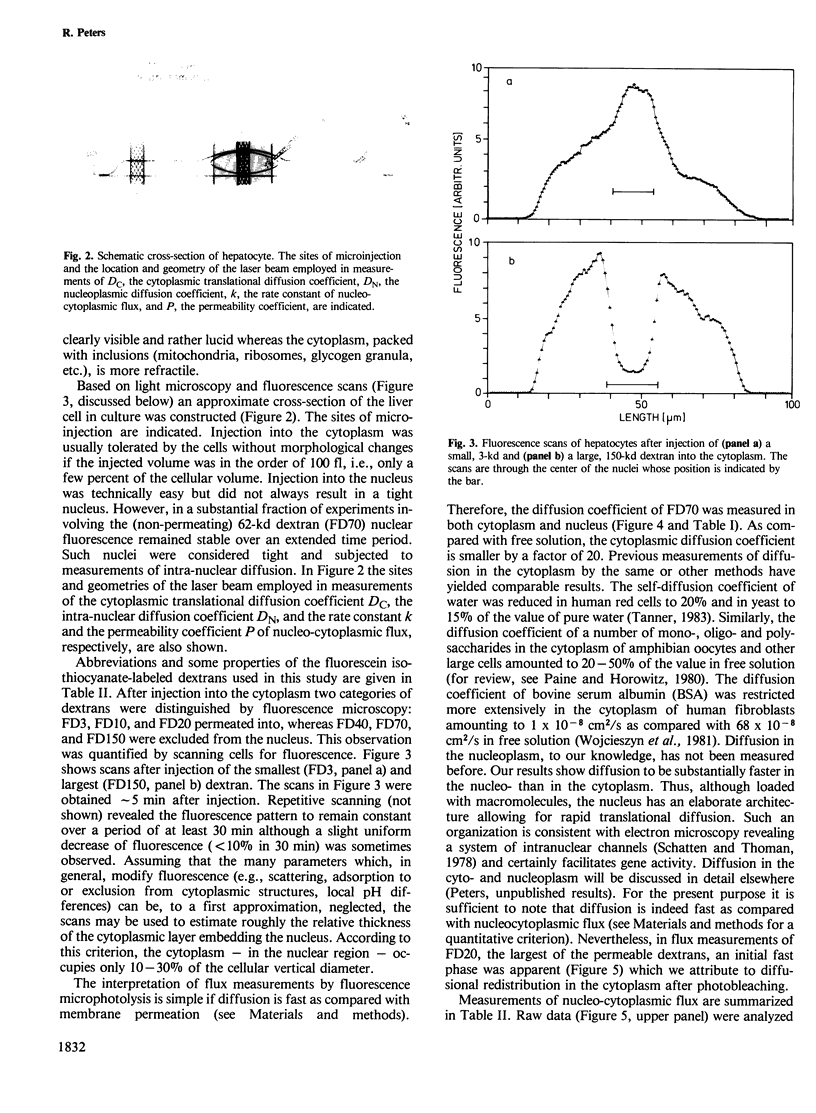
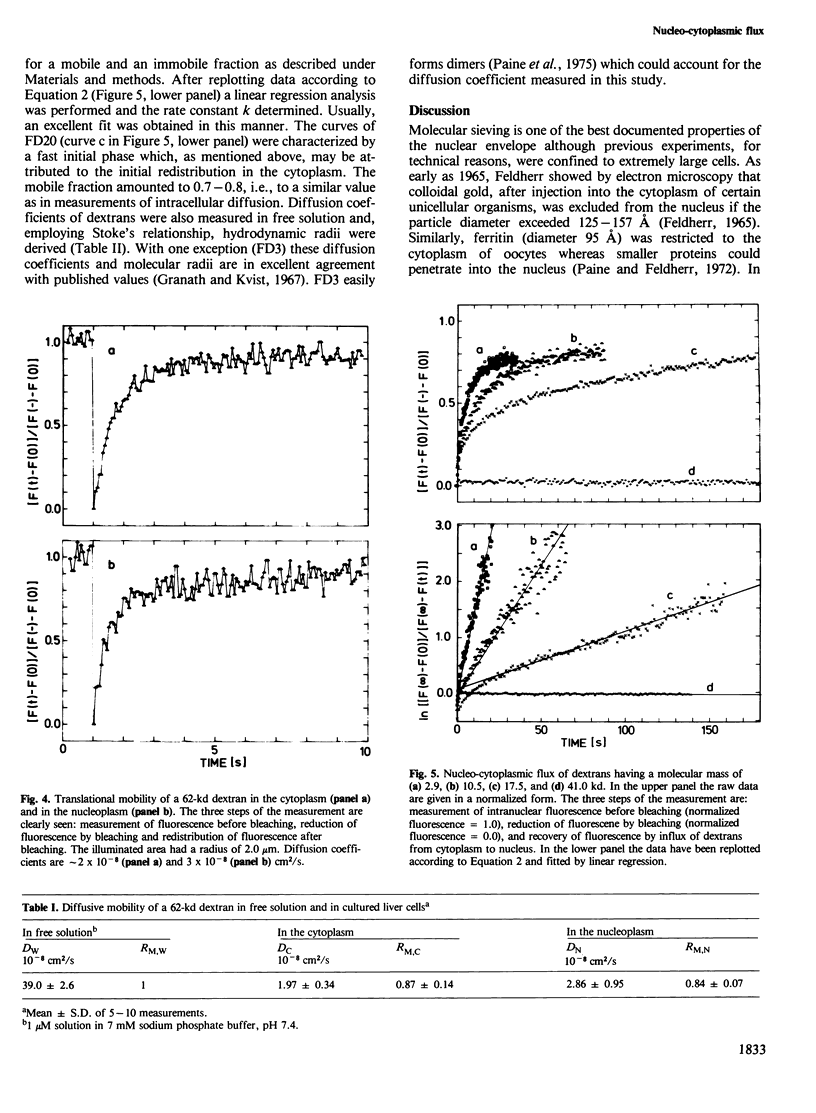
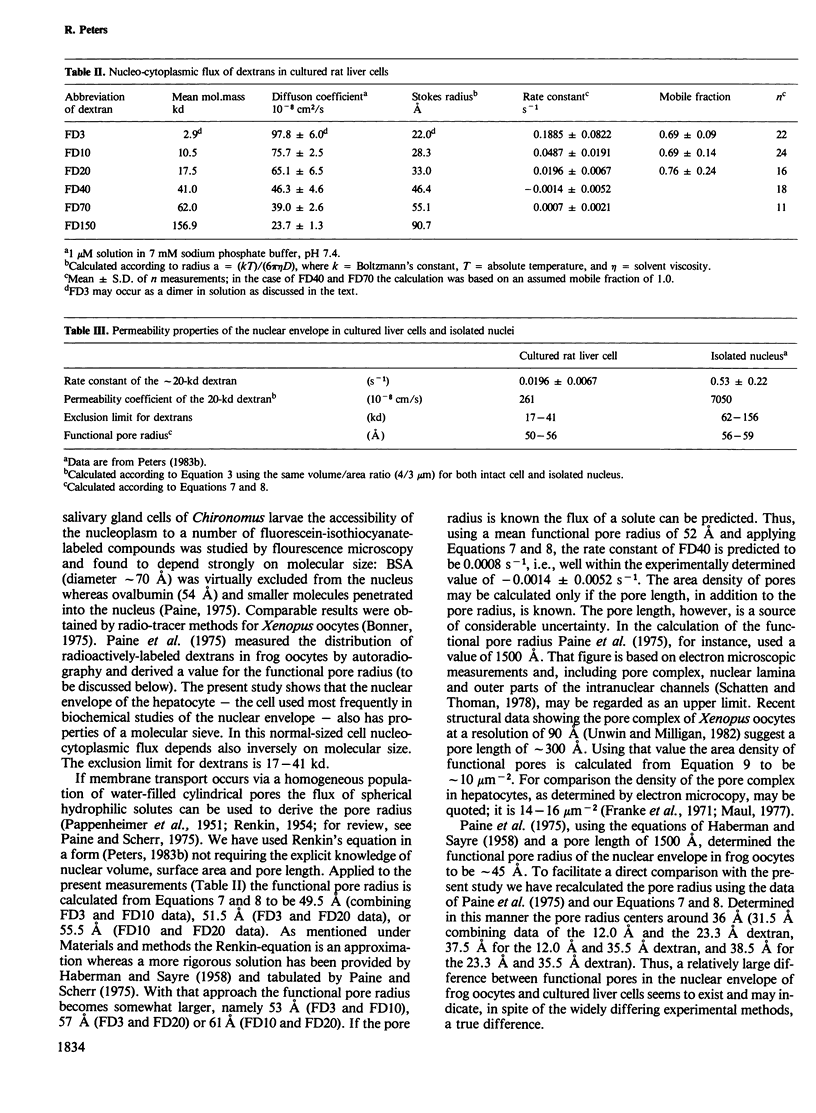
Abstract
Fluorescence microphotolysis was used to measure nucleocytoplasmic flux in single rat hepatocytes for a series of dextrans ranging in molecular mass from 3 to 150 kd. The cytoplasmic translational diffusion coefficient DC and the nucleoplasmic diffusion coefficient DN of a 62-kd dextran were also determined. DC was approximately 2 X 10(-8) and DN approximately 3 X 10(-8) cm2/s, i.e., 1/20-1/15 of the value in free solution. The mobile fraction amounted to 0.7-0.8 in measurements of both intracellular diffusion and nucleo-cytoplasmic flux. The flux of dextrans from cytoplasm to nucleus depended inversely on molecular mass with an exclusion limit between 17 and 41 kd suggesting that the nuclear envelope has functions of a molecular sieve. Employing the Pappenheimer-Renkin equations, a functional pore radius of 50-56 A was derived. By comparison with recent measurements on isolated liver cell nuclei, large quantitative differences between the intracellularly located and the isolated nucleus were revealed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansorge W. Improved system for capillary microinjection into living cells. Exp Cell Res. 1982 Jul;140(1):31–37. doi: 10.1016/0014-4827(82)90152-5. [DOI] [PubMed] [Google Scholar]
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry M. N., Friend D. S. High-yield preparation of isolated rat liver parenchymal cells: a biochemical and fine structural study. J Cell Biol. 1969 Dec;43(3):506–520. doi: 10.1083/jcb.43.3.