Abstract
Temporomandibular joint disorder (TMD) is a complex musculoskeletal disorder that presents with pain, limited jaw opening, and abnormal noises in the temporomandibular joint. Despite the significant impact that TMD has in terms of suffering and financial burden, relatively few new treatments have emerged; therefore, development of novel treatments to treat TMD pain remains a high priority. The rationale of this study was to use a double-blind, vehicle-controlled clinical trial to evaluate the effects of a high-concentration (8%) capsaicin cream on TMD. This is based on the hypothesis that targeting TRP vanilloid subfamily member 1 (TRPV1) for pain control may provide a novel method for pain relief in TMD patients. TRPV1 is primarily expressed on a population of nociceptive-specific neurons and provides a candidate target for the development of pain treatments. Capsaicin is the primary agonist for TRPV1 and has been used previously in relatively low doses (0.025% to 0.075%) as a therapeutic for a variety of pain disorders, including postherpetic neuralgia and osteoarthritis; however, analgesic efficacy remains equivocal. TMD and healthy control subjects were assigned to either an active capsaicin or vehicle control group. The treatments were applied for 2 h and then removed. Quantitative sensory testing (QST) was completed prior to drug application (baseline), 2 h after drug application, and 1 wk later. Perceived pain intensity was measured using a visual analog scale (VAS) following capsaicin or vehicle cream application. Significantly lower pain was reported in the week after application in the capsaicin-treated TMD subjects. For QST measures, there was a decreased thermal pain threshold 2 h after capsaicin application for both the control and TMD groups, but this resolved within a week. Capsaicin had no effect on pressure pain threshold or mechanical sensitivity in both TMD and healthy individuals. This study demonstrates that 8% topical capsaicin therapy is a relatively safe, simple, and effective treatment for patients with TMD.
Knowledge Transfer Statement: This study evaluated a novel topical capsaicin therapy for reducing orofacial pain. The results of this study can be used to provide another treatment option for patients with TMD.
Keywords: orofacial pain, TRPV1, clinical study, pain, neuroscience/neurobiology, neuropharmacology
Introduction
Orofacial pain disorders are estimated to affect 20% of the US population (Lipton et al. 1993) and are made up largely of temporomandibular joint disorders (TMDs). TMD can encompass a complex musculoskeletal disorder presenting with pain, limited opening, and abnormal noises in the temporomandibular joint (TMJ). Reports from the Orofacial Pain Prospective Evaluation and Risk Assessment (OPPERA) study, an ongoing, prospective study on the development of TMD, indicates an incidence rate for examiner-verified first-onset TMD of 3.9% per annum, and a meta-analysis by Al-Jundi et al. (2008) reported a prevalence of at least 1 sign or symptom in 16% of the US population (Slade et al. 2013). Women are affected more frequently than men by an approximately 2 to 1 margin (Solberg 1983; Lipton et al. 1993; LeResche 1997). Despite the significant impact of TMD in terms of suffering and financial burden, relatively few new treatments have emerged, and development of novel treatments to treat TMD pain remains a high priority.
A promising target for pain control is the transient receptor potential (TRP) channel, a family of receptors that mediates both temperature and pain (Jordt et al. 2003; Tominaga and Caterina 2004; Patapoutian et al. 2009). The TRP vanilloid subfamily member 1 (TRPV1) is a ligand-gated ion channel involved with heat and inflammatory pain (Caterina and Julius 2001; Karai et al. 2004; Szallasi and Appendino 2004). TRPV1 responds to protons, temperatures greater than 43°C, and capsaicin (Gunthorpe et al. 2002; Szallasi and Appendino 2004; Tominaga and Caterina 2004) and is primarily expressed by Aδ and C-fiber primary afferent neurons. These neurons are responsible for the erythema, burning, and sensitivity to other stimuli after application of capsaicin. The expectation of TRPV1 may be a target for pain control (Caterina and Julius 2001) and, if realized, a novel therapeutic approach inhibiting certain nociceptive neurons while maintaining other motor and sensory functions (Neubert et al. 2003; Karai et al. 2004).
