Abstract
Treatment failure and resistance amplification are common among patients with rifampin-resistant tuberculosis (TB). Drug susceptibility testing (DST) for second-line drugs is recommended for these patients, but logistical difficulties have impeded widespread implementation of second-line DST in many settings. To provide a quantitative perspective on the decision to scale up second-line DST, we synthesize literature on the prevalence of second-line drug resistance, the expected clinical and epidemiologic benefits of using second-line DST to ensure that patients with rifampin-resistant TB receive effective regimens, and the costs of implementing (or not implementing) second-line DST for all individuals diagnosed with rifampin-resistant TB. We conclude that, in most settings, second-line DST could substantially improve treatment outcomes for patients with rifampin-resistant TB, reduce transmission of drug-resistant TB, prevent amplification of drug resistance, and be affordable or even cost-saving. Given the large investment made in each patient treated for rifampin-resistant TB, these payoffs would come at relatively small incremental cost. These anticipated benefits likely justify addressing the real challenges faced in implementing second-line DST in most high-burden settings.
Keywords: Drug resistance, diagnostics, regimen selection, treatment outcomes, cost effectiveness
Introduction
Each year, more than half a million new cases of rifampin-resistant tuberculosis – including multidrug-resistant tuberculosis (MDR TB, resistant to rifampin and isoniazid) – occur worldwide. These strains are exceedingly difficult to treat; the per-patient cost of treatment exceeds the per-capita gross domestic product in most countries, and only half of treated patients are successfully cured.1 As tests that detect rifampin resistance are scaled up globally,2 the number of patients diagnosed with rifampin-resistant TB is growing dramatically. Unfortunately, despite the large investment made in each rifampin-resistant TB patient treated, the majority of these patients still receive suboptimal therapy.
In countries with surveillance data, 9.5% of MDR TB cases also had resistance to the two most important classes of second-line TB drugs [fluoroquinolones and second-line injectable drugs (SLIs)] – or extensively drug resistant (XDR) TB – in 2015,1 and many more have resistance to other combinations of the drugs in the regimens typically used to treat rifampin-resistant TB.3,4 This drug resistance is associated with worse treatment outcomes,5 and DST to second-line drugs has therefore been recommended for all rifampin-resistant TB patients “where possible”.6
Uptake of second-line DST remains limited by economic and logistical challenges, however. Second-line DST costs more than other TB diagnostics7,8 and requires specialized laboratory facilities and expertise9 as well as infrastructure to transport specimens and relay results. Accurate, rapid assays for fluoroquinolones and SLIs are now available,10,11 but for most other drugs used to treat rifampin-resistant TB, the only well-established methods are growth-based, phenotypic assays – which take weeks or months to complete and require stringent biosafety containment procedures.12,13 Moreover, developing individualized regimens on the basis of second-line DST can be logistically challenging (for example, to maintain continuous drug supplies14) and expensive.15 In light of these challenges, many high-burden countries opt not to maintain capacity for routine second-line DST. Even for fluoroquinolones and SLIs – the easiest second-line drugs to test – susceptibility results were reported for only 36% of MDR TB patients in 2015.1
Although standardized treatment regimens for rifampin-resistant TB can cure many patients, regimens containing ineffective drugs reduce the probability of success,5 expose patients to toxicity without benefit, amplify drug resistance,16 and waste resources. The only way to reliably prevent these outcomes is to verify susceptibility to any drug being used. In this commentary, we review and synthesize data regarding the expected return on investment in second-line DST for all patients with rifampin-resistant TB – from clinical, public-health, and economic perspectives.
The clinical impact of second-line resistance
Guidelines for treatment of rifampin-resistant TB recommend at least five effective TB medicines, including, when possible, a newer-generation fluoroquinolone, an SLI, and pyrazinamide.6 Each of the five drugs in such a regimen makes an important contribution. In meta-analyses of observational data, susceptibility to the fluoroquinolone was associated with a three-fold increase in the adjusted odds of treatment success of a conventional MDR TB regimen,17 and an effective SLI17 and pyrazinamide17,18 each increased success roughly two-fold. Beyond these three critical drugs, each additional effective agent still adds value: inclusion of 5 effective drugs rather than 4, and of 6 effective drugs rather than 5, further improves treatment response,18 and six effective drugs add benefit even in XDR TB.5 Knowing that treatment response improves with each effective agent, ensuring that patients with rifampin-resistant TB receive an adequate number of effective drugs should be a high priority; susceptibility to empiric regimens should only be assumed when resistance to each component is rare in the underlying population.
