Abstract
Messenger RNA for yeast cytosolic polypeptide chain elongation factor 1 alpha (EF-1 alpha) was partially purified from Saccharomyces cerevisiae. Double-stranded complementary DNA (cDNA) was synthesized and cloned in Escherichia coli with pBR327 as a vector. Recombinant plasmid carrying yEF-1 alpha cDNA was identified by cross-hybridization with the E. coli tufB gene and the yeast mitochondrial EF-Tu gene (tufM) under non-stringent conditions. A yeast gene library was then screened with the EF-1 alpha cDNA and several clones containing the chromosomal gene for EF-1 alpha were isolated. Restriction analysis of DNA fragments of these clones as well as the Southern hybridization of yeast genomic DNA with labelled EF-1 alpha cDNA indicated that there are two EF-1 alpha genes in S. cerevisiae. The nucleotide sequence of one of the two EF-1 alpha genes (designated as EF1 alpha A) was established together with its 5'- and 3'-flanking sequences. The sequence contained 1374 nucleotides coding for a protein of 458 amino acids with a calculated mol. wt. of 50 300. The derived amino acid sequence showed homologies of 31% and 32% with yeast mitochondrial EF-Tu and E. coli EF-Tu, respectively.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amons R., Pluijms W., Roobol K., Möller W. Sequence homology between EF-1 alpha, the alpha-chain of elongation factor 1 from Artemia salina and elongation factor EF-Tu from Escherichia coli. FEBS Lett. 1983 Mar 7;153(1):37–42. doi: 10.1016/0014-5793(83)80115-x. [DOI] [PubMed] [Google Scholar]
- An G., Friesen J. D. The nucleotide sequence of tufB and four nearby tRNA structural genes of Escherichia coli. Gene. 1980 Dec;12(1-2):33–39. doi: 10.1016/0378-1119(80)90013-x. [DOI] [PubMed] [Google Scholar]
- Arai K., Clark B. F., Duffy L., Jones M. D., Kaziro Y., Laursen R. A., L'Italien J., Miller D. L., Nagarkatti S., Nakamura S. Primary structure of elongation factor Tu from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1326–1330. doi: 10.1073/pnas.77.3.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arai K., Kawakita M., Nakamura S., Ishikawa K., Kaziro Y. Studies on the polypeptide elongation factors form E. coli. VI. Characterization of sulfhydryl groups in EF-Tu and EF-Ts. J Biochem. 1974 Sep;76(3):523–534. doi: 10.1093/oxfordjournals.jbchem.a130596. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp P. M., Horn E. W., Gross S. R. Proposed involvement of an internal promoter in regulation and synthesis of mitochondrial and cytoplasmic leucyl-tRNA synthetases of Neurospora. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1172–1176. doi: 10.1073/pnas.74.3.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
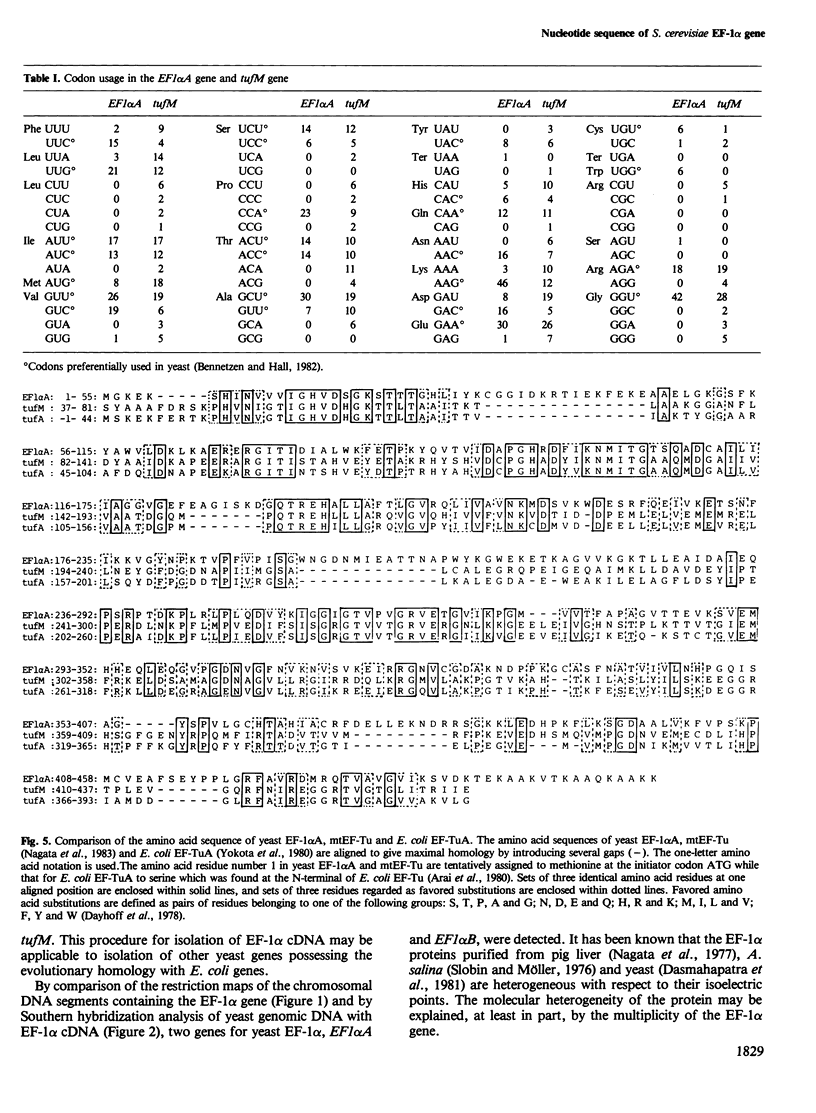