A number of studies evaluated the targeting of TRPV1 for pain control. Capsaicin activates TRPV1 but also desensitizes, inactivates, and even eliminates TRPV1-expressing neurons with prolonged exposure (Yaksh et al. 1979; Nolano et al. 1999; Malmberg et al. 2004). Clinical trials in diseased individuals indicate that capsaicin can produce analgesia when applied topically at relatively low doses (0.025% to 0.075%), and capsaicin has shown some efficacy for the treatment of chronic pain disorders such as rheumatoid arthritis (RA) and osteoarthritis (OA) (Deal et al. 1991; McCarthy and McCarty 1992). In addition, studies have shown favorable results for patients with postherpetic neuralgia (PHN) (Peikert et al. 1991; Watson et al. 1993), and capsaicin is one of the only Food and Drug Administration (FDA)–approved drugs for treatment of PHN. Healthy subjects who applied capsaicin (0.075%) 4 times daily for 3 wk had degeneration of epidermal nerve fibers (ENFs) by approximately 80% at the epidermis (Nolano et al. 1999). In contrast, a meta-analysis evaluating the efficacy of low-dose capsaicin indicated a moderate to poor efficacy when treating chronic neuropathic or musculoskeletal pain (Mason et al. 2004). In a randomized, double-blind, vehicle-controlled trial, there was no difference in reduction of symptoms between the 0.025% capsaicin and placebo groups with TMD (Winocur et al. 2000). Malmberg et al. (2004) compared the effectiveness of low-dose capsaicin (0.04%) to a high dose (8%) topically applied for up to 120 min. They found that the low dose had no effect on reduction of ENFs or reduction of heat sensitivity compared to placebo, but a single dose of 8% capsaicin reduced the number of ENFs and produced desensitization to heat stimuli for 1 wk. Many recent studies have evaluated the 8% capsaicin patch for the treatment of neuropathic pain (Maihöfner and Heskamp 2013, 2014; Zis et al. 2014). A recent case series on the use of the 8% capsaicin patch reports a decrease in neuropathic pain for patients with various orofacial neuropathic pain (Gaul and Resch 2015). While many studies have been completed evaluating 8% capsaicin as a pain therapy, using it as a therapy provides a conundrum, as it is a pain stimulus that then relieves pain. We were interested in the role of sensitization produced by capsaicin in both “healthy” and “diseased” (chronic pain) subjects.
Since orofacial pain is a hallmark of many different disorders and there are a multitude of different groups also within the TMD classification, we chose to recruit subjects who were classified within the “group IIIa, arthralgia of the TMJ criteria” to narrow the focus of the study. This group represents patients who commonly present to health care professionals for treatment, and we believe that this is the first study describing the use of 8% capsaicin in a TMD population. The goal of our study was 2-fold: 1) to evaluate the effects of a high-concentration (8%) capsaicin cream on normal pain sensitivity in healthy, control subjects and 2) to evaluate the effects of 8% capsaicin cream on patients with chronic orofacial pain. We completed a double-blind, vehicle-controlled clinical trial to evaluate the analgesic efficacy of capsaicin in chronic TMD patients.
Methods
Subject Recruitment and Randomization
The study protocol complied with the guidelines of the Institutional Review Board at the University of Florida (UF). We enrolled TMD and healthy, control subjects (“non-TMD subjects”) from May 2006 to January 2009, who were identified through advertising within the greater Gainesville, Florida, region. Treatments were assigned via a random-number generator, and syringes were labeled with a study protocol number. Both the subject and investigator were blinded to the treatment as the pharmacist did not break the study number code until the final analysis was completed. All subjects received participation compensation ($50 gift cards).
Inclusion Criteria
Healthy female volunteers (18 to 65 y old) with American Society of Anesthesiologists (ASA) status 1 or 2 and deemed in good general health were recruited. This represents the age range of patients with TMD, and the exclusive sex was selected given the higher proportion of females (>2:1 female/male) who develop TMD. The TMD patients’ inclusion criteria also included 1) subjects to have TMJ pain of greater than 6 mo duration, 2) episodes of pain with an average rating of at least 3 out of 10 on a visual analog scale (VAS) for the week that immediately preceded the initial testing date or the day of testing, and 3) fulfilling the Research Diagnostic Criteria (RDC), group IIIa, arthralgia of the TMJ criteria (Dworkin and LeResche 1992). Subjects had to have a joint component with or without muscle pain but were excluded if they only had myalgia. By this RDC definition, these subjects had no crepitus, had verified joint pain with palpation at either the lateral pole or at the posterior attachment, and met one of the following conditions: 1) be able to point to either muscle or joint pain, 2) joint or muscle pain on assisted/unassisted opening, or 3) joint or muscle pain in right or left excursive movements. A single examiner completed all of the clinical examinations on both TMD and healthy subjects.
Exclusion Criteria
Exclusion criteria were as follows: ASA status of 3 to 5, pregnant or breastfeeding mothers, allergy to capsaicin or red chili peppers, and presence of chronic disease(s) other than TMD. Subjects with course crepitus (by subject report or examination) of the TMJ or any subjects who had taken any pain medications (e.g., ibuprofen, acetaminophen, opioids) within 48 h prior to participating in the trial for either testing day were also excluded.