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M. Protein migration into nuclei. I. Frog oocyte nuclei in vivo accumulate microinjected histones, allow entry to small proteins, and exclude large proteins. J Cell Biol. 1975 Feb;64(2):421–430. doi: 10.1083/jcb.64.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Robertis E. M., Longthorne R. F., Gurdon J. B. Intracellular migration of nuclear proteins in Xenopus oocytes. Nature. 1978 Mar 16;272(5650):254–256. doi: 10.1038/272254a0. [DOI] [PubMed] [Google Scholar]
- FELDHERR C. M. THE EFFECT OF THE ELECTRON-OPAQUE PORE MATERIAL ON EXCHANGES THROUGH THE NUCLEAR ANNULI. J Cell Biol. 1965 Apr;25:43–53. doi: 10.1083/jcb.25.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke W. W., Kartenbeck J., Deumling B. Nuclear pore flow rates of ribonucleic acids in the mature rat hepatocyte. Experientia. 1971 Apr 15;27(4):372–373. doi: 10.1007/BF02137254. [DOI] [PubMed] [Google Scholar]
- Gebhardt R., Jung W., Robenek H. Primary cultures of rat hepatocytes as a model system of canalicular development, biliary secretion, and intrahepatic cholestasis. I. Distribution of filipin-cholesterol complexes during de novo formation of bile canaliculi. Eur J Cell Biol. 1982 Nov;29(1):68–76. [PubMed] [Google Scholar]
- Gebhardt R., Mecke D. Perifused monolayer cultures of rat hepatocytes as an improved in vitro system for studies on ureogenesis. Exp Cell Res. 1979 Dec;124(2):349–359. doi: 10.1016/0014-4827(79)90210-6. [DOI] [PubMed] [Google Scholar]
- Granath K. A., Kvist B. E. Molecular weight distribution analysis by gel chromatography on Sephadex. J Chromatogr. 1967 May;28(1):69–81. doi: 10.1016/s0021-9673(01)85930-6. [DOI] [PubMed] [Google Scholar]
