Abstract
Interest in nutrient-rich berry juices is growing, but their high polyphenol levels render them sensorily unappealing. Fifty adults, who were assessed for sensory phenotype and dietary behaviors, provided sensory and palatability ratings of juices from ‘Viking’ aronia berries for each of seven harvest weeks. By peak harvest, juice preference increased two-fold, averaging neither like/dislike. This hedonic shift was associated with: increases in juice sugars paralleling increases in perceived sweetness (maximum = weak); reductions in percent acidity paralleling reductions in sourness (minimum = moderate), astringency (minimum = to just above weak) and bitterness (minimum = just below weak). About 25% of adults liked the aronia juice, including adults who also liked an aqueous citric acid solution (average rating = moderately sour) or those who reported adventurous eating behaviors. Bitter taste phenotype, measured by propylthiouracil or quinine bitterness, failed to explain significant variation in juice sensation or preference. We also collected sensory and preference ratings from juice collected at peak harvest blended with sugar and/or sweet olfactory flavoring (10 ppm ethyl butyrate). Increasing juice sweetness by adding 5% sucrose decreased sourness and improved preference from weak dislike to weak like. Adding sweet olfactory flavoring decreased juice sourness without changing preference. Adding sweet flavoring and 3% sucrose resulted in reduction of sourness and improvements in preference ratings comparable to 5% added sucrose. Neither added sugar nor flavoring blocked juice astringency. In summary, these findings suggest that aronia juice, even from berries picked at peak harvest, appealed to only a few adults (sour likers or adventurous eaters). Although enhanced sweetness, with added sugar and sweet olfactory flavoring, improved aronia juice preference, broader sensory approaches are required to blunt astringency for greater consumer appeal.
Keywords: Food preferences, Beverages/*analysis, Psychophysics, Fruit, Surveys and questionnaires, Polyphenols
1. Introduction
Dark-colored aronia berry (chokeberry) has significant quantities of health-promoting polyphenols, including anthocyanins and proanthocyanidins (Bolling et al., 2015; Taheri, Connolly, Brand, & Bolling, 2013). Yet, as the common name of chokeberry implies, it has pronounced bitterness, sourness and astringency (drying/puckering sensation in the oral cavity (Bajec & Pickering, 2008)). Similar to wines (Gonzalo-Diago, Dizy, & Fernandez-Zurbano, 2014), the high levels of aronia phenols and non-volatile organic acids contribute to these oral sensations. While strong bitterness, astringency and sourness are generally disliked sensations in berry juices and products (Suomela et al., 2012), low levels of sourness can positively influence preference for berry products (Laaksonen, Ahola, & Sandell, 2013). Aronia berry is cultivated in Europe for use in wine, preserves, juice, and other food products. The demand for aronia berry has increased in the U.S. because of its health promoting potential (Brand, 2010). The contribution of berries to U.S. polyphenol intake has increased (Kim, Vance, & Chun, 2016) and diets higher in polyphenols are associated with lower risk of chronic conditions such as heart disease (Wang, Ouyang, Liu, & Zhao, 2014).
Variation in oral sensory abilities influences the acceptance of beverages with pronounced sour and bitter characteristics (Gonzalo-Diago et al., 2014). Twin studies show that variation in taste and liking for sour foods is mediated by genetic influences, and these exceed environmental influences (Tornwall et al., 2012; Wise, Hansen, Reed, & Breslin, 2007). The most widely studied phenotypic marker of taste genetics is perceived bitterness of propylthiouracil (PROP). Individuals who taste PROP as more bitter also taste more bitterness or sourness (Prescott, Soo, Campbell, & Roberts, 2004) and perceive more astringency (Pickering, Simunkova, & DiBattista, 2004) in solutions or food products. These heightened oral sensations suppress naturally occurring sweetness in bitter vegetables and alcoholic beverages to decrease liking and consumption (Dinehart, Hayes, Bartoshuk, Lanier, & Duffy, 2006; Lanier, Hayes, & Duffy, 2005). Those who taste PROP as most bitter are sensitive to changes in taste concentration, which in turn influences their preference for foods/beverages (Hayes, Sullivan, & Duffy, 2010; Lee, Prescott, & Kim, 2008). For example, changes in the level of sodium chloride in sodium-containing foods resulted in greater changes in preference among those who taste PROP as most bitter versus least bitter (Hayes et al., 2010).
Much of the ability to taste the bitterness of PROP is mediated by the bitter taste receptor gene, TAS2R38 (Kim et al., 2003). Laaksonen and colleagues (Laaksonen, Ahola, et al., 2013) reported differences in bitterness, sourness and astringency of juices from bilberry and crowberry by three common polymorphisms in TAS2R38. Contrary to what they expected to find, nontasters by genotype perceived greater intensities than did homozygous tasters. These taste gene polymorphisms may or may not explain variability in the consumption of berries. A population-based study of adults from Finland showed no significant TAS2R38 effects on berry or fruit consumption (Sandell et al., 2014). However, in a smaller, sensory-focused study, TAS2R38 tasters were the least frequent consumers of lingonberries (Sandell et al., 2015), which, similar to aronia berries, have intense sourness/bitterness that blocks natural sweetness. Importantly however, polymorphisms of the TAS2R38 gene do not explain heightened oral sensations (i.e., the supertasting phenomenon) (Hayes, Bartoshuk, Kidd, & Duffy, 2008). Bitterness of compounds such as quinine may be a better marker of variation in orosensation. Quinine is broadly tuned to multiple bitter receptors (Reed et al., 2010), correlates well with intensity of multiple taste qualities (S. Rawal, Hoffman, Honda, Huedo-Medina, & Duffy, 2015), and is a good probe of taste alterations (Coldwell et al., 2013). Relationship between taste genes and behaviors can be influenced by sex hormones, which associate with differences in taste intensity and preference (Duffy, Bartoshuk, Striegel-Moore, & Rodin, 1998). Finally, it may not be the intensity of the sour taste that influences liking for sour sensations but rather the salivary response to the sour foods; in a study with children, those with higher salivary responses had higher sour liking (Liem, Westerbeek, Wolterink, Kok, & de Graaf, 2004).
Strategies to reduce the unpleasant oral sensations of nutrient-dense berry juices are needed to increase consumer acceptance. Additives block bitterness by different mechanisms—at the receptor level through chemical (e.g., salts) and physical (e.g., milk solids, fat) interactions, and at the central processing level (e.g., perceived sweetness) (Bennett, Zhou, & Hayes, 2012). Multiple mechanisms of bitter blocking are required to improve the flavor and acceptability of functional foods and beverages with high levels of polyphenols (Gaudette & Pickering, 2012). Added sweetness improves the preference for bitter vegetables when added at a level that considers individual differences in taste perception and is most effective for vegetable dislikers (Sharafi, Hayes, & Duffy, 2013). As repeated exposure to sour alone does not increase preference in adults or children (Liem & de Graaf, 2004), adding sucrose can help condition a preference for orangeade (Liem & de Graaf, 2004) and white grapefruit juice (Capaldi & Privitera, 2008), which extends to their unsweetened form after the conditioning period. Astringency is a complex sensation that interacts with other tastes in complex mixtures like berry juices and may be attenuated with changes in pH and sweetness (Bajec & Pickering, 2008). While added sugars block unpleasant sensations, they have been linked to increased risk of many chronic diseases (Bray & Popkin, 2014) and beverages with added sugars are less acceptable to health-conscious consumers (Pohjanheimo & Sandell, 2009). Olfactory flavors perceived through the mouth (i.e., retronasal olfaction) increase perceived sweetness if they have a sweet quality, even when added in subthreshold levels (Labbe, Rytz, Morgenegg, Ali, & Martin, 2007). Olfactory flavors have been previously shown to add to sweetness and acceptability of complex foods like strawberries and tomatoes (Bartoshuk & Klee, 2013).