Unfortunately, among patients with rifampin-resistant TB, resistance to second-line drugs is highly prevalent. As detailed in Table 1 for several illustrative settings with population-based data, resistance to a fluoroquinolone, SLI, and/or pyrazinamide is typically present in half of rifampin-resistant TB cases; this number can approach 90% in some settings such as Eastern Europe. The prevalence of resistance to other commonly-used agents, including ethionamide/prothionamide,5 cycloserine/terizidone,5 ethambutol,16 and high-dose isoniazid19,20 is also substantial. Therefore, without routine use of second-line DST, using five agents in a standardized regimen is likely to result in therapy with four or fewer truly effective drugs – and thus in poorer clinical outcomes. Although there are settings where second-line resistance is rare,21–23 in most cases, the prevalence of additional resistance in rifampin-resistant TB has expanded to the point that regimens with 5 effective drugs cannot be reliably constructed without the use of second-line DST.6 Furthermore, two recently approved novel agents (bedaquiline and delamanid) offer unprecedented potential to effectively treat such patients when second-line resistance is identified.24,25 Thus, the clinical value of second-line DST is greater now than ever before.
Table 1.
Prevalence of resistance to drugs used in treatment of rifampin-resistant TB, in illustrative high-burden settings
| China | South Africa | Pakistan | Belarus (Minsk city) | |
|---|---|---|---|---|
| Fluoroquinolone (FQ) resistance | ||||
| Ofloxacin | 25%45 | 12–18%3 | 22%3 | 31%3 |
| Moxifloxacin at 0.5 μg/mL | 8–12%3 | 14%3 | 27%3 | |
| Moxifloxacin at 2 μg/mL | 0–4%3 | 1.4%3 | 9%3 | |
| Second-line injectable (SLI) resistancea | 15%45 | 12%46 | 1% 47,48 | 27%49 |
| FQ and SLI resistance | 8%45 | 7–10%16,46 | 1% 47,48 | 14%49 |
| Pyrazinamide (PZA) resistance | 43%50 | 39–49%3 | 38%3 | 81%3 |
| FQ, SLI and/or PZA resistance | 56% | 52% | 47% | 89% |
| Susceptible to all 3 classesb | 44% | 48% | 53% | 11% |
Estimates in Pakistan are based on limited data in non-population-based samples, with testing of only some SLIs.
Public health consequences of missed second-line resistance
From a public health standpoint, treating large numbers of patients for rifampin-resistant TB without accounting for second-line drug resistance introduces concerning long-term risks. Specifically, at the population level, ineffective treatment prolongs the window of potential transmission and increases the risk that transmitted strains will carry additional drug resistance.
These population-level dynamics can be understood in terms of the effective reproduction number Reff, which describes the average number of secondary infectious TB cases generated per infectious index case. This quantity has four components: (1) the duration of infectiousness for the index case, (2) the rate at which respiratory contacts sufficient for transmission are made, (3) the probability that such contact results in infection, and (4) the probability that an infection progresses to active (infectious) TB. The value of Reff determines whether an epidemic is in advance (Reff>1) or retreat (Reff<1). While the data are far from complete, global estimates suggest that the incidence of rifampin-resistant TB is roughly constant (though with wide geographic variation), suggesting a value of Reff near 1.1 Since the frequency of contact and probability of transmission are difficult to alter, and therapies to prevent progression among those infected with rifampin-resistant strains are not well established, reducing the duration of infectiousness is critical in order to reduce the effective reproduction number of rifampin-resistant TB below 1. Since patients with MDR TB who receive ineffective therapy likely remain at least partially infectious,26 rapidly initiating effective treatment is our best weapon to reduce the duration of infectiousness, and thus Reff, for rifampin-resistant TB.
Not only can ineffective regimens allow for additional transmission of rifampin-resistant TB, but the selective pressure of treatment may result in transmission of increasingly resistant strains.27 Studies of serial sputum isolates have revealed high rates of acquired drug resistance during treatment of rifampin-resistant TB, especially among those who already have some second-line drug resistance at baseline; of patients with baseline resistance to 3 or more second-line drugs without meeting XDR criteria in one multi-center study, 44% acquired XDR during treatment.28 In some settings, the compounding of drug resistance during ineffective treatment has led to outbreaks of extremely difficult-to-treat disease.29,30 Regimens with at least five – as opposed to even three or four – effective drugs are associated with substantial reductions in acquired drug resistance.16 Given the likelihood that novel regimens of the foreseeable future (e.g. those containing nitroimidazoles31,32 or bedaquiline33) will continue to include conventional drugs such as fluoroquinolones and pyrazinamide, minimizing the transmission of isolates resistant to these agents is an important public health goal – a goal that will be more difficult to achieve without wider use of second-line DST.