- Bennetzen J. L., Hall B. D. Codon selection in yeast. J Biol Chem. 1982 Mar 25;257(6):3026–3031. [PubMed] [Google Scholar]
- Carlson M., Botstein D. Two differentially regulated mRNAs with different 5' ends encode secreted with intracellular forms of yeast invertase. Cell. 1982 Jan;28(1):145–154. doi: 10.1016/0092-8674(82)90384-1. [DOI] [PubMed] [Google Scholar]
- Cryer D. R., Eccleshall R., Marmur J. Isolation of yeast DNA. Methods Cell Biol. 1975;12:39–44. doi: 10.1016/s0091-679x(08)60950-4. [DOI] [PubMed] [Google Scholar]
- Dasmahapatra B., Skogerson L., Chakraburtty K. Protein synthesis in yeast. II. Purification and properties of the elongation factor 1 from Saccharomyces cerevisiae. J Biol Chem. 1981 Oct 10;256(19):10005–10011. [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hoeijmakers J. H., Borst P., van den Burg J., Weissmann C., Cross G. A. The isolation of plasmids containing DNA complementary to messenger RNA for variant surface glycoproteins of Trypanosoma brucei. Gene. 1980 Mar;8(4):391–417. doi: 10.1016/0378-1119(80)90043-8. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hopper A. K., Furukawa A. H., Pham H. D., Martin N. C. Defects in modification of cytoplasmic and mitochondrial transfer RNAs are caused by single nuclear mutations. Cell. 1982 Mar;28(3):543–550. doi: 10.1016/0092-8674(82)90209-4. [DOI] [PubMed] [Google Scholar]
- Jaskunas S. R., Lindahl L., Nomura M. Identification of two copies of the gene for the elongation factor EF-Tu in E. coli. Nature. 1975 Oct 9;257(5526):458–462. doi: 10.1038/257458a0. [DOI] [PubMed] [Google Scholar]
- Kaziro Y. The role of guanosine 5'-triphosphate in polypeptide chain elongation. Biochim Biophys Acta. 1978 Sep 21;505(1):95–127. doi: 10.1016/0304-4173(78)90009-5. [DOI] [PubMed] [Google Scholar]
- Leberman R., Egner U. Homologies in the primary structure of GTP-binding proteins: the nucleotide-binding site of EF-Tu and p21. EMBO J. 1984 Feb;3(2):339–341. doi: 10.1002/j.1460-2075.1984.tb01808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyajima A., Shibuya M., Kaziro Y. Construction and characterization of the two hybrid Co1E1 plasmids carrying Escherichia coli tufB gene. FEBS Lett. 1979 Jun 15;102(2):207–210. doi: 10.1016/0014-5793(79)80001-0. [DOI] [PubMed] [Google Scholar]
- Nagata S., Iwasaki K., Kaziro Y. Purification and properties of polypeptide chain elongation factor-1alpha from pig liver. J Biochem. 1977 Dec;82(6):1633–1646. doi: 10.1093/oxfordjournals.jbchem.a131859. [DOI] [PubMed] [Google Scholar]
- Nagata S., Taira H., Hall A., Johnsrud L., Streuli M., Ecsödi J., Boll W., Cantell K., Weissmann C. Synthesis in E. coli of a polypeptide with human leukocyte interferon activity. Nature. 1980 Mar 27;284(5754):316–320. doi: 10.1038/284316a0. [DOI] [PubMed] [Google Scholar]