General
Subjects were seen at the UF College of Dentistry, explained and signed the informed consent form, and then completed a health history and RDC for TMD History Questionnaires. Subjects were informed that they could stop participating in the study or have the cream removed at any point. If they asked for removal of the cream earlier than the designated time, then the time was noted and they were then given the option to continue with the rest of the study or stop all together. Blood pressure, temperature, results of pregnancy test, and VAS and verbal ratings of that day’s TMD pain were recorded initially prior to any testing. We tried to include as many participants as possible, but due to recruitment and funding issues, only 16 TMD subjects were enrolled, and these subjects were then split (n = 8/group) into the capsaicin and control groups.
Quantitative Sensory Testing
Quantitative sensory testing (QST) for mechanical and thermal sensitivity were completed prior to drug application (baseline), 2 h after drug application, and at the second visit 1 wk later for select groups. All QSTs were demonstrated on the nondominant arm to familiarize the subject to the testing procedures.
Pressure pain was evaluated using a clinical grade pressure algometer (FPX 50—25 × 0.2N; Wagner). The subjects’ pressure pain threshold was determined by placing the probe tip perpendicular to the skin testing sites (lateral pole of the TMJ, mid-body of the superficial masseter) and applying pressure in an increasing manner at a rate of 1 kg/s. The subject signified the time of first painful response, at which point the pressure stimulus was terminated and the value recorded. After testing both sites bilaterally, VAS ratings of global pain were immediately recorded. Global pain was defined as clinical (TMD) pain (spontaneous, evoked) plus experimental pain. This test was repeated 3 times unless 2 of the 3 tests were not within 0.5 kg, necessitating a fourth trial.
Changes in thermal sensitivity were assessed using the Medoc Thermal Testing Device (Medoc Advanced Medical Systems). Pain threshold was assessed using an ascending method of limits with a rate of rise equaling 0.5°C/s, starting at 32°C, with the maximum limit set at 53°C. The 3 × 3-cm² thermode was applied on the skin overlying the TMJ lateral pole and in the mid-region of the superficial masseter. Subjects indicated when they first felt pain by pressing a button immediately stopping the stimulus and the value recorded. The contralateral side of the face was then tested and a global pain rating using a VAS was taken 3 min later. This test was repeated 3 times unless 2 of the 3 tests were not within 1°C, necessitating a fourth trial. The threshold for each patient was calculated as the mean of the individual trials.
Drug Application
Capsaicin powder (Formosa Laboratories) was compounded into an 8% (w/v) topical emollient cream base (andydrous ointment, polysorbate 80, butylated hydroxytoleune NF, water) by Westlab Pharmacy. Capsaicin content was verified by high-performance liquid chromatography analysis (Analytical Research Laboratories). The vehicle cream was the same topical solution but contained no capsaicin. The investigator spread a 0.1-mL dollop of cream to a standardized area overlying the affected TMJ and superficial masseter and then covered the site with a cotton gauge for 2 h. This area extended 3 cm anteriorly and inferiorly from the posterior aspect of the TMJ. A square 3 × 3-cm template was cut into a rubber dental dam to standardize the application site. The drug was applied to the right or left side randomly for subjects without TMD, whereas for the TMD sufferers, the side that was reported as more painful was studied.
Capsaicin-evoked pain intensity VAS ratings were collected every 5 min for the first half hour of the experiment and then every 30 min for the 2-h duration, at which point the subjects’ face was wiped clean with soaked gauze of half and half cream followed by alcohol swabs to remove residual capsaicin. QST was then repeated, and then the patients were discharged. All subjects were phoned 24 h following the study drug application to record their pain levels and any adverse events. TMD patients rated their clinical pain upon awakening for 7 days using a VAS home diary. These subjects were retested 1 wk from their initial visit, and this consisted of performing the same QST followed by the RDC for TMD Physical Assessment (Axis I). A subset of non-TMD subjects (n = 10) were tested again at this 1-wk time point to evaluate the effects of capsaicin on QST. This separate control group was completed in a single-blinded fashion, as the investigator was not blinded.
Measures
Perceived pain intensity was measured using a slide algometer VAS (Price et al. 1983) following capsaicin or vehicle cream application. In using the pain sensation intensity scale, the patient would slide the middle sliding part of the device to the right for an indicator of greater pain sensation intensity. The arrow at the extreme left meant no pain sensation at all, and the arrow at the extreme right indicated a pain sensation intensity that subject imagined to be the most intense that she could possibly experience.