Economic considerations
While the clinical and public health benefits of second-line DST may not be controversial, there remains concern that these benefits do not justify the additional cost and logistical challenges of performing routine second-line DST. In settings where resources are clearly constrained, these costs (around $100 per patient34) and feasibility barriers (such as the need to maintain laboratory expertise) cannot be ignored. They should be understood, however, within the full context of TB and MDR/rifampin-resistant TB treatment programs, including the total costs of treatment and of other diagnostics such as Xpert MTB/RIF, as illustrated in Figure 1. At an average of more than $6000, per-patient costs of care for rifampin-resistant TB exceed those of drug-susceptible TB more than tenfold in low-income countries.1 Patients’ out-of-pocket expenses are frequently devastating as well.35,36 Second-line DST – at about 2% of the total cost to treat a patient with rifampin-resistant TB8,34 – represents a relatively small budgetary outlay, one that likely will be recovered. For example, if performing second-line DST on 50 patients with known rifampin-resistant TB can reduce the amount spent on ineffective TB therapy by only one treatment course (likely a substantial underestimate), then second-line DST could be cost-neutral to a rifampin-resistant TB treatment program. Of course, diagnostics are not the only cost involved in individualizing therapy, as switching to effective drugs may add to regimen cost. Yet even substitutions such as clofazamine,32 bedaquiline, and/or newly-generic linezolid,37 are likely to cost no more than a few hundred dollars per patient when averaged over a rifampin-resistant TB cohort,38 and such proactive regimen changes can avoid fruitless investment in standard therapy for patients who are unlikely to benefit from it.5 In the short term, implementing DST will require substantial up-front investment, especially in laboratory and sputum transport infrastructure. But given the frequency at which therapy with standardized regimens is ineffective, this investment could pay for itself by reducing rifampin-resistant TB treatment costs within as little as two years. Given the benefits to patients and populations of routine second-line DST, this is an investment arguably worth making.
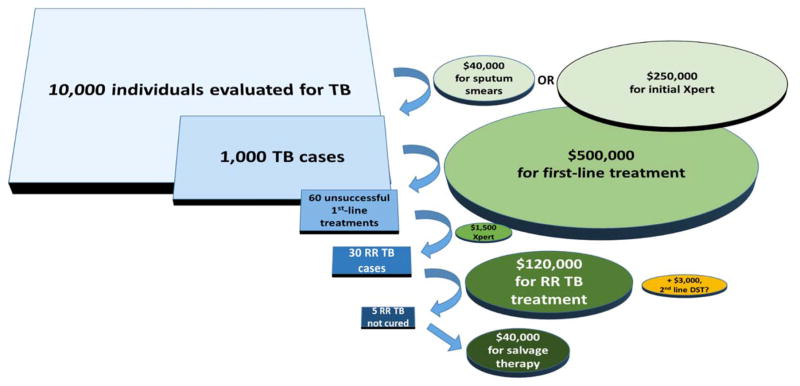
Figure 1. Rifampin-resistant tuberculosis (RR TB) patients and spending in context.
By applying estimated outcomes and costs for a high-burden, low-income setting, we compare expenditures on overall and drug-resistant TB diagnostics and treatment in a hypothetical cohort of 10,000 individuals evaluated for TB, using sputum smear or Xpert MTB/RIF as the initial diagnostic test. Assumptions about procedures and outcomes include: initial TB diagnosis by sputum smear, 2 smears (or one Xpert) collected per suspect, 10 suspects per TB case,57,58 3% prevalence of rifampin-resistant TB among TB cases (near the global average),1 6% failure or relapse after first-line treatment,1,59 rifampin susceptibility testing (e.g. by Xpert) after unsuccessful first-line therapy (if initial diagnosis is by smear), and 15% failure or relapse after treatment for rifampin-resistant TB.60 Cost estimates are in US dollars and include $2 per smear,34 $500 per first-line treatment,1 $25 per Xpert,34 $4000 per initial MDR TB treatment,1 $8,000 per salvage regimen, and $100 per rifampin-resistant case for second-line DST (second-line line probe assay plus culture and phenotypic second-line DST, including fixed costs, in a low-income, high-burden setting)34. The yellow oval shows the relatively small cost of performing second-line DST for all individuals diagnosed with rifampin resistance, relative to total TB and rifampin-resistant TB spending. Note that secondary drug-resistant cases – an added potential cost of ineffective treatment for rifampin-resistant TB – are not included in this figure.