- Nagata S., Tsunetsugu-Yokota Y., Naito A., Kaziro Y. Molecular cloning and sequence determination of the nuclear gene coding for mitochondrial elongation factor Tu of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6192–6196. doi: 10.1073/pnas.80.20.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Kaziro Y. Selective photooxidation of histidine residues in polypeptide chain elongation factor Tu from E. coli. J Biochem. 1981 Oct;90(4):1117–1124. doi: 10.1093/oxfordjournals.jbchem.a133563. [DOI] [PubMed] [Google Scholar]
- Shank P. R., Cohen J. C., Varmus H. E., Yamamoto K. R., Ringold G. M. Mapping of linear and circular forms of mouse mammary tumor virus DNA with restriction endonucleases: evidence for a large specific deletion occurring at high frequency during circularization. Proc Natl Acad Sci U S A. 1978 May;75(5):2112–2116. doi: 10.1073/pnas.75.5.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibuya M., Nashimoto H., Kaziro Y. Cloning of an EcoRI fragment carrying E. coli tufA gene. Mol Gen Genet. 1979 Feb 26;170(2):231–234. doi: 10.1007/BF00337801. [DOI] [PubMed] [Google Scholar]
- Slobin L. I., Clark R. V., Olson M. O. Functional and structural studies on a tryptic fragment of eucaryotic elongation factor Tu from rabbit reticulocytes. Biochemistry. 1981 Sep 29;20(20):5761–5767. doi: 10.1021/bi00523a019. [DOI] [PubMed] [Google Scholar]
- Slobin L. I. The role of eucaryotic factor Tu in protein synthesis. The measurement of the elongation factor Tu content of rabbit reticulocytes and other mammalian cells by a sensitive radioimmunoassay. Eur J Biochem. 1980 Sep;110(2):555–563. doi: 10.1111/j.1432-1033.1980.tb04898.x. [DOI] [PubMed] [Google Scholar]
- Struhl K., Davis R. W. Transcription of the his3 gene region in Saccharomyces cerevisiae. J Mol Biol. 1981 Nov 5;152(3):535–552. doi: 10.1016/0022-2836(81)90267-9. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Gillespie D. Preparative and analytical purification of DNA from agarose. Proc Natl Acad Sci U S A. 1979 Feb;76(2):615–619. doi: 10.1073/pnas.76.2.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkie N. M., Clements J. B., Boll W., Mantei N., Lonsdale D., Weissmann C. Hybrid plasmids containing an active thymidine kinase gene of Herpes simplex virus 1. Nucleic Acids Res. 1979 Oct 25;7(4):859–877. doi: 10.1093/nar/7.4.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota T., Sugisaki H., Takanami M., Kaziro Y. The nucleotide sequence of the cloned tufA gene of Escherichia coli. Gene. 1980 Dec;12(1-2):25–31. doi: 10.1016/0378-1119(80)90012-8. [DOI] [PubMed] [Google Scholar]
- van Hemert F. J., van Ormondt H., Möller W. A bacterial clone carrying sequences coding for elongation factor EF-1 alpha from Artemia. FEBS Lett. 1983 Jul 4;157(2):289–293. doi: 10.1016/0014-5793(83)80563-8. [DOI] [PubMed] [Google Scholar]