Statistical Analyses
Individual subjects who were assigned to control and treatment groups provided repeated observations for subjective pain measurements during 5, 10, . . . 180 min and, later, 1 to 7 days. Because the serial measurements and distinction between relatively short-term versus long-term effects were correlated according to the individual subjects, multilevel linear mixed modeling was applied. Bonferroni adjustment was used to compare multiple mean differences. All values were considered significant when P < 0.05.
Results
A total of 44 control (non-TMD) subjects were recruited with 21 receiving capsaicin and the remaining 23 receiving vehicle. An additional 10 non-TMD subjects were recruited to evaluate the effects of capsaicin on QST performed 1 wk later, as described in the Methods. Sixteen TMD subjects were recruited, and upon evaluation, they met the criteria for RDC, group IIIa. Eight received capsaicin and 8 vehicle cream. None of the participants dropped out, but 2 (1 from each of the TMD and non-TMD capsaicin groups) requested the cream to be removed before the 2-h time limit after drug application due to excess pain, and their data were not included in the results. Tables 1 and 2 summarize the total number of subjects enrolled to date tabulated by age and by race, excluding the 2 participants who did not complete all requirements.
Table 1.
Subject Demographics: Age Distribution.
| Normal |
TMD |
|||||
|---|---|---|---|---|---|---|
| Treatment | n | Mean | SD | n | Mean | SD |
| Age distribution | ||||||
| Control vehicle | 23 | 22.5 | 6.9 | 8 | 25.8 | 12.2 |
| Capsaicin 8% | 30 | 28.0 | 10.6 | 7 | 29.3 | 12.3 |
| Total | 53 | 25.6 | 9.5 | 15 | 27.4 | 11.9 |
Two-way (2-factorial) analysis of variance revealed that there is no significant difference in the age distribution between normal and TMD subjects (P = 0.437) or between control and treatment groups (P = 0.127).
TMD, temporomandibular joint disorder.
Table 2.
Subject Demographics: Ethnic Distribution.
| Normal Subjects, n (%) |
TMD Patients, n (%) |
|||||
|---|---|---|---|---|---|---|
| Ethnicity | Control | Capsaicin | Total | Control | Capsaicin | Total |
| Caucasian | 12 (52.2) | 20 (66.6) | 32 (60.5) | 5 (62.5) | 5 (71.4) | 10 (66.6) |
| African American | 2 (8.7) | 2 (6.7) | 4 (7.5) | 1 (14.3) | 1 (6.7) | |
| Hispanic | 8 (34.8) | 5 (16.7) | 13 (24.5) | 3 (37.5) | 1 (14.3) | 4 (26.7) |
| Asian | 1 (4.3) | 3 (10.0) | 4 (7.5) | |||
Fisher’s exact statistic showed that there is no significant difference in the ethnicity distribution neither between normal and TMD subjects (P = 0.459) or between control and treatment groups (P = 0.569).
TMD, temporomandibular joint disorder.
Effects of Capsaicin on Experimental and Global Pain
Application of capsaicin increased pain ratings compared to vehicle treatment in both TMD symptomatic (Fig. 1A) and non-TMD subjects (Fig. 1B). When the short-term effects of capsaicin versus vehicle treatments were evaluated up to 2 h postapplication for the TMD and non-TMD groups, there was a significant increase in VAS ratings over time for the capsaicin-treated groups compared to vehicle-treated subjects (analysis of variance [ANOVA], P < 0.001). The peak value (mean ± SEM) of this increase was similar for both groups, 5.3 ± 0.6 and 4.8 ± 0.6 for the TMD and non-TMD groups, respectively. There was a similar response pattern for both groups, with the peak rating occurring 15 min after capsaicin and then returning to baseline after 2 h.
Figure 1.