Implementation challenges, options, and strategies
If second-line DST is to become the norm in programmatic management of MDR/rifampin-resistant TB, it is important to understand the inherent challenges. An initial challenge is simply deciding which drugs to evaluate and by what methods. Currently, rapid molecular testing is limited to line probe assays for fluoroquinolones and SLIs, as well as for first-line drugs such as isoniazid that may have a role in treating some patients with rifampin-resistant TB;11 the use of line probe assays for these two key second-line drug classes was endorsed by WHO in 2016.39 Phenotypic DST currently provides more complete and accurate results,11 but phenotypic results typically remain unavailable during the initial months of treatment. Therefore, local epidemiology, drug availability, resources, and existing infrastructure should guide the choice of diagnostic algorithms. Options may include line probe assay screening for fluoroquinolone and SLI susceptibility, with follow-up phenotypic testing for those found to have resistance; up-front phenotypic DST, perhaps in conjunction with high-intensity regimens designed to de-escalate once susceptibilities are confirmed; or both line-probe assay and phenotypic DST in order to maximize rapidity and depth of results in settings of highest resistance prevalence. Regardless of the second-line DST algorithm chosen, clinical, laboratory, pharmacy, and ancillary capacity will be required; this capacity includes physicians who can review results of testing and tailor regimens accordingly, guidance for interpreting (discordant) results, laboratory staff with continuing training to maintain proficiency, maintenance of a continuous supply of multiple drugs, and networks to transport specimens and results between clinics and centralized laboratories.
Overcoming these challenges is a difficult task, especially in settings with already very limited infrastructure. The optimal implementation strategy will vary by locale, but a number of settings and programs illustrate possible components of an approach to second-line drug resistance in the steps they have taken to build laboratory capacity, develop clinical diagnostic infrastructure, harness novel drugs, and curb recognized drug-resistant TB epidemics (Table 2). Where second-line DST has been successfully implemented in conjunction with effective treatment regimens and broader public health efforts (e.g., infection control, intensified case finding), drug-resistant TB epidemics from New York City40 to Tomsk Oblast, Russia,41–43 have declined within a few years. Any initial investment required to implement second-line DST is likely to pay off quickly, as treating patients with ineffective regimens represents an enormous waste of resources – and most importantly, exposes patients to very real toxicity with little clinical benefit. Moreover, to some extent, programs must address DST challenges regardless of whether they implement up-front DST for all patients with rifampin-resistant TB. Patients who are not cured will require assessment for drug resistance, and many will require retreatment with alternative drugs. With the recent introduction of a 9-month regimen for rifampin-resistant TB,44 second-line DST will also be able to rapidly identify appropriate candidates for this simpler, cheaper, and potentially more effective regimen. Tailoring regimens based on DST at the start of treatment (before additional acquisition of resistance occurs) may be no more difficult, on average, than managing retreatments of highly drug-resistant disease – and will result in better outcomes for patients.
Table 2.
Expanding capacity for the response to drug-resistant TB: Examples from high burden countries
| Areas of focus | Examples | Steps taken |
|---|---|---|
| Establishment of a biosafety level 3 reference laboratory | Uganda51 | A successful lab in another low-income country provided standard operating procedures and trained key personnel. National university and international research partnerships increase the sustainability of laboratory funding and expertise. |
| Creation of a national TB diagnostic program (including second-line DST) | Peru52 | Decentralized first-line DST was combined with centralized second-line DST. Computerized laboratory information systems and provider training reduced transport and reporting delays. |
| Expansion of TB diagnostic technologies (line probe assays, culture-based DST) also applicable to second-line DST | EXPAND TB partnership (multiple low-income countries53) | International partners assisted with initial laboratory start-up and technology. Domestic sources provide long-term funding; local political commitment is essential. |
| Increased use of novel agents for treatment of drug-resistant TB | South Africa54,55 | Genotypic second-line DST and clear treatment guidelines are allowing rapid scale-up of bedaquiline +/− linezolid for pre-XDR and XDR TB. |
| Scale up of bedaquiline and delamanid for treatment of drug-resistant TB | endTB Project56 | This combined implementation project and effectiveness study aims to accelerate importation, financing, and clinical use of novel drugs in participating countries; routine use of some form of second-line DST is required in participating countries. |
| Comprehensive management of drug-resistant TB including aggressive case detection, individualized regimens, and social support | Tomsk Oblast, Russia41,43 | Second-line DST and aggressive individualized regimens with at least 5 effective drugs resulted in high rates of durable treatment success. These measures were followed by stabilization of a large and growing MDR TB epidemic. |
Conclusion
Treatment of rifampin-resistant TB is already expensive and complex. Although places exist where second-line drug resistance is relatively rare, the use of standard regimens for rifampin-resistant TB in most settings will result in the use of at least one ineffective drug in the majority of cases. When treatment costs thousands of dollars per patient and carries grave clinical toxicities, it is essential that we optimize the probability that those treatment regimens will work. Second-line DST has the capacity to increase the number of effective drugs in each regimen – an intervention demonstrated to improve treatment outcomes in patients and likely to reduce transmission and acquisition of further resistance in populations. Implementing second-line DST presents real logistical challenges but has the potential to be cost-saving in the long run as the (unnecessary) cost of ineffective treatment is reduced. From a quantitative perspective – but also from the perspective of patients – incorporating second-line DST into the routine care of rifampin-resistant TB seems a justifiable investment to make.