- Howard R. B., Christensen A. K., Gibbs F. A., Pesch L. A. The enzymatic preparation of isolated intact parenchymal cells from rat liver. J Cell Biol. 1967 Dec;35(3):675–684. doi: 10.1083/jcb.35.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohen E., Siebert G., Kohen C. Transfer of metabolites across the nuclear membrane. A microfluorometric study. Hoppe Seylers Z Physiol Chem. 1971 Jul;352(7):927–937. doi: 10.1515/bchm2.1971.352.2.927. [DOI] [PubMed] [Google Scholar]
- Maul G. G. The nuclear and the cytoplasmic pore complex: structure, dynamics, distribution, and evolution. Int Rev Cytol Suppl. 1977;(6):75–186. [PubMed] [Google Scholar]
- Paine P. L., Feldherr C. M. Nucleocytoplasmic exchange of macromolecules. Exp Cell Res. 1972 Sep;74(1):81–98. doi: 10.1016/0014-4827(72)90483-1. [DOI] [PubMed] [Google Scholar]
- Paine P. L., Moore L. C., Horowitz S. B. Nuclear envelope permeability. Nature. 1975 Mar 13;254(5496):109–114. doi: 10.1038/254109a0. [DOI] [PubMed] [Google Scholar]
- Paine P. L. Nucleocytoplasmic movement of fluorescent tracers microinjected into living salivary gland cells. J Cell Biol. 1975 Sep;66(3):652–657. doi: 10.1083/jcb.66.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine P. L., Scherr P. Drag coefficients for the movement of rigid spheres through liquid-filled cylindrical pores. Biophys J. 1975 Oct;15(10):1087–1091. doi: 10.1016/S0006-3495(75)85884-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R. Fluorescence microphotolysis. Diffusion measurements in single cells. Naturwissenschaften. 1983 Jun;70(6):294–302. doi: 10.1007/BF00404836. [DOI] [PubMed] [Google Scholar]
- Peters R. Nuclear envelope permeability measured by fluorescence microphotolysis of single liver cell nuclei. J Biol Chem. 1983 Oct 10;258(19):11427–11429. [PubMed] [Google Scholar]
- Peters R., Richter H. P. Translational diffusion in the plasma membrane of sea urchin eggs. Dev Biol. 1981 Sep;86(2):285–293. doi: 10.1016/0012-1606(81)90186-x. [DOI] [PubMed] [Google Scholar]