The first objective of the present study was to describe the oral sensations and palatability of aronia berry juice harvested over seven weeks, which allowed for the comparison of changes in juice chemical composition with changes in oral sensation and preference. The second objective was to compare the sensory profile and preference of the juice with prototypical oral sensations in solution and in common foods/beverages. The third objective was to determine if oral sensory phenotype could explain differences in sensory and hedonic responses to the aronia juice. We hypothesized that those with heightened oral sensory ability would demonstrate a greater dislike for aronia juice due to their increased perception of bitter, sour, and astringent sensations. With this third objective and recognizing that liking for sour/bitter fruits and vegetables varies with health seeking and adventurous dietary behaviors (Laaksonen, Ahola, et al., 2013; Tornwall et al., 2014), we also examined associations between preference for the aronia juice and dietary quality as well as adventurous eating behaviors. The final objective was to assess the ability of different concentrations of sugar, with or without the sweet olfactory flavoring ethyl butyrate (EB), to improve the sensory profile of the juice for increased acceptability. We hypothesized that the sweet olfactory flavoring, either alone or with minimal added sugar, would increase the perceived sweetness enough to increase the acceptability of the aronia juice.
2. Methods
2.1. Participants
A convenience sample of adults was recruited for this laboratory-based study through posters distributed around the University of Connecticut, in Storrs, CT. Recruitment posters asked for participation “in a study looking at why people eat what they do.” The exclusion criteria were reported pregnancy, allergies to quinine, and history of thyroid problems (to avoid individuals with Grave’s Disease who might have developed hypersensitivity to PROP). The University Institutional Review Board approved all methods; subjects provided informed and written consent and were paid for participating.
Fifty adults (36 females), between the ages of 18 and 62 (mean age = 26.9 ± 11.7 years) participated. All but one reported their health as excellent, very good or good, 86% reported their race category as white, and, from body mass index (BMI) calculated with measured weight and height, most were normal adiposity [nine were overweight (BMI between 25 and 30), five were obese (BMI ≥ 30)].
2.2. Procedure
The data were collected in three sessions. The first session included collection of demographic, health information, rating the intensity and level of liking/disliking of prototypical aqueous oral stimuli, sensory phenotyping, and assessment of dietary quality and adventurous eating. During the second session, participants rated the oral sensations and liking of all aronia juice samples across the 7 harvest weeks as well as the food items, and were phenotyped for PROP bitterness. In the third session, the effects of added sucrose and/or the olfactory flavoring, ethyl butyrate, on oral sensations from aronia juice and prototypical sour and astringent stimuli were assessed. In all sessions, the sensory stimuli were presented in random order.
In all three sessions, the participants were oriented to the general Labeled Magnitude Scale (gLMS), which they used to rate the intensity and level of liking/disliking of oral and non-oral sensations (Bartoshuk et al., 2004). For intensity scaling, the gLMS scale was vertical, ranging from ‘no sensation’ (score = 0) to ‘strongest sensation of any kind’ at the top (score = 100). Participants were oriented that the top of the scale applied to sensations across all sensory domains (i.e., generalized). Intermediate labels were ‘barely detectable’ (1.4) ‘weak’ (6), ‘moderate’ (17), ‘strong’ (35), and ‘very strong’ (53). They practiced using the scale by judging remembered, common light sensations (intensity of light in a dimly lit restaurant, the intensity of the light in the testing room, and the brightest light ever experienced). All participants correctly ordered these light intensities, supporting that they understood how to use the gLMS. For hedonic scaling, the gLMS was bidirectional and horizontal, with labels applied to liking (0 to +100) and disliking (0 to −100) with intermediate label (0) as ‘neither like or dislike.’
2.3. Taste and oral sensory function and phenotype
Standard measures of taste functioning, intensities of prototypical stimuli, and bitterness of PROP were completed on each participant. The National Health and Nutrition Examination Survey (NHANES) protocol (Rawal et al., 2015) [similar to the NIH Toolbox project (Coldwell et al., 2013)] assessed regional and whole mouth taste by scaling the intensity of 1 mM quinine hydrochloride and two concentrations of NaCl (1 M and 0.32 M) painted across the tongue tip and/or sampled with the whole mouth. A ratio of whole-mouth to tongue tip quinine bitterness was constructed; a greater ratio suggests genetic variation (Rawal, Hayes, Wallace, Bartoshuk, & Duffy, 2013) or altered oral sensations with depressed taste on the tongue tip relative to whole mouth (Bartoshuk et al., 2005).
Participants reported the intensities of sweetness, sourness, saltiness, bitterness, astringency, and liking/disliking of NaCl (0.32 M), sucrose (0.32, 1 M), citric acid (1 mM, 32 mM), quinine hydrochloride (0.32 mM), sucralose (0.1 M), 25% (v/v) ethanol, sodium acetate (1.3 M), PROP (3.2 mM), and alum (1 g/L). These aqueous solutions were served room-temperature and in random order. At the end of the second session, participants reported the intensities of three concentrations of NaCl (0.1, 0.32,1 M) and PROP (0.32, 1, 3.2 mM), presented in random order, to construct a ratio of PROP to NaCl intensities [(1 mM PROP/.32 M NaCl + 3.2 mM PROP/1 M NaCl)/2]. For describing the study sample, nontasters had a ratio <0.4, supertasters ≥1.2 and medium tasters in between (Bartoshuk, Duffy, & Miller, 1994).
2.4. Smell functioning
A 40-item odor identification test generated by an olfactometer (OLFACT-ID™, Osmic Enterprises, Inc.) provided an assessment of olfactory dysfunction, similar to the University of Pennsylvania Smell Identification Test (Doty, Shaman, & Dann, 1984). As additional measures of smell function, participants also reported intensities of these 40 odors and a single coffee jellybean. The jellybean was chewed, first with and then without the nose plugged, as a probe of retronasal olfaction.
2.5. Ratings of aronia juice and food items
Aronia mitchurinii ‘Viking’ was first harvested on August 1, 2012 and every 7th day thereafter until September 9, 2012 at a research orchard in Storrs, CT, USA. The berries were cleaned, dried, and frozen at −20 °C and stored in polyethylene bags to maintain proper quality for analysis. The berries were processed into juice on October 24, 2012. On the day of the juicing, the frozen berries were thawed, mashed, and then placed on a cider press for juice extraction. The juices were portioned into 2oz covered containers, frozen to −20 °C, and removed from the freezer on the day before testing to thaw in the refrigerator.
After rating the intensity of their hunger on the gLMS, participants sampled and rated the intensities of sweetness, sourness, saltiness, bitterness, astringency, and liking/disliking of seven aronia juices from harvest time-points (Week 1 to Week 7), interspersed with food items (white grapefruit juice, white cake icing, apple juice, heavy cream, instant espresso coffee, soy sauce); all presented in random order. Right before testing, the aronia juice, unsweetened white grapefruit juice (Ocean Spray, Lakeville/Middleborough, MA), and apple juice without added sugar (Mott’s, Plano, TX) were removed from the refrigerator for testing and served along with white icing (Betty Crocker, Golden Valley, MN) and reconstituted instant espresso (Cafe Bustelo, Rowland Coffee Roasters, Inc, Miami, FL) served room temperature. All samples were served in 10 mL medicine cups. The participants orally sampled and palpated the stimuli, expectorated what remained in the mouth, reported the intensities on the gLMS, and rinsed their mouth with deionized water.