Acknowledgments
Funding: This research was supported by the Bill and Melinda Gates Foundation, Seattle, WA [Work Order 10 to DWD]. The sponsors had no role in study design, data analysis, writing, or decision to publish.
Footnotes
Ethical Approval: No ethical approval was required for this work.
Conflicts of interest: Carole Mitnick previously served as a member of the Scientific Advisory Board for Otsuka’s development of delamanid. Carole Mitnick was a co-investigator on a research grant from Janssen to Harvard Medical School’s Department of Global Health and Social Medicine, entitled, “Forming the building blocks for a paradigm shift in the delivery of care for drug resistant TB”. Carole Mitnick is co-principal investigator on the trial portion of the UNITAID-funded endTB project.
References
- 1.Global Tuberculosis Report 2016 [Internet] Geneva: World Health Organization; 2016. [cited 2016 Oct 27]. Available from: http://www.who.int/tb/publications/global_report/en/ [Google Scholar]
- 2.Lawn SD, Mwaba P, Bates M, Piatek A, Alexander H, Marais BJ, et al. Advances in tuberculosis diagnostics: the Xpert MTB/RIF assay and future prospects for a point-of-care test. Lancet Infect Dis. 2013;13:349–61. doi: 10.1016/S1473-3099(13)70008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zignol M, Dean AS, Alikhanova N, Andres S, Cabibbe AM, Cirillo DM, et al. Population-based resistance of Mycobacterium tuberculosis isolates to pyrazinamide and fluoroquinolones: results from a multicountry surveillance project. Lancet Infect Dis. 2016;16:1185–92. doi: 10.1016/S1473-3099(16)30190-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kurbatova EV, Dalton T, Ershova J, Tupasi T, Caoili JC, Van Der Walt M, et al. Additional drug resistance of multidrug-resistant tuberculosis in patients in 9 countries. Emerg Infect Dis. 2015;21:977–83. doi: 10.3201/eid2106.141329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Falzon D, Gandhi N, Migliori GB, Sotgiu G, Cox HS, Holtz TH, et al. Resistance to fluoroquinolones and second-line injectable drugs: impact on multidrug-resistant TB outcomes. Eur Respir J. 2013;42:156–68. doi: 10.1183/09031936.00134712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. WHO treatment guidelines for drug-resistant tuberculosis: 2016 update [Internet] 2016 [cited 2016 Jul 13];Available from: http://www.who.int/tb/areas-of-work/drug-resistant-tb/treatment/resources/en/ [PubMed]
- 7.Fitzpatrick C, Floyd K. A systematic review of the cost and cost effectiveness of treatment for multidrug-resistant tuberculosis. PharmacoEconomics. 2012;30:63–80. doi: 10.2165/11595340-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.FIND-Negotiated Product Pricing [Internet] [cited 2016 Jul 26];Available from: http://www.finddx.org/pricing/
- 9.Hillemann D, Hoffner S, Cirillo D, Drobniewski F, Richter E, Rüsch-Gerdes S, et al. First Evaluation after Implementation of a Quality Control System for the Second Line Drug Susceptibility Testing of Mycobacterium tuberculosis Joint Efforts in Low and High Incidence Countries. PLOS ONE. 2013;8:e76765. doi: 10.1371/journal.pone.0076765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brossier F, Guindo D, Pham A, Reibel F, Sougakoff W, Veziris N, et al. Performance of the New Version (v2. 0) of the GenoType MTBDRsl Test for Detection of Resistance to Second-Line Drugs in Multidrug-Resistant Mycobacterium tuberculosis Complex Strains. J Clin Microbiol. 2016;54:1573–80. doi: 10.1128/JCM.00051-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Domínguez J, Boettger EC, Cirillo D, Cobelens F, Eisenach KD, Gagneux S, et al. Clinical implications of molecular drug resistance testing for Mycobacterium tuberculosis: a TBNET/RESIST-TB consensus statement. Int J Tuberc Lung Dis. 2016;20:24–42. doi: 10.5588/ijtld.15.0221. [DOI] [PubMed] [Google Scholar]
- 12.Cambau E, Viveiros M, Machado D, Raskine L, Ritter C, Tortoli E, et al. Revisiting susceptibility testing in MDR-TB by a standardized quantitative phenotypic assessment in a European multicentre study. J Antimicrob Chemother. 2015;70:686–96. doi: 10.1093/jac/dku438. [DOI] [PubMed] [Google Scholar]
- 13.Foongladda S, Klayut W, Chinli R, Pholwat S, Houpt ER. Use of mycobacteriophage quantitative PCR on MGIT broths for a rapid tuberculosis antibiogram. J Clin Microbiol. 2014;52:1523–8. doi: 10.1128/JCM.03637-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Institute of Medicine (US) Forum on Drug Discovery, Development, and Translation. Developing and Strengthening the Global Supply Chain for Second-Line Drugs for Multidrug-Resistant Tuberculosis: Workshop Summary [Internet] Washington (DC): National Academies Press (US); 2013. [cited 2016 Sep 19]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK115060/ [PubMed] [Google Scholar]
- 15.Medicins Sans Frontieres. DR-TB Drugs Under the Microscope. (4) 2016 [Internet] [cited 2016 Sep 13]. Available from: https://issuu.com/msf-org/docs/dr-tb_drugs_under_the_microscope.
