016/j.ygyno.2013.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wright JD, Herzog TJ, Siddiq Z, Arend R, Neugut AI, Burke WM, et al. Failure to rescue as a source of variation in hospital mortality for ovarian cancer. J Clin Oncol. 2012;30(32):3976–3982. doi: 10.1200/JCO.2012.43.2906. [DOI] [PubMed] [Google Scholar]
- 26.Cliby WA, Powell MA, Al-Hammadi N, Chen L, Miller J Philip, Roland PY, et al. Ovarian cancer in the United States: contemporary patterns of care associated with improved survival. Gynecol Oncol. 2015;136(1):11–17. doi: 10.1016/j.ygyno.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bristow RE, Chang J, Ziogas A, Randall LM, Anton-Culver H. High-volume ovarian cancer care: survival impact and disparities in access for advanced-stage disease. Gynecol Oncol. 2014;132(2):403–410. doi: 10.1016/j.ygyno.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Kumpulainen S, Kuoppala T, Leminen A, Penttinen J, Puistola U, Pukkala E, et al. Surgical treatment of ovarian cancer in different hospital categories–a prospective nation-wide study in Finland. Eur J Cancer. 2006;42(3):388–395. doi: 10.1016/j.ejca.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 29.Woo YL, Kyrgiou M, Bryant A, Everett T, Dickinson HO. Centralisation of services for gynaecological cancers - a Cochrane systematic review. Gynecol Oncol. 2012;126(2):286–290. doi: 10.1016/j.ygyno.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 30.Phippen NT, Barnett JC, Lowery WJ, Miller CR, Leath CA., 3rd Surgical outcomes and national comprehensive cancer network compliance in advanced ovarian cancer surgery in a low volume military treatment facility. Gynecol Oncol. 2013;131(1):158–162. doi: 10.1016/j.ygyno.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Bristow RE, Santillan A, Diaz-Montes TP, Gardner GJ, Giuntoli RL, 2nd, Meisner BC, et al. Centralization of care for patients with advanced-stage ovarian cancer: a cost-effectiveness analysis. Cancer. 2007;109(8):1513–1522. doi: 10.1002/cncr.22561. [DOI] [PubMed] [Google Scholar]
- 32.Dahm-Kahler P, Palmqvist C, Staf C, Holmberg E, Johannesson L. Centralized primary care of advanced ovarian cancer improves complete cytoreduction and survival - a population-based cohort study. Gynecol Oncol. 2016;142(2):211–216. doi: 10.1016/j.ygyno.2016.05.025. [DOI] [PubMed] [Google Scholar]
- 33.Paulson EC, Mitra N, Sonnad S, Armstrong K, Wirtalla C, Kelz RR, et al. National Cancer Institute designation predicts improved outcomes in colorectal cancer surgery. Ann Surg. 2008;248(4):675–686. doi: 10.1097/SLA.0b013e318187a757. [DOI] [PubMed] [Google Scholar]
- 34.Wolfson JA, Sun CL, Wyatt LP, Hurria A, Bhatia S. Impact of care at comprehensive cancer centers on outcome: results from a population-based study. Cancer. 2015;121(21):3885–3893. doi: 10.1002/cncr.29576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chan JK, Kapp DS, Shin JY, Husain A, Teng NN, Berek JS, et al. Influence of the gynecologic oncologist on the survival of ovarian cancer patients. Obstet Gynecol. 2007;109(6):1342–1350. doi: 10.1097/01.AOG.0000265207.27755.28. [DOI] [PubMed] [Google Scholar]
- 36.Earle CC, Schrag D, Neville BA, Yabroff KR, Topor M, Fahey A, et al. Effect of surgeon specialty on processes of care and outcomes for ovarian cancer patients. J Natl Cancer Inst. 2006;98(3):172–180. doi: 10.1093/jnci/djj019. [DOI] [PubMed] [Google Scholar]