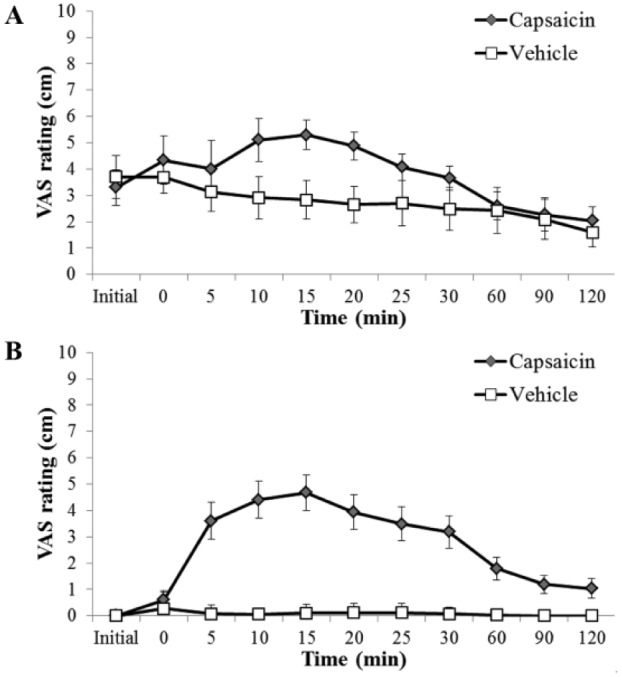
Global pain (experimental + clinical pain) ratings. (A) Temporomandibular joint disorder (TMD) and (B) non-TMD group global pain ratings for up to 2 h after capsaicin or vehicle cream application. Application of capsaicin increased reported pain compared to vehicle in both healthy and TMD symptomatic subjects, with a significant increase in visual analog scale (VAS) ratings over time for the capsaicin-treated group compared to vehicle-treated subjects (analysis of variance, P < 0.001).
Since the TMD group had existing pain, we used a global pain rating that was a combination of their existing clinical pain plus the experimentally induced pain. The TMD group global baseline pain rating (prior to initial QST) was compared to post–study drug (capsaicin or vehicle) pain ratings. The VAS measures during 1 to 7 days (Fig. 2) were significantly greater in the control vehicle group than in the capsaicin treatment group (P = 0.001). The VAS ratings were significantly lower for the capsaicin-treated subjects over the 1-wk postapplication period (P = 0.033).
Figure 2.
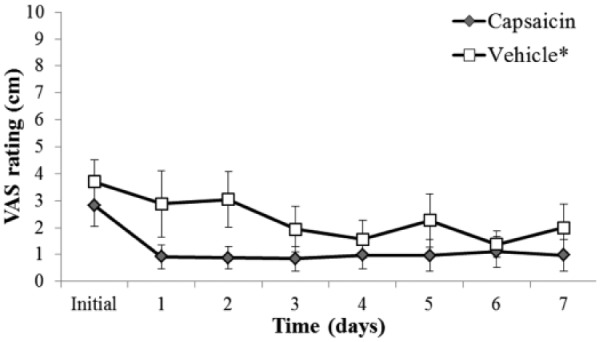
One-week temporomandibular joint disorder (TMD) group global (TMD) pain ratings posttreatment. The visual analog scale (VAS) measures during 1 to 7 days were significantly greater in the control vehicle group than in the capsaicin treatment group (*P = 0.001).
Thermal Pain Threshold
Capsaicin and vehicle TMD groups had similar baseline thermal pain thresholds on their affected side, and there was no difference with the no/less pain contralateral side threshold, and this comparison holds true for the non-TMD groups. Both TMD and non-TMD groups (Fig. 3) demonstrated a significant decrease in thermal pain threshold at 2 h after capsaicin cream application. The thermal thresholds for the capsaicin-treated groups returned to baseline levels at the 1-week follow-up for the control subjects (baseline: 44.5°C ± 0.8°C; 1 wk: 45.3°C ± 0.8°C; P = 0.27) and TMD groups (baseline: 40.4°C ± 1.5°C; 1 wk: 41.6°C ± 1.7°C; P = 0.51). No significant thermal pain threshold differences were observed at any time of the postvehicle test sessions (+2 h, 1 wk) compared to baseline levels between the normal controls and TMD groups.
Figure 3.
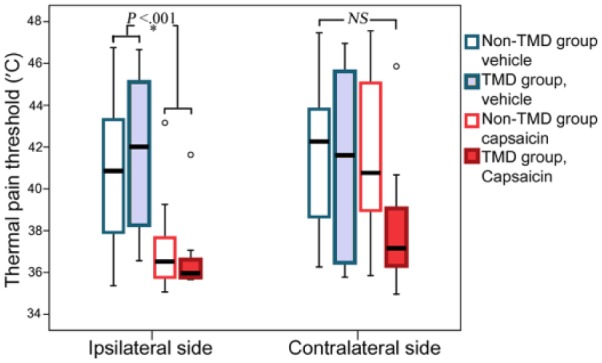
Thermal pain threshold responses for temporomandibular joint disorder (TMD) and non-TMD subjects. There was a significant (*P < 0.001) decrease in the threshold for both TMD and non-TMD groups that received capsaicin treatment compared to vehicle and baseline predrug values. There were no significant (NS) differences for any of the treatment or study groups when the contralateral (nontreated) side was evaluated.