- 16.Cegielski JP, Kurbatova E, van der Walt M, Brand J, Ershova J, Tupasi T, et al. Multidrug-Resistant Tuberculosis Treatment Outcomes in Relation to Treatment and Initial Versus Acquired Second-Line Drug Resistance. Clin Infect Dis. 2016;62:418–30. doi: 10.1093/cid/civ910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bastos ML, Hussain H, Weyer K, Garcia-Garcia L, Leimane V, Leung CC, et al. Treatment outcomes of patients with multidrug-resistant and extensively drug-resistant tuberculosis according to drug susceptibility testing to first- and second-line drugs: an individual patient data meta-analysis. Clin Infect Dis. 2014;59:1364–74. doi: 10.1093/cid/ciu619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yuen CM, Kurbatova EV, Tupasi T, Caoili JC, Walt MVD, Kvasnovsky C, et al. Association between Regimen Composition and Treatment Response in Patients with Multidrug-Resistant Tuberculosis: A Prospective Cohort Study. PLOS Med. 2015;12:e1001932. doi: 10.1371/journal.pmed.1001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niehaus AJ, Mlisana K, Gandhi NR, Mathema B, Brust JCM. High Prevalence of inhA Promoter Mutations among Patients with Drug-Resistant Tuberculosis in KwaZulu-Natal, South Africa. PloS One. 2015;10:e0135003. doi: 10.1371/journal.pone.0135003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yin Q-Q, Jiao W-W, Li Q-J, Xu F, Li J-Q, Sun L, et al. Prevalence and molecular characteristics of drug-resistant Mycobacterium tuberculosis in Beijing, China: 2006 versus 2012. BMC Microbiol. 2016;16:85. doi: 10.1186/s12866-016-0699-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Philippine Nationwide Tuberculosis Drug Resistance Survey Team. Nationwide drug resistance survey of tuberculosis in the Philippines. Int J Tuberc Lung Dis. 2009;13:500–7. [PubMed] [Google Scholar]
- 22.Orikiriza P, Tibenderana B, Siedner MJ, Mueller Y, Byarugaba F, Moore CC, et al. Low resistance to first and second line anti-tuberculosis drugs among treatment naive pulmonary tuberculosis patients in southwestern Uganda. PloS One. 2015;10:e0118191. doi: 10.1371/journal.pone.0118191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umubyeyi A, Rigouts L, Shamputa IC, Dediste A, Struelens M, Portaels F. Low levels of second-line drug resistance among multidrug-resistant Mycobacterium tuberculosis isolates from Rwanda. Int J Infect Dis. 2008;12:152–6. doi: 10.1016/j.ijid.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Pym AS, Diacon AH, Tang S-J, Conradie F, Danilovits M, Chuchottaworn C, et al. Bedaquiline in the treatment of multidrug- and extensively drug-resistant tuberculosis. Eur Respir J. 2016;47:564–74. doi: 10.1183/13993003.00724-2015. [DOI] [PubMed] [Google Scholar]
- 25.Gler MT, Skripconoka V, Sanchez-Garavito E, Xiao H, Cabrera-Rivero JL, Vargas-Vasquez DE, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med. 2012;366:2151–60. doi: 10.1056/NEJMoa1112433. [DOI] [PubMed] [Google Scholar]
- 26.Dharmadhikari AS, Mphahlele M, Venter K, Stoltz A, Mathebula R, Masotla T, et al. Rapid impact of effective treatment on transmission of multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2014;18:1019–25. doi: 10.5588/ijtld.13.0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kempker RR, Kipiani M, Mirtskhulava V, Tukvadze N, Magee MJ, Blumberg HM. Acquired Drug Resistance in Mycobacterium tuberculosis and Poor Outcomes among Patients with Multidrug-Resistant Tuberculosis. Emerg Infect Dis. 2015;21:992–1001. doi: 10.3201/eid2106.141873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cegielski JP, Dalton T, Yagui M, Wattanaamornkiet W, Volchenkov GV, Via LE, et al. Extensive drug resistance acquired during treatment