- Pitot H. C., Sirica A. E. Methodology and utility of primary cultures of hepatocytes from experimental animals. Methods Cell Biol. 1980;21B:441–456. doi: 10.1016/s0091-679x(08)60697-4. [DOI] [PubMed] [Google Scholar]
- RENKIN E. M. Filtration, diffusion, and molecular sieving through porous cellulose membranes. J Gen Physiol. 1954 Nov 20;38(2):225–243. [PMC free article] [PubMed] [Google Scholar]
- Schatten G., Thoman M. Nuclear surface complex as observed with the high resolution scanning electron microscope. Visualization of the membrane surfaces of the neclear envelope and the nuclear cortex from Xenopus laevis oocytes. J Cell Biol. 1978 May;77(2):517–535. doi: 10.1083/jcb.77.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Sirica A. E., Richards W., Tsukada Y., Sattler C. A., Pitot H. C. Fetal phenotypic expression by adult rat hepatocytes on collagen gel/nylon meshes. Proc Natl Acad Sci U S A. 1979 Jan;76(1):283–287. doi: 10.1073/pnas.76.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner J. E. Intracellular diffusion of water. Arch Biochem Biophys. 1983 Jul 15;224(2):416–428. doi: 10.1016/0003-9861(83)90228-x. [DOI] [PubMed] [Google Scholar]
- Unwin P. N., Milligan R. A. A large particle associated with the perimeter of the nuclear pore complex. J Cell Biol. 1982 Apr;93(1):63–75. doi: 10.1083/jcb.93.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcieszyn J. W., Schlegel R. A., Wu E. S., Jacobson K. A. Diffusion of injected macromolecules within the cytoplasm of living cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4407–4410. doi: 10.1073/pnas.78.7.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]