2.6. Analysis of Brix, acidity and sugars
As reported previously (Bolling et al., 2015), a handheld refractometer was used to determine the Brix at each of the seven harvest times. The level of acidity was determined using a standardized base for juice titration and citric acid equivalents based on AOAC Official method 932.15. An Acument AB15 pH meter (Fisher Scientific, Pittsburgh, PA, USA) provided pH values. A commercial laboratory analyzed juices for sugar and sugar alcohol levels using high performance anion exchange chromatography with pulsed amperometric detection and GC methods (Covance Laboratories, Madison, WI, USA). The polyphenols within the juice were assessed by UHPLC-UV-MS (Bolling et al., 2015). Polymeric color was assessed by spectrophotometry and antioxidant activity within the juice by Ferric Reducing Antioxidant Power and the 2,2-diphenyl-1-picrylhydrazyl assays (Bolling et al., 2015).
2.7. Dietary quality and adventurous eating
Using a bi-directional, horizontally-oriented gLMS, participants reported liking/disliking for 57 foods/beverages, 7 physical activities, and 6 pleasurable/unpleasurable common experiences. The items were interspersed on a single survey. The food items fell into major food groups (fruits, vegetables, high-fat, grains, dairy products, meats, alcoholic beverages) as well as sensory groups of sweet, salty, sour/bitter, and spicy. Survey liking serves as a proxy for dietary intake as it correlates with reported intake (Drewnowski & Hann, 1999; Duffy et al., 2007; H; Tuorila et al., 2008), biomarkers of nutritional status (Pallister et al., 2015; Scarmo et al., 2012; Sharafi et al., 2015), serum lipids (Sharafi, Duffy, Miller, Winchester, & Sullivan, 2016), and adiposity (Duffy et al., 2007; Sharafi et al., 2015). The non-food items in the survey and directions for rating the level of liking/disliking reinforce that the scale applies to all experiences (i.e., generalized) and supports the ability to compare liking/disliking ratings between individuals (Bartoshuk, Duffy, Hayes, Moskowitz, & Snyder, 2006).
A dietary quality score was formed from the food groups and overall score for the liking of healthy foods as reported previously (Sharafi et al., 2015, 2016). Liking scores were treated continuously (±100 points) and conceptually grouped, a variety score formed (# healthy foods rated as at least liked and mathematically converted to a ±100 score), and weights applied for averaging into a dietary quality index. Previous latent variable analysis (Sharafi et al., 2016) has shown adequate factor structure and internal reliability. Conceptual weights were assigned prior to averaging into a dietary quality index: fruits/vegetables (+3), protein (+1), fat (−3), sweets (−3), salty food (−3), and variety score (+2), consistent with dietary quality indices (Kourlaba & Panagiotakos, 2009). Consistent with previous research (Sharafi et al., 2015), score ≥25 was assigned as optimal dietary quality (weak to moderate liking for negative weighted high fat/sweet foods; strong liking for positive weighted foods) in the present study sample. From our laboratory, the dietary quality index generated from a liking survey was consistent with a similar index derived from a frequency survey (Sharafi et al., 2015) and a significant predictor of carotenoid status and/or adiposity (Sharafi et al., 2015, 2016).
An Adventurous Eating Score was formed similar to previous research (Byrnes & Hayes, 2013; Tornwall et al., 2014). The participant-reported acceptance for six spicy foods/condiments, bitter foods/beverages was averaged into a single score (Cronbach’s alpha = 0.61).
2.8. Additive procedure
The effect of added sucrose and ethyl butyrate (EB), an olfactory flavoring, on sweetness, sourness, astringency and level of liking/disliking of prototypical sour and astringent stimuli as well as aronia juice was assessed on the last session. Participants sampled EB with 0.15 M sucrose (~5% w/v) and rated the sweetness and flavor during three conditions, first with the nose plugged (blocking retronasal olfaction), next with the nose unplugged, and finally swallowed. In the additive procedures, participants rated the taste qualities, astringency and level of liking of stimuli that were presented in randomized order. Solutions of 6 mM and 12 mM citric acid were tested with sucrose [0.15 M (approximately 5% w/v), 0.3 M (approximately 10% w/v)] alone or with EB (10 ppm, ≥98%, FCC, FG, Sigma-Aldrich, St. Louis, MO). Based on pilot testing and matching sweetness to level of astringency, tannic acid (1 g/L) was tested with 5% sucrose and 10 ppm EB. Aronia juice from the week 5 harvest was tested with 5% sucrose, 10 ppm EB, and 3% sucrose plus 10 ppm EB.
2.9. Data management and statistical analysis
SPSS 22.0 (Chicago, IL) was used to conduct the statistical analysis; significance criterion was P ≤ 0.05. Data are presented as means ± standard deviations (SD), unless otherwise noted. All tests were examined for age and sex differences and reported only when significant. The first two objectives were tested with descriptive statistics and then principal component analysis (JMP Pro software (11.2.0) using the default estimation number) to identify constructs of sensory and chemical relationships (i.e., factors) and then show how the juice mapped onto to these factors across the harvest times. The Spearman rho statistic was used to compare individual sensory and hedonic ratings and chemical analysis of the aronia juices with common juices as well as with the prototypical oral stimuli. Stepwise multiple regression analysis was used to assess the contributions of the sensory ratings in predicting level of aronia juice liking, assessing for multi-collinearity with tolerance values. For the third objective, exploratory factor analysis was used to assess the ability of a single intensity score to represent taste across the prototypical tastants and smell functioning across the 40 odors (maximum likelihood estimation and varimax rotations). The third objective was assessed through single and multiple regression analysis predicting sensory or level of liking of the aronia juice (averaged across the seven weeks) from measures of taste or smell functioning, dietary quality and adventurous eating, controlling for age, gender, and hunger ratings that could affect sensory and hedonic ratings as appropriate (Haase, Cerf-Ducastel, Buracas, & Murphy, 2007). Variables were assessed for normality and transformed if required for the analysis. The fourth objective was tested with repeated analysis of covariance, controlling for age, and sex, and hunger ratings as appropriate, with pair-wise comparisons made with the student’s t-test.
3. Results
3.1. Sensory profile of the participants
There were 28% nontasters, 42% medium tasters, and 30% supertasters as classified by the ratio of PROP to NaCl intensities (Bartoshuk et al., 1994). Following the NHANES taste protocol, the sample captured the expected variability in ratings for taste measures applied to the anterior tongue, and sampled with the whole mouth. The tongue tip averaged near strong for quinine and NaCl and near very strong for whole mouth (Rawal et al., 2015). The participants also showed good variability in taste functioning assessed by the ratio of whole mouth to tongue tip quinine intensity (average 2.07 ± 1.23). Exploratory factor analysis showed that the intensity of salt (1 M NaCl), sweet (1 M sucrose), sour (32 mM citric acid), and bitter (1 mM quinine hydrochloride) loaded on a single factor that explained 50% of the variability in taste intensity with an average taste intensity score of 41.06 ± 17.77 SD (between strong and very strong).