of multidrug-resistant tuberculosis. Clin Infect Dis. 2014;59:1049–63. doi: 10.1093/cid/ciu572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Klopper M, Warren RM, Hayes C, Gey van Pittius NC, Streicher EM, Müller B, et al. Emergence and spread of extensively and totally drug-resistant tuberculosis, South Africa. Emerg Infect Dis. 2013;19:449–55. doi: 10.3201//EID1903.120246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Müller B, Chihota VN, Pillay M, Klopper M, Streicher EM, Coetzee G, et al. Programmatically selected multidrug-resistant strains drive the emergence of extensively drug-resistant tuberculosis in South Africa. PloS One. 2013;8:e70919. doi: 10.1371/journal.pone.0070919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dawson R, Diacon AH, Everitt D, van Niekerk C, Donald PR, Burger DA, et al. Efficiency and safety of the combination of moxifloxacin, pretomanid (PA-824), and pyrazinamide during the first 8 weeks of antituberculosis treatment: a phase 2b, open-label, partly randomised trial in patients with drug-susceptible or drug-resistant pulmonary tuberculosis. Lancet. 2015;385:1738–47. doi: 10.1016/S0140-6736(14)62002-X. [DOI] [PubMed] [Google Scholar]
- 32.Diacon AH, Dawson R, von Groote-Bidlingmaier F, Symons G, Venter A, Donald PR, et al. Bactericidal activity of pyrazinamide and clofazimine alone and in combinations with pretomanid and bedaquiline. Am J Respir Crit Care Med. 2015;191:943–53. doi: 10.1164/rccm.201410-1801OC. [DOI] [PubMed] [Google Scholar]
- 33.Moodley R, Godec TR STREAM Trial Team. Short-course treatment for multidrug-resistant tuberculosis: the STREAM trials. Eur Respir Rev Off J Eur Respir Soc. 2016;25:29–35. doi: 10.1183/16000617.0080-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vassall A, van Kampen S, Sohn H, Michael JS, John KR, den Boon S, et al. Rapid diagnosis of tuberculosis with the Xpert MTB/RIF assay in high burden countries: a cost-effectiveness analysis. PLoS Med. 2011;8:e1001120. doi: 10.1371/journal.pmed.1001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Laurence YV, Griffiths UK, Vassall A. Costs to Health Services and the Patient of Treating Tuberculosis: A Systematic Literature Review. PharmacoEconomics. 2015;33:939–55. doi: 10.1007/s40273-015-0279-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Hof S, Collins D, Hafidz F, Beyene D, Tursynbayeva A, Tiemersma E. The socioeconomic impact of multidrug resistant tuberculosis on patients: results from Ethiopia, Indonesia and Kazakhstan. BMC Infect Dis. 2016;16:470. doi: 10.1186/s12879-016-1802-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee M, Lee J, Carroll MW, Choi H, Min S, Song T, et al. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med. 2012;367:1508–18. doi: 10.1056/NEJMoa1201964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stop TB Partnership. Global Drug Facility Product Catalogue [Internet] 2016 Available from: http://www.stoptb.org/assets/documents/gdf/drugsupply/GDF%20product%20catalog_25%20Jul%202016_final.pdf.
- 39.World Health Organization. The use of molecular line probe assays for the detection of resistance to second-line anti-tuberculosis drugs: Policy Guidance [Internet] 2016 [cited 2016 Dec 2]. Available from: http://www.who.int/tb/WHOPolicyStatementSLLPA.pdf.
- 40.Sterling TR. Drug-Resistant Tuberculosis in New York City: Lessons to Remember. Clin Infect Dis. 2006;42:1711–2. doi: 10.1086/504332. [DOI] [PubMed] [Google Scholar]
- 41.Partners in Health. Small Team, Big Impact: 15 Years of Better Care for Russian TB Patients [Internet] 2015 [cited 2016 Nov 30]. Available from: http://www.pih.org/blog/small-team-big-impact-15-years-of-better-care-for-tb-patients.