Pressure Pain Threshold
TMD subjects (n = 14) had similar baseline thresholds (2.0 ± 0.4 g) compared to non-TMD (n = 53) controls (2.2 ± 0.6 g) on the treated/symptomatic side. No significant changes in pressure pain threshold across time emerged for normal controls or TMD subjects regardless of treatment, site (TMJ or superficial masseter), or side of face compared to baseline. There was no statistical significance between TMD and normal subjects, regardless of treatment at baseline and after 2 h, or between baseline and 2 h for Von Frey QST. Analysis was done using Fisher’s exact test and the Cochran-Mantel-Haenzel statistic.
Discussion
The concept of targeting TRPV1 due to its role in inflammation and pain has provided a rationale for the treatment of chronic pain disorders. Findings from previous studies are mixed, with some studies indicating efficacy in the treatment of OA, RA, and PHN. Other reports indicate that capsaicin’s analgesic effectiveness at lower concentration may be insufficient as a viable therapy. With only 1 study evaluating capsaicin’s resolution of TMD pain, there is a definite need to explore the effects of higher dose topical capsaicin before conclusions can be drawn about targeting TRPV1 as an effective method for controlling TMD pain and ultimately chronic pain.
The purpose of this study was to evaluate a high-concentration (8%) topical capsaicin cream on TMD pain. Our data suggest that capsaicin may have some clinical benefits of relieving TMD pain. Significantly lower pain was reported in the week postapplication in the capsaicin-treated subjects compared to the vehicle subjects. This contrasts the previous capsaicin TMD study that found no difference between the TMD and healthy control groups (Winocur et al. 2000). This discrepancy could be attributed to a lower dose of capsaicin and in the weekly reporting interval, whereas our study collected measures daily. Our data showed a significant difference between the TMD capsaicin and vehicle groups over the entire 7 d postapplication.
Our approach used established QST techniques to evaluate sensory changes and pain. There was a decreased thermal pain threshold 2 h after capsaicin application, but this resolved 1 wk postapplication with return of normal thermal pain threshold responses. Thermal pain thresholds showed that regardless of disease state of the group (TMD vs. non-TMD), the threshold decreased only on the side ipsilateral to the capsaicin and was only seen at the 2-h time point rather than 7 d out. This finding seemingly contradicts previous findings of desensitization 1 wk later after a 2-h application of 8% capsaicin or 3 wk later after multiple daily low-dose capsaicin applications at the same skin site (Nolano et al. 1999; Malmberg et al. 2004). Their findings suggest thermal sensitization determines when a subject first feels warmth (sensitization) rather than pain due to heat (thermal pain threshold), which we evaluated.
Capsaicin had no effect on pressure pain threshold for TMD and healthy individuals. This contrasts reports of pressure pain threshold differences in TMD and controls. Our study focused on arthralgia rather than myalgia; therefore, this selection bias may account for the lack of pressure sensitivity between the treatment groups. We also found no difference in punctate mechanical sensitivity between baseline and the 2-h time points between normal control and TMD groups. These findings indicate that effects of this high-concentration topical capsaicin cream do not affect the mechanoreceptors and either pressure or punctate pain. This contrasts findings regarding mechanical allodynia induced by topically applied 0.075% capsaicin (Petersen et al. 2000). However, a different mechanical stimulus (light paintbrush touch) differs substantially from the pressure and Von Frey stimuli used in the current protocol.
Not surprisingly, capsaicin produced pain in both the TMD and healthy subject groups during the postapplication testing period. An hour after capsaicin application, the TMD group pain returned to or was below initial pain levels. The TMD group receiving vehicle had no increase in pain; rather, they showed stable pain levels that declined. As noted, 2 of 38 individuals felt like the pain was too much and did not want to continue suggesting that some of the population may have poor compliance with the present treatment modality.
There exists a somewhat paradoxical finding that a topical therapy can affect an essentially deep tissue (TMJ/masseter muscle) pain input. While it is possible for the capsaicin to penetrate and directly activate TRPV1 receptors within the joint or muscle, other actions may occur due to central modulation. The central nervous system is susceptible to peripheral, nociceptive stimuli and responds with sensitization of the peripheral receptive fields. Our data suggest that this occurs following capsaicin application, as those subjects (both TMD and healthy controls) experienced thermal allodynia at the 2-h postapplication time point. This manifested as a significant lowering of the heat pain threshold. Conversely, central inhibitory mechanisms can be triggered such that the painful stimuli can actually lower pain responses via compensatory inhibitory pathways. Our data for the TMD subjects support this phenomenon, as these subjects demonstrated a week-long decrease in global pain rating following a severe noxious insult. This is consistent with the theory of diffuse noxious inhibitory control (DNIC) systems, which implies that a noxious stimulus can activate global inhibitory systems to reduce pain (Le Bars 2002). This pathway is likely mediated by endogenous opioid pathways, such as found in the periacqueductal gray (Basbaum and Jessell 2000). The findings from our current study suggest that initial algesic response following 8% topical capsaicin cream application may activate inhibitory systems to produce long-duration effects. This may complement the desensitization concept proposed by previous topical capsaicin studies and together support the use of capsaicin as a beneficial therapy for patients.
Overall, we found that there was a significant effect for reducing TMD pain over the 1-wk period following a single application of capsaicin; however, the main limitation of this study still remains the relatively low sample size for the TMD groups. While promising, further studies with larger cohorts that include other RDC diagnostic groups are needed. We acknowledge that male patients also have facial pain and TMD in particular, albeit at a much lower occurrence. Due to practical experimental issues, including funding and difficulty in recruiting subjects, we chose to focus on female subjects, and this limits the generalizability of our results. Future studies will include male subjects, evaluation of duration of analgesic effect, optimal application (e.g., frequency, duration), and tolerability, which are important factors that require further evaluation for 8% topical capsaicin therapy.
Author Contributions
B.K. Campbell, contributed to design, data acquisition, and analysis, drafted the manuscript; R.B. Fillingim, contributed to conception and data interpretation, critically revised the manuscript; S. Lee, contributed to data analysis, critically revised the manuscript; R. Brao, contributed to design and data acquisition, critically revised the manuscript; D.D. Price, contributed to conception, design, and data interpretation, critically revised the manuscript; J.K. Neubert, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
The authors thank Prince Hinson for compounding and preparing the study drugs.
Footnotes
Support for this research was provided by grant 1K22DE014865-01A1, National Institute of Dental and Craniofacial Research, National Institutes of Health, Department of Health and Human Services, Bethesda, MD, USA.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
In memoriam: Donald D. Price, PhD
References
- Al-Jundi MA, John MT, Setz JM, Szentpétery A, Kuss O. 2008. Meta-analysis of treatment need for temporomandibular disorders in adult nonpatients. J Orofac Pain. 22(2):97–107. [PubMed] [Google Scholar]
- Basbaum A, Jessell T. 2000. The perception of pain. New York (NY): Elsevier Science. [Google Scholar]
- Caterina MJ, Julius D. 2001. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 24:487–517. [DOI] [PubMed] [Google Scholar]
- Deal CL, Schnitzer TJ, Lipstein E, Seibold JR, Stevens RM, Levy MD, Albert D, Renold F. 1991. Treatment of arthritis with topical capsaicin: a double-blind trial. Clin Ther. 13(3):383–395. [PubMed] [Google Scholar]
- Dworkin SF, LeResche L. 1992. Research diagnostic criteria for temporomandibular disorders: review, criteria, examinations and specifications, critique. J Craniomandib Disord. 6(4):301–355. [PubMed] [Google Scholar]
- Gaul C, Resch S. 2015. Application of the capsaicin 8% cutaneous patch in neuropathic pain of the head and face: a case series. Cephalalgia. 35(6):545–550. [DOI] [PubMed] [Google Scholar]
- Gunthorpe MJ, Benham CD, Randall A, Davis JB. 2002. The diversity in the vanilloid (TRPV) receptor family of ion channels. Trends Pharmacol Sci. 23(4):183–191. [DOI] [PubMed] [Google Scholar]
- Jordt SE, McKemy DD, Julius D. 2003. Lessons from peppers and peppermint: the molecular logic of thermosensation. Curr Opin Neurobiol. 13(4):487–492. [DOI] [PubMed] [Google Scholar]
- Karai L, Brown DC, Mannes AJ, Connelly ST, Brown J, Gandal M, Wellisch OM, Neubert JK, Olah Z, Iadarola MJ. 2004. Deletion of vanilloid receptor 1–expressing primary afferent neurons for pain control. J Clin Invest. 113(9):1344–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bars D. 2002. The whole body receptive field of dorsal horn multireceptive neurones. Brain Res Brain Res Rev. 40(1–3):29–44. [DOI] [PubMed] [Google Scholar]
- LeResche L. 1997. Epidemiology of temporomandibular disorders: implications for the investigation of etiologic factors. Crit Rev Oral Biol Med. 8(3):291–305. [DOI] [PubMed] [Google Scholar]
- Lipton JA, Ship JA, Larach-Robinson D. 1993. Estimated prevalence and distribution of reported orofacial pain in the United States. J Am Dent Assoc. 124(10):115–121. [DOI] [PubMed] [Google Scholar]
- Maihöfner CG, Heskamp ML. 2013. Prospective, non-interventional study on the tolerability and analgesic effectiveness over 12 weeks after a single application of capsaicin 8% cutaneous patch in 1044 patients with peripheral neuropathic pain: first results of the QUEPP study. Curr Med Res Opin. 29(6):673–683. [DOI] [PubMed] [Google Scholar]
- Maihöfner CG, Heskamp ML. 2014. Treatment of peripheral neuropathic pain by topical capsaicin: impact of pre-existing pain in the QUEPP-study. Eur J Pain. 18(5):671–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmberg AB, Mizisin AP, Calcutt NA, von Stein T, Robbins WR, Bley KR. 2004. Reduced heat sensitivity and epidermal nerve fiber immunostaining following single applications of a high-concentration capsaicin patch. Pain. 111(3):360–367. [DOI] [PubMed] [Google Scholar]
- Mason L, Moore RA, Derry S, Edwards JE, McQuay HJ. 2004. Systematic review of topical capsaicin for the treatment of chronic pain. BMJ. 328(7446):991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy GM, McCarty DJ. 1992. Effect of topical capsaicin in the therapy of painful osteoarthritis of the hands. J Rheumatol. 19(4):604–607. [PubMed] [Google Scholar]
- Neubert JK, Karai L, Jun JH, Kim HS, Olah Z, Iadarola MJ. 2003. Peripherally induced resiniferatoxin analgesia. Pain. 104(1–2): 219–228. [DOI] [PubMed] [Google Scholar]
- Nolano M, Simone DA, Wendelschafer-Crabb G, Johnson T, Hazen E, Kennedy WR. 1999. Topical capsaicin in humans: parallel loss of epidermal nerve fibers and pain sensation. Pain. 81(1–2):135–145. [DOI] [PubMed] [Google Scholar]
- Patapoutian A, Tate S, Woolf CJ. 2009. Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov. 8(1):55–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peikert A, Hentrich M, Ochs G. 1991. Topical 0.025% capsaicin in chronic post-herpetic neuralgia: efficacy, predictors of response and long-term course. J Neurol; 238(8):452–456. [DOI] [PubMed] [Google Scholar]
- Petersen KL, Fields HL, Brennum J, Sandroni P, Rowbotham MC. 2000. Capsaicin evoked pain and allodynia in post-herpetic neuralgia. Pain. 88(2):125–133. [DOI] [PubMed] [Google Scholar]
- Price DD, McGrath PA, Rafii A, Buckingham B. 1983. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 17(1):45–56. [DOI] [PubMed] [Google Scholar]
- Slade GD, Bair E, Greenspan JD, Dubner R, Fillingim RB, Diatchenko L, Maixner W, Knott C, Ohrbach R. 2013. Signs and symptoms of first-onset TMD and sociodemographic predictors of its development: the OPPERA prospective cohort study. J Pain. 14(12 Suppl):T20–32.e1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solberg W. 1983. Epidemiology, incidence and prevalence of temporomandibular disorders.In: Laskin D, Greenfield W, Gale W, editors. The president’s conference of the examination, diagnosis, and management of temporomandibular disorders. Chicago: (IL): American Dental Association; 1983. [Google Scholar]
- Szallasi A, Appendino G. 2004. Vanilloid receptor TRPV1 antagonists as the next generation of painkillers: are we putting the cart before the horse? J Med Chem. 47(11):2717–2723. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ. 2004. Thermosensation and pain. J Neurobiol. 61(1):3–12. [DOI] [PubMed] [Google Scholar]
- Watson CP, Tyler KL, Bickers DR, Millikan LE, Smith S, Coleman E. 1993. A randomized vehicle-controlled trial of topical capsaicin in the treatment of postherpetic neuralgia. Clin Ther. 15(3):510–526. [PubMed] [Google Scholar]
- Winocur E, Gavish A, Halachmi M, Eli I, Gazit E. 2000. Topical application of capsaicin for the treatment of localized pain in the temporomandibular joint area. J Orofac Pain. 14(1):31–36. [PubMed] [Google Scholar]
- Yaksh TL, Farb DH, Leeman SE, Jessell TM. 1979. Intrathecal capsaicin depletes substance p in the rat spinal cord and produces prolonged thermal analgesia. Science. 206(4417):481–483. [DOI] [PubMed] [Google Scholar]
- Zis P, Apsokardos A, Isaia C, Sykioti P, Vadalouca A. 2014. Posttraumatic and postsurgical neuropathic pain responsive to treatment with capsaicin 8% topical patch. Pain Physician. 17(2):E213–E218. [PubMed] [Google Scholar]