According to the orthonasal odor identification task and scoring (Doty, 1995), 76% were normosmic, 22% mild microsmic, and 2% severe microsmic. Exploratory factor analysis with the intensity of the 40 odors and single jellybean revealed a single factor that explained 50% of the variability across the intensities. Thus, an average odor intensity score was calculated and was just below strong intensity (30.9 ± 13.1), ranging from weak to above very strong.
3.2. Sensory and hedonic ratings of aronia juice by harvest time and chemical composition
Fig. 1 shows the sensory and hedonic profiles of the aronia juice across 7 weeks of harvest. Juices from harvest weeks 1, 2, and 3 had the most unfavorable sensory profiles (lower sweetness; greater sourness and astringency), weeks 5, 6 and 7 had the most favorable (greater sweetness; lower sourness and astringency). The preference for the juice averaged across harvest times was tied most strongly to the average perceived sweetness (rho = 0.96), sourness (rho = −0.86) and astringency (−0.93) and less so with bitterness (−0.54). In stepwise regression only sweetness was a significant predictor of juice liking (b = 0.46, t(47) = 3.496, p = 0.001), with non-significant contributions of sourness, astringency or bitterness. For example, examining juice liking by 1st versus 4th quartiles of juice liking, the juice dislikers reported significantly less sweetness from the juice than the juice likers (averaging 38.23 ± 4.43 and 18.19 ± 1.77, respectively, p < 0.01) with no significant differences in sourness, bitterness or astringency.
Fig. 1.

Perceived intensity (±S.E.M.) of sensations or degree of liking/disliking of aronia juice across 7 weeks of harvest on the general Labeled Magnitude Scale (gLMS) (Bartoshuk et al., 2004), where 0 = neutral, ±1.4 barely detectable, ±6 weak, ±17 moderate, ±35 strong, ±53 very strong.
The chemical analysis of the aronia juice across harvest time has been reported previously (Bolling et al., 2015). Juice composition varied by week, with percent acidity starting at 1.15%, peaking at week 4 (1.22%) and falling to weeks 6 and 7 (0.85–0.91%) and Brix/acid ratio peaking at 15.6 and 15.7 at weeks 6 and 7. pH was more stable, (3.4 ± 0.1), and sugars/Brix increased through week 7, with sorbitol, glucose and fructose as the primary sugars. Polyphenol content also changed by harvest date (Bolling et al., 2015) with the largest changes in the anthocyanins (lowest at week 1, increasing approximately 3.25-fold to week 5, dropping 0.25 fold to week 7) and hydroxycinnamic acids (highest at week 1, dropping 33% to week 3, remaining depressed through week 7). Flavonol content was variable by harvest date but did not follow a specific pattern across harvest. Proanthocyanidins, polymeric color and total phenols all generally increased 2–3-fold from week 1 to week 7.
By Spearman’s analysis, average juice liking at each week showed significant positive associations with sugar level (glucose, fructose, sorbitol) or Brix/acid ratio (rho’s between 0.77 and 0.82, p ≤ 0.05). Conversely, average juice liking at each week decreased with increasing level of acidity (rho = −0.75, p = 0.05). Level of sweetness showed the same pattern of association/significance with the chemical analysis, except that the sweetness-acidity relationship did not reach significance. As expected, average sourness at each week correlated strongly with the acidity (rho = 0.86, p = 0.01) and negatively with sorbitol (rho = −0.75, p = 0.05), while astringency only correlated negatively with sorbitol (rho = −0.78, p = 0.05). Perceived bitterness failed to correlate significantly with any of the chemical constituents.
Principal component analysis created two factors from average sensory and hedonic ratings of the aronia juices in relationship to the juice chemical analysis (Bolling et al., 2015) and how the juices at each week loaded onto these factors (Fig. 2B). The factors explained nearly 80% of the variance in the data. Factor 1 was comprised of sugars, anthocyanins, total phenols, and proanthocyanidins, sweetness and hedonics (Fig. 2A); harvest weeks 5, 6 and 7 loaded onto this factor (Fig. 2B). Flavonols, hydroxycinnamic acids, and titratable acidity were most closely associated with sourness, astringency, and bitterness in factor 2 (Fig. 2A); weeks 1, 2 and 3 loaded onto this factor (Fig. 2B).
Fig. 2.
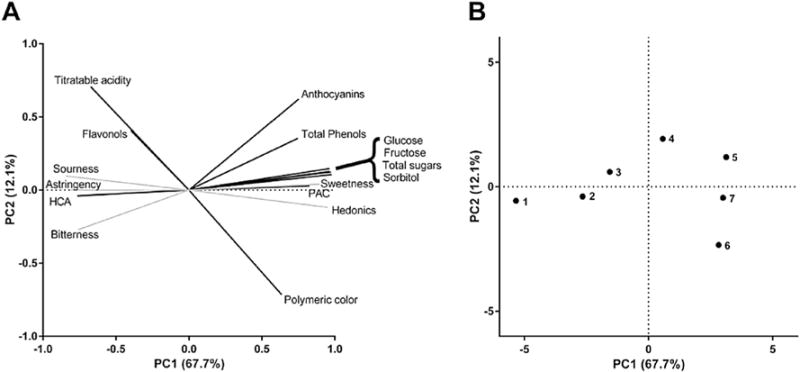
Principal component analysis on correlations of aronia juice chemical composition and sensory/hedonic ratings. A.) Loading plot of principal components, where two principal components (PC), PC1 and PC2, explained 67.7% and 12.1% of variance, respectively; B.) Score plot of juices labeled by harvest week. Anthocyanins, flavonols, and hydroxycinnamic acids (HCA) by UHPLC-MS, total phenols, proanthocyanidins (PAC), polymeric color, total sugars, sorbitol, glucose, and fructose as reported by Bolling et al. (2015).
3.3. Sensory and hedonic comparisons between aronia juice, prototypical sweet, sour, astringent and bitter stimuli identifies a sub-group of sour likers
Aronia juice, averaged across all harvest weeks, was most like the prototypical sour (citric acid) and astringent (alum, tannic acid) stimuli, being perceived as having multiple sensory qualities (Fig. 3). The juice sweetness was about 33% that of 0.32 M sucrose. Sourness fell between that of 1 and 32 mM citric acid, astringency was close to that of 32 mM citric acid but below 1 g/L alum or tannic acid, and bitterness was much less than that of tannic acid or the concentrated bitter (quinine, PROP) stimuli.
Fig. 3.
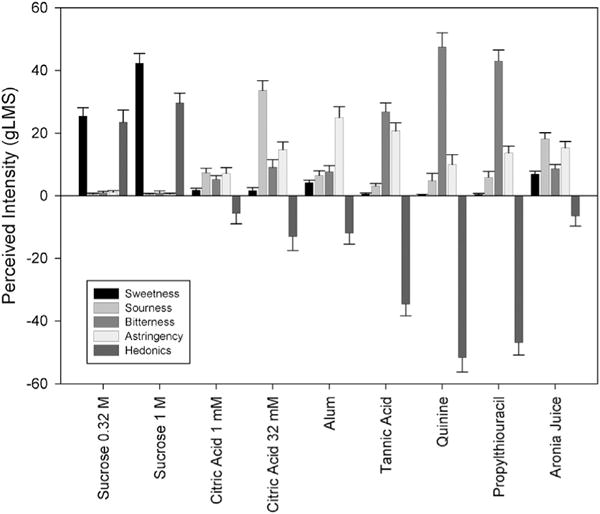
Perceived intensity (±S.E.M.) of sensation or degree of liking/disliking of aqueous sweet (0.32, 1 M sucrose), sour (1, 32 mM citric acid), astringent (1 g/L alum, 1 g/L tannic acid) and bitter (0.32 mM quinine hydrochloride, 3.2 mM propylthiouracil), and aronia juice averaged across the 7 harvest weeks on the general Labeled Magnitude Scale (gLMS) (Bartoshuk et al., 2004), where 0 = neutral, ±1.4 barely detectable, ±6 weak, ±17 moderate, ±35 strong, ±53 very strong.
The preference ratings for the aronia juice averaged weakly disliked, which was close in ratings to 1 mM citric acid, but less disliked than either 32 mM citric acid or tannic acid. There were significant associations between liking of aronia juice and citric acid. In multiple regression analysis, liking of aronia juice was significantly associated with liking of both 1 mM (b = 0.34, t(47) = 2.21, p < 0.05) and 32 mM (b = 0.39, t(47) = 2.86, p < 0.01) citric acids, controlling for the level of hunger. In sub-group analysis of the lowest and highest quartile of aronia juice liking, the two citric acid concentrations averaged strongly disliked for the lowest quartile and moderately liked for the highest quartile of aronia juice, yet did not differ significantly on intensity of specific qualities from the citric acids. Conversely, by examining citric acid dislikers (averaged strong disliking) versus citric acid likers (averaged moderate liking), citric acid dislikers were skewed to < weak liking (11 of 13 individuals) of the aronia juice whereas citric acid likers were skewed to ≥ weak liking (8 of 13 individuals) (Pearson chi square = 5.85, p < 0.05). The citric acid liker versus disliker groups did not differ on intensity of specific qualities from the aronia juice except for bitterness: the citric acid likers reported less bitterness from the aronia juice than the dislikers (t = 2.052, p = 0.05).
Compared to foods/beverages with pronounced sweet, sour and bitter sensations (Fig. 4), the aronia juice was most similar to the grapefruit juice in sensory profile yet approximately double in astringency. Being less astringent and familiar in the food supply, the grapefruit juice was greater than moderately liked compared with the weakly disliked aronia juice.
Fig. 4.
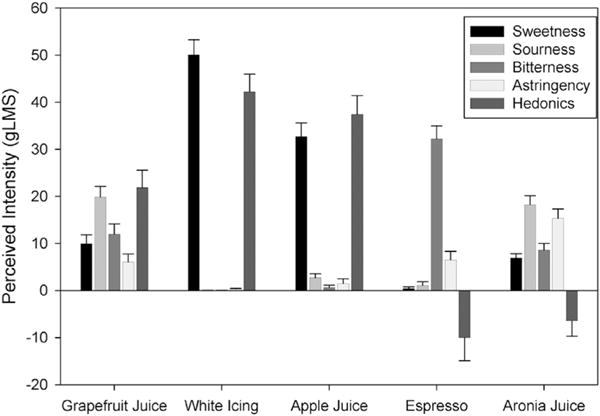
Perceived intensity (±S.E.M.) of sensation or degree of liking/disliking of white grapefruit juice, white cake icing, apple juice, reconstituted instant espresso, and aronia juice (averaged across the 7 weeks) on the general Labeled Magnitude Scale (gLMS) (Bartoshuk et al., 2004), where 0 = neutral, ±1.4 barely detectable, ±6 weak, ±17 moderate, ±35 strong, ±53 very strong.
3.4. Taste phenotype and functioning versus aronia juice sensations and preference
There were no significant correlations between PROP bitterness (PROP or PROP ratio), the quinine ratio, the average odor intensity and the sensory characteristics of the aronia juice, including sweetness, sourness, bitterness, astringency, or level of liking/disliking. However, participants with higher taste functioning varied on oral intensities from the juice. In multiple regression analysis, higher average taste intensity score was associated with greater juice sourness (r = 0.35, p < 0.05) yet lower bitterness (r = −0.39, p = 0.01).
3.5. Dietary quality and adventurous eating versus aronia juice sensations and preference
The dietary quality scores were normally distributed. Twelve percent of the sample reported liking a healthier diet, with women reporting greater liking for a healthy diet than did men (t = 2.893, p < 0.05). Dietary quality showed a nearly flat association with liking for aronia juice. The Adventurous Eating score was normally distributed with men reporting significantly higher liking scores than did women (t = 2.72, p < 0.05): men averaged just below moderately like and women reported just below weakly dislike. Independent of other demographic factors and hunger ratings, participants who were more adventuresome in their eating tended to report greater liking for the aronia juice (r = 0.28, p = 0.056) as well as reported significantly greater liking for citric acid (r = 0.34, p < 0.05).
3.6. Effect of additives on sensations and preference on prototypical sour and astringent stimuli and aronia juice
We first assessed the sweetness of a mixture of EB and 0.15 M sucrose perceived with the nose plugged (suppressing retronasal olfaction), and then nose unplugged plus swallowing (facilitating retronasal olfaction). Sweetness increased significantly in the latter condition (F = 17.79, p < 0.01) with 70% of participants reporting increases that paralleled increases perceived flavor (F = 19.59, p < 0.01).
Next the effects of two levels of sucrose without/with EB on the sweetness, sourness, astringency and level of liking/disliking of 12 mM citric acid were tested. Sweetness increased significantly with added sugar and the addition of EB added to the sweetness in a marginally significant (F = 3.6, p < 0.05) but not synergistic fashion (Supplemental Fig. 1). Sourness and astringency of citric acid decreased significantly with increasing sucrose concentration. Adding EB did not show additive effect with sweetness (F = 0.945, p < 0.36, F = 0.679, p < 0.51, respectively). Adding sucrose increased the liking of citric acid, yet adding EB did not add to the liking (F = 0.418, p < 0.521).
For tannic acid, the addition of 5% sucrose significantly increased the sweetness and shifted disliking to weak liking, while decreasing astringency and with no effect on sourness (Supplemental Fig. 2). Adding the flavoring plus sucrose did not result in gains in sweetness, further reductions in astringency or improvements in the level of liking.
The week 5 aronia juice was used for testing the effects of additives on juice sensations and preference. Sweetness only increased with the addition of 5% sucrose or the 3% sucrose and flavor, and not with the flavor alone (Fig. 5). Sourness decreased significantly with addition of the EB flavor, without additional significant reductions with the 5% sucrose or 3% sucrose and flavor. For astringency, only the added 5% sucrose significantly decreased its intensity. There was large variability in change in preference ratings with the additives. Both 5% sucrose and 3% sucrose and EB flavor significantly shifted near weak disliking and to above weak liking, with non-significant differences between the two additives. Adding the olfactory flavor alone didn’t shift liking significantly across all participants. Those who reported greater disliking of the plain aronia juice were more likely to report greater improvements in liking of the aronia juice with the additives (r = −0.38, p < 0.01, averaged across the additives). Neither PROP bitterness nor measures of taste explained significant differences in preference for aronia juice with any of the additives.
Fig. 5.
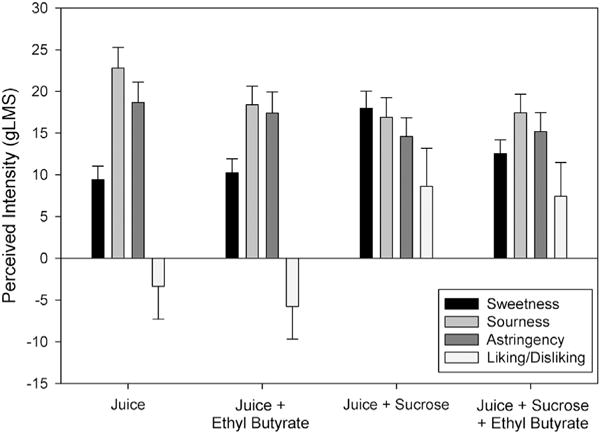
Perceived intensity (±S.E.M.) of sensations or degree of liking/disliking of week 5 aronia juice alone, or with 10 ppm (86 μM) ethyl butyrate, or 0.15 M sucrose, or 0.09 M sucrose plus 10 ppm (86 μM) ethyl butyrate on the general Labeled Magnitude Scale (gLMS) (Bartoshuk et al., 2004), where 0 = neutral, ±1.4 barely detectable, ±6 weak, ±17 moderate, ±35 strong, ±53 very strong.
4. Discussion
While aronia juice has high levels of antioxidant polyphenols and potential health benefits (Bolling et al., 2015; Chrubasik, Li, & Chrubasik, 2010), it reached only low acceptance across harvest, which was related to low levels of sweetness coupled with moderate sourness and astringency. The sensory profile of the aronia juice was matched best with moderate concentrations of citric acid and had the complexity of unsweetened grapefruit juice, yet with lower preference ratings. Despite our hypothesis, propylthiouracil (PROP) bitterness phenotype failed to explain significant variability in aronia juice sensations and preference. However, we identified a subset of the study sample which reported liking moderate citric acid concentration as well as the aronia juice. In testing the effects of additives on the enhancement of aronia juice sweetness and preference, we found that the addition of 5% sucrose increased the juice sweetness and shifted weak disliking to above weak liking across all participants. Adding the sweet olfactory flavoring, ethyl butyrate, with 3% sucrose maintained the level of liking to nearly that of the 5% added sucrose. Those who initially disliked the aronia juice reported the greatest gains in liking of juice with these additives, similar to our previous findings with vegetables (Sharafi et al., 2013).
4.1. Sensory and hedonic ratings of aronia juice by harvest time and chemical composition
Across seven weeks of harvest, the preference ratings for the juice peaked at the fifth and sixth weeks, reaching only neither like/nor dislike, and the juice was characterized as having greater sweetness, less sourness and lower astringency (Bolling et al., 2015). Aronia juice, similar to other high-nutrient berry juices, has significant sour/bitter/astringent properties that inhibit liking (D. Hartvig, Hausner, Wendin, & Bredie, 2014). There was a strong correlation between sensory ratings averaged across the participants and the chemically-analyzed characteristics of the juice, which is consistent with that reported in the literature (Bett-Garber et al., 2015; Goldenberg et al., 2015; Laaksonen, Makila, Tahvonen, Kallio, & Yang, 2013). We found good association between the sensory characteristics of perceived sourness, astringency and bitterness with the chemical composition of flavonols and hydroxycinnamic acids. As reported for other fruits and juices, those that have more sweetness and lower sourness and acidity are liked more (Goldenberg et al., 2015; Koppel et al., 2014), which is consistent with our findings that liking of aronia juice was associated best with the sugars, anthocyanins and proanthocyanins.
4.2. Sensory and hedonic comparisons between aronia juice and sour stimuli identifies a sub-group of sour likers
The present study identified about 25% of the sample who liked citric acid and, as well, liked the aronia juice. Sour likers and dislikers reported similar qualities from the citric acid (e.g., more sweetness) or aronia juice, except for some less bitterness. Previous sensory studies have identified minority groups of individuals who liked sourness, including across geographic regions (Rødbotten et al., 2009), among children (Kildegaard, Tønning, & Thybo, 2011), and in berry products (Laaksonen, Ahola, et al., 2013). Part of the liking for sourness could relate to individual eating behaviors. Consistent with Tornwall and colleagues (Tornwall et al., 2014), we found that those who liked sour berries tended to be characterized as adventurous eaters. Unlike Tornwall and colleagues (Tornwall et al., 2014), we did not find gender differences in this eating pattern or liking of the aronia juice. Sour likers among children also show more adventurous food behaviors (Liem et al., 2004) and, conversely, those with food neophobia report lower liking for foods with complex flavor profiles (Olabi et al., 2015). Liking for a sour/bitter/astringency juice can show improvements with repeated exposure that addresses food neophobia as shown by Hartvig and colleagues (D. L. Hartvig, Hausner, Wendin, Ritz, & Bredie, 2015). In a conditioning experiment with children, initial dislikers of aronia juice showed some gains in liking following repeated exposure to aronia juice (Hartvig et al., 2015). Liking for a product such as aronia juice can be modulated by non-sensory influences; such as the time of day, thirst level, while doing an activity (Hein, Hamid, Jaeger, & Delahunty, 2012).
Health consciousness assessed by level of dietary quality did not explain significant difference in liking for the aronia juice in the present study, which is similar to findings from a cross-cultural study on pomegranate juice (Koppel et al., 2014). Thus, consumers may not show longer-term liking for a nutritional beverage with a strong unpleasant taste (Tuorila & Cardello, 2002), even if marketed as a healthy beverage.
4.3. Taste phenotype and functioning versus aronia juice sensations and preference
None of the typical measures of taste phenotyping (level of bitterness from propylthiouracil or quinine) or smell acuity were able to explain significant variation in the sensory or preference ratings of the juices. Despite our hypothesis, PROP bitterness did not associate significantly with either sensations from or the liking of the aronia berry juice. Hartvig and colleagues (Hartvig et al., 2014) also did not find significant associations among children between thresholds to either quinine, sweet, sour, or salt and the taste sensations from aronia juice. The association between one common genotype for bitter taste, TAS2R38 taste receptor, and sensation, liking or use of berry products is inconsistent in the literature, including that bitter insensitive individuals rate greater sour/bitter/astringency (Laaksonen, Ahola, et al., 2013), report no differences in consumption (Sandell et al., 2014), or report lower intakes among those who carried the bitter sensitive gene (Sandell et al., 2015). Many taste genetic factors likely influence preferences for sour/bitter/astringent products. For example, a number of other bitter taste receptor genes associate with sensation from or liking of sour/bitter beverages, such as grapefruit juice (Hayes, Feeney, Nolden, & McGeary, 2015; Hayes et al., 2011). Twin studies support strong genetic influences in sour taste perception (Wise et al., 2007) and liking for sour foods (Tornwall et al., 2012).
4.4. Effect of additives on sensations and preference of aronia juice
The liking for the aronia juice was improved somewhat by simply adding sugar alone or with a sweet olfactory flavoring to suppress the sourness and astringency. The sweet olfactory flavoring was congruent to the sweet flavor of the juice and added to the sweetness ratings of the juice, supporting the ability of sweet olfactory flavorings to enhance sensory profiles of fruits (Bartoshuk & Klee, 2013). Importantly, the sweet olfactory flavoring, in the present study, allowed less added sugar to maintain the same level of liking, which could appeal to consumers wanting less added sugar consumption. Although adding sweetness decreased astringency and sourness as shown previously (Lyman & Green, 1990; Pelletier, Lawless, & Horne, 2004), the sucrose additive was insufficient to block astringency enough to bring aronia juice beyond just weakly to moderately liked. The sweet olfactory flavoring also did not mitigate the sour and astringent sensations (Pelletier et al., 2004). Perceived astringency can be lowered by increasing viscosity and acidity (Peleg & Noble, 1999). For example, berries with significant sour/bitter/astringency can be more preferred in a yogurt (Suomela et al., 2012). Foamy or emulsified structures, such as ganache or sabayon sauces, can lower perceived sourness, bitterness and astringency in products made with unsweetened pressed lingonberry juice, which has natural bitter, sour and astringent characteristics (Sandell et al., 2015). Interdisciplinary collaborations between sensory, nutrition, and plant scientists are key to producing palatable products that appeal to health conscious consumers and grown with agricultural practices that are sustainable, as has been as shown in optimizing tomatoes (Bartoshuk & Klee, 2013) and strawberries (Schwieterman et al., 2014).
4.5. Limitations and conclusions
This study has a number of limitations. The sample study was relatively small and homogeneous, consisted mostly of healthy adults, and testing occurred in a laboratory setting. Therefore, results may not generalize to children; those of diverse ethnic, racial and geographic regions; as well as to consumers in more naturalistic settings. The findings may not apply to other berry products, including aronia berries that are grown in different regions and of different genetic make-up. Only two additives were used to improve sweetness and juice acceptability. Additional experimentation is required to reduce the negative astringent sensations. Finally, the present study did not test a ‘combination-therapy’ approach, which uses sodium ions and a sweetener simultaneously to exploit peripheral and unpleasant sensations to improve aronia berry liking (Gaudette & Pickering, 2012).
In conclusion, aronia berry juice was not widely accepted because of its low sweet and high sour/astringent qualities. Approximately 1 in 4 participants liked the juice and also liked a moderate to strong aqueous citric acid. The liking for aronia juice tended to associate with being a more adventurous eater. Additives to improve sweetness, either directly through added sugar or indirectly through a sweet olfactory flavoring, showed potential to increase juice acceptability, particularly for those who did not like the juice without additives. However these additives were insufficient to completely block the unpleasant astringency. Further research is needed to improve the aronia juice acceptability for a wider range of consumers.
Supplementary Material
Acknowledgments
Supported by the National Institute of Food and Agriculture, U.S. Department of Agriculture, Hatch Formula Funds, under award number 2015-31200-06009, accession number 1001056 and the USDA Northeast Sustainable Agriculture Research & Education. Also, Dr. S. Rawal was partially supported by the Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD).
Abbreviations
- BMI
body mass index
- EB
ethyl butyrate
- gLMS
general Labeled Magnitude Scale
- NHANES
National Health and Nutrition Examination Survey
- NaCl
sodium chloride
- ppm
parts per million
- PROP
propylthiouracil
- SD
standard deviation
- SEM
standard error of the mean
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.appet.2016.07.026.
References
- Bajec MR, Pickering GJ. Astringency: Mechanisms and perception. Critical Reviews In Food Science and Nutrition. 2008;48(9):858–875. doi: 10.1080/10408390701724223. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, Weiffenbach JM. Valid across-group comparisons with labeled scales: The gLMS versus magnitude matching. Physiology & Behavior. 2004;82(1):109–114. doi: 10.1016/j.physbeh.2004.02.033. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Hayes JE, Moskowitz HR, Snyder DJ. Psychophysics of sweet and fat perception in obesity: Problems, solutions and new perspectives. Philosophical Transactions of the Royal Society of London B Biological Sciences. 2006;361(1471):1137–1148. doi: 10.1098/rstb.2006.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartoshuk LM, Duffy VB, Miller IJ., Jr PTC/PROP Tasting: Anatomy, psychophysics, and sex effects. Physiology & Behavior. 1994;56(6):1165–1171. doi: 10.1016/0031-9384(94)90361-1. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Klee HJ. Better fruits and vegetables through sensory analysis. Current Biology. 2013;23(9):R374–R378. doi: 10.1016/j.cub.2013.03.038. [DOI] [PubMed] [Google Scholar]
- Bartoshuk LM, Snyder DJ, Grushka M, Berger AM, Duffy VB, Kveton JF. Taste damage: Previously unsuspected consequences. Chemical Senses. 2005;30(Suppl. 1):i218–i219. doi: 10.1093/chemse/bjh192. [DOI] [PubMed] [Google Scholar]
- Bennett SM, Zhou L, Hayes JE. Using milk fat to reduce the irritation and bitter taste of ibuprofen. Chemosensory Perception. 2012;5(3–4):231–236. doi: 10.1007/s12078-012-9128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bett-Garber KL, Lea JM, Watson MA, Grimm CC, Lloyd SW, Beaulieu JC, Marshall DA. Flavor of fresh blueberry juice and the comparison to amount of sugars, acids, anthocyanidins, and physicochemical measurements. Journal of Food Science. 2015;80(4):S818–S827. doi: 10.1111/1750-3841.12821. [DOI] [PubMed] [Google Scholar]
- Bolling BW, Taheri R, Pei R, Kranz S, Yu M, Durocher SN, Brand MH. Harvest date affects aronia juice polyphenols, sugars, and antioxidant activity, but not anthocyanin stability. Food Chemistry. 2015;187:189–196. doi: 10.1016/j.foodchem.2015.04.106. [DOI] [PubMed] [Google Scholar]
- Brand M. Aronia: Native shrubs with untapped potential. Arnoldia. 2010;67:14–25. [Google Scholar]
- Bray GA, Popkin BM. Dietary sugar and body weight: Have we reached a crisis in the epidemic of obesity and diabetes?: Health be damned! Pour on the sugar. Diabetes Care. 2014;37(4):950–956. doi: 10.2337/dc13-2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes N, Hayes J. Personality factors predict spicy food liking and intake. Food Quality and Preference. 2013;28(1):213–221. doi: 10.1016/j.foodqual.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capaldi ED, Privitera GJ. Decreasing dislike for sour and bitter in children and adults. Appetite. 2008;50(1):139–145. doi: 10.1016/j.appet.2007.06.008. [DOI] [PubMed] [Google Scholar]
- Chrubasik C, Li G, Chrubasik S. The clinical effectiveness of chokeberry: A systematic review. Phytotherapy Research. 2010;24(8):1107–1114. doi: 10.1002/ptr.3226. [DOI] [PubMed] [Google Scholar]
- Coldwell SE, Mennella JA, Duffy VB, Pelchat ML, Griffith JW, Smutzer G, Hoffman HJ. Gustation assessment using the NIH Toolbox. Neurology. 2013;80(11 Suppl 3):S20–S24. doi: 10.1212/WNL.0b013e3182872e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinehart ME, Hayes JE, Bartoshuk LM, Lanier SL, Duffy VB. Bitter taste markers explain variability in vegetable sweetness, bitterness, and intake. Physiology & Behavior. 2006;87(2):304–313. doi: 10.1016/j.physbeh.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Doty R. The smell identification test administration manual. Haddon Heights; NJ: 1995. Retrieved from. [Google Scholar]
- Doty R, Shaman P, Dann M. Development of the university of Pennsylvania smell identification test: A standardized microencapsulated test of olfactory function. Physiology & Behavior. 1984;34:489–502. doi: 10.1016/0031-9384(84)90269-5. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Hann C. Food preferences and reported frequencies of food consumption as predictors of current diet in young women. American Journal of Clinical Nutrition. 1999;70(1):28–36. doi: 10.1093/ajcn/70.1.28. [DOI] [PubMed] [Google Scholar]
- Duffy VB, Bartoshuk LM, Striegel-Moore R, Rodin J. Taste changes across pregnancy. Annals of the New York Academy of Sciences. 1998;855:805–809. doi: 10.1111/j.1749-6632.1998.tb10663.x. [DOI] [PubMed] [Google Scholar]
- Duffy VB, Lanier SA, Hutchins HL, Pescatello LS, Johnson MK, Bartoshuk LM. Food preference questionnaire as a screening tool for assessing dietary risk of cardiovascular disease within health risk appraisals. Journal of the American Dietetic Association. 2007;107(2):237–245. doi: 10.1016/j.jada.2006.11.005. [DOI] [PubMed] [Google Scholar]
- Gaudette NJ, Pickering GJ. Optimizing the orosensory properties of model functional beverages: The influence of novel sweeteners, odorants, bitter blockers, and their mixtures on (+)-catechin. Journal of Food Science. 2012;77(6):S226–S232. doi: 10.1111/j.1750-3841.2012.02707.x. [DOI] [PubMed] [Google Scholar]
- Goldenberg L, Yaniv Y, Kaplunov T, Doron-Faigenboim A, Carmi N, Porat R. Diversity in sensory quality and determining factors influencing mandarin flavor liking. Journal of Food Science. 2015;80(2):S418–S425. doi: 10.1111/1750-3841.12742. [DOI] [PubMed] [Google Scholar]
- Gonzalo-Diago A, Dizy M, Fernandez-Zurbano P. Contribution of low molecular weight phenols to bitter taste and mouthfeel properties in red wines. Food Chemistry. 2014;154:187–198. doi: 10.1016/j.foodchem.2013.12.096. [DOI] [PubMed] [Google Scholar]
- Haase L, Cerf-Ducastel B, Buracas G, Murphy C. On-line psychophysical data acquisition and event-related fMRI protocol optimized for the investigation of brain activation in response to gustatory stimuli. Journal of Neuroscience Methods. 2007;159(1):98–107. doi: 10.1016/j.jneumeth.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Hartvig D, Hausner H, Wendin K, Bredie WL. Quinine sensitivity influences the acceptance of sea-buckthorn and grapefruit juices in 9- to 11-year-old children. Appetite. 2014;74:70–78. doi: 10.1016/j.appet.2013.11.015. [DOI] [PubMed] [Google Scholar]
- Hartvig DL, Hausner H, Wendin K, Ritz C, Bredie WL. Initial liking influences the development of acceptance learning across repeated exposures to fruit juices in 9–11 year-old children. Food Quality and Preference. 2015;39:228–235. [Google Scholar]
- Hayes JE, Bartoshuk LM, Kidd JR, Duffy VB. Supertasting and PROP bitterness depends on more than the TAS2R38 gene. Chemical Senses. 2008;33(3):255–265. doi: 10.1093/chemse/bjm084. [DOI] [PubMed] [Google Scholar]
- Hayes JE, Feeney EL, Nolden AA, McGeary JE. Quinine bitterness and grapefruit liking associate with allelic variants in TAS2R31. Chemical Senses. 2015;40(6):437–443. doi: 10.1093/chemse/bjv027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JE, Sullivan BS, Duffy VB. Explaining variability in sodium intake through oral sensory phenotype, salt sensation and liking. Physiology & Behavior. 2010;100(4):369–380. doi: 10.1016/j.physbeh.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes JE, Wallace MR, Knopik VS, Herbstman DM, Bartoshuk LM, Duffy VB. Allelic variation in TAS2R bitter receptor genes associates with variation in sensations from and ingestive behaviors toward common bitter beverages in adults. Chemical Senses. 2011;36(3):311–319. doi: 10.1093/chemse/bjq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein KA, Hamid N, Jaeger SR, Delahunty CM. Effects of evoked consumption contexts on hedonic ratings: A case study with two fruit beverages. Food Quality and Preference. 2012;26(1):35–44. [Google Scholar]
- Kildegaard H, Tønning E, Thybo AK. Preference, liking and wanting for beverages in children aged 9–14 years: Role of sourness perception, chemical composition and background variables. Food Quality and Preference. 2011;22(7):620–627. [Google Scholar]
- Kim UK, Jorgenson E, Coon H, Leppert M, Risch N, Drayna D. Positional cloning of the human quantitative trait locus underlying taste sensitivity to phenylthiocarbamide. Science. 2003;299(5610):1221–1225. doi: 10.1126/science.1080190. [DOI] [PubMed] [Google Scholar]
- Kim K, Vance TM, Chun OK. Estimated intake and major food sources of flavonoids among US adults: Changes between 1999–2002 and 2007–2010 in NHANES. European Journal of Nutrition. 2016 Mar;55(2):833–843. doi: 10.1007/s00394-015-0942-x. [DOI] [PubMed] [Google Scholar]
- Koppel K, Chambers E, Vázquez-Araujo L, Timberg L, Carbonell-Barrachina A, Suwansichon S. Cross-country comparison of pomegranate juice acceptance in Estonia, Spain, Thailand, and United States. Food Quality and Preference. 2014;31:116–123. [Google Scholar]
- Kourlaba G, Panagiotakos DB. Dietary quality indices and human health: A review. Maturitas. 2009;62(1):1–8. doi: 10.1016/j.maturitas.2008.11.021. [DOI] [PubMed] [Google Scholar]
- Laaksonen O, Ahola J, Sandell M. Explaining and predicting individually experienced liking of berry fractions by the hTAS2R38 taste receptor genotype. Appetite. 2013;61(1):85–96. doi: 10.1016/j.appet.2012.10.023. [DOI] [PubMed] [Google Scholar]
- Laaksonen O, Makila L, Tahvonen R, Kallio H, Yang B. Sensory quality and compositional characteristics of blackcurrant juices produced by different processes. Food Chemistry. 2013;138(4):2421–2429. doi: 10.1016/j.foodchem.2012.12.035. [DOI] [PubMed] [Google Scholar]
- Labbe D, Rytz A, Morgenegg C, Ali S, Martin N. Subthreshold olfactory stimulation can enhance sweetness. Chemical Senses. 2007;32(3):205–214. doi: 10.1093/chemse/bjl040. [DOI] [PubMed] [Google Scholar]
- Lanier SA, Hayes JE, Duffy VB. Sweet and bitter tastes of alcoholic beverages mediate alcohol intake in of-age undergraduates. Physiology & Behavior. 2005;83(5):821–831. doi: 10.1016/j.physbeh.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Lee YM, Prescott J, Kim KO. PROP taster status and the rejection of foods with added tastants. Food Science and Biotechnology. 2008;17(5):1066–1073. [Google Scholar]
- Liem DG, de Graaf C. Sweet and sour preferences in young children and adults: Role of repeated exposure. Physiology & Behavior. 2004;83(3):421–429. doi: 10.1016/j.physbeh.2004.08.028. [DOI] [PubMed] [Google Scholar]
- Liem DG, Westerbeek A, Wolterink S, Kok FJ, de Graaf C. Sour taste preferences of children relate to preference for novel and intense stimuli. Chemical Senses. 2004;29(8):713–720. doi: 10.1093/chemse/bjh077. [DOI] [PubMed] [Google Scholar]
- Lyman BJ, Green BG. Oral astringency: Effects of repeated exposure and interactions with sweeteners. Chemical Senses. 1990;15(2):151–164. [Google Scholar]
- Olabi A, Neuhaus T, Bustos R, Cook-Camacho M, Corvi T, Abdouni L. An investigation of flavor complexity and food neophobia. Food Quality and Preference. 2015;42:123–129. [Google Scholar]
- Pallister T, Sharafi M, Lachance G