- 42.Cohen T, Jenkins HE, Lu C, McLaughlin M, Floyd K, Zignol M. On the spread and control of MDR-TB epidemics: an examination of trends in anti-tuberculosis drug resistance surveillance data. Drug Resist Updat. 2014;17:105–23. doi: 10.1016/j.drup.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmad Khan F, Gelmanova IY, Franke MF, Atwood S, Zemlyanaya NA, Unakova IA, et al. Aggressive Regimens Reduce Risk of Recurrence After Successful Treatment of MDR-TB. Clin Infect Dis Off Publ Infect Dis Soc Am. 2016;63:214–20. doi: 10.1093/cid/ciw276. [DOI] [PubMed] [Google Scholar]
- 44.Aung KJM, Van Deun A, Declercq E, Sarker MR, Das PK, Hossain MA, et al. Successful “9-month Bangladesh regimen” for multidrug-resistant tuberculosis among over 500 consecutive patients. Int J Tuberc Lung Dis. 2014;18:1180–7. doi: 10.5588/ijtld.14.0100. [DOI] [PubMed] [Google Scholar]
- 45.Zhao Y, Xu S, Wang L, Chin DP, Wang S, Jiang G, et al. National survey of drug-resistant tuberculosis in China. N Engl J Med. 2012;366:2161–70. doi: 10.1056/NEJMoa1108789. [DOI] [PubMed] [Google Scholar]
- 46.Said HM, Kock MM, Ismail NA, Mphahlele M, Baba K, Omar SV, et al. Molecular characterization and second-line antituberculosis drug resistance patterns of multidrug-resistant Mycobacterium tuberculosis isolates from the northern region of South Africa. J Clin Microbiol. 2012;50:2857–62. doi: 10.1128/JCM.00358-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rao NA, Irfan M, Soomro MM, Mehfooz Z. Drug resistance pattern in multidrug resistance pulmonary tuberculosis patients. J Coll Physicians Surg--Pak JCPSP. 2010;20:262–5. [PubMed] [Google Scholar]
- 48.Akhtar AM, Arif MA, Kanwal S, Majeed S. Prevalence and drug resistance pattern of MDR TB in retreatment cases of Punjab, Pakistan. JPMA J Pak Med Assoc. 2016;66:989–93. [PubMed] [Google Scholar]
- 49.Skrahina A, Hurevich H, Zalutskaya A, Sahalchyk E, Astrauko A, van Gemert W, et al. Alarming levels of drug-resistant tuberculosis in Belarus: results of a survey in Minsk. Eur Respir J. 2012;39:1425–31. doi: 10.1183/09031936.00145411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu P, Wu J, Yang C, Luo T, Shen X, Zhang Y, et al. Prevalence and transmission of pyrazinamide resistant Mycobacterium tuberculosis in China. Tuberc Edinb Scotl. 2016;98:56–61. doi: 10.1016/j.tube.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ssengooba W, Gelderbloem SJ, Mboowa G, Wajja A, Namaganda C, Musoke P, et al. Feasibility of establishing a biosafety level 3 tuberculosis culture laboratory of acceptable quality standards in a resource-limited setting: an experience from Uganda. Health Res Policy Syst. 2015;13:4. doi: 10.1186/1478-4505-13-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shin SS, Yagui M, Ascencios L, Yale G, Suarez C, Quispe N, et al. Scale-up of multidrug-resistant tuberculosis laboratory services, Peru. Emerg Infect Dis. 2008;14:701–8. doi: 10.3201/eid1405.070721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.World Health Organization. EXPAND TB Project: Progress in Diagnostics [Internet] 2014 [cited 2016 Nov 29]. Available from: http://www.who.int/tb/publications/factsheet_expand_tb.pdf.
- 54.Cariem R, Cox V, de Azevedo V, Hughes J, Mohr E, Durán LT, et al. The experience of bedaquiline implementation at a decentralised clinic in South Africa. Public Health Action. 2016;6:190–2. doi: 10.5588/pha.16.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ndjeka N. Incorporation of Bedaquiline in the South African National TB Programme [Internet] 2016 [cited 2016 Nov 29];Available from: http://www.croiconference.org/sites/default/files/posters-2016/754.pdf.
- 56.Rich M. The endTB Project: Expanding New Drugs for TB [Internet] 2015 [cited 2016 Nov 28];Available from: http://www.stoptb.org/wg/gli/assets/documents/M7/3.%20RICH_endTB%20Project.pdf.
- 57.Durovni B, Saraceni V, van den Hof S, Trajman A, Cordeiro-Santos M, Cavalcante S, et al. Impact of replacing smear microscopy with Xpert MTB/RIF for diagnosing tuberculosis in Brazil: a stepped-wedge cluster-randomized trial. PLoS Med. 2014;11:e1001766. doi: 10.1371/journal.pmed.1001766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Luetkemeyer AF, Firnhaber C, Kendall MA, Wu X, Mazurek GH, Benator DA, et al. Evaluation of Xpert MTB/RIF Versus AFB Smear and Culture to Identify Pulmonary Tuberculosis in Patients With Suspected Tuberculosis From Low and Higher Prevalence Settings. Clin Infect Dis. 2016;62:1081–8. doi: 10.1093/cid/ciw035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gillespie SH, Crook AM, McHugh TD, Mendel CM, Meredith SK, Murray SR, et al. Four-month moxifloxacin-based regimens for drug-sensitive tuberculosis. N Engl J Med. 2014;371:1577–87. doi: 10.1056/NEJMoa1407426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ahuja SD, Ashkin D, Avendano M, Banerjee R, Bauer M, Bayona JN, et al. Multidrug Resistant Pulmonary Tuberculosis Treatment Regimens and Patient Outcomes: An Individual Patient Data Meta-analysis of 9,153 Patients. PLoS Med. 2012;9:e1001300. doi: 10.1371/journal.pmed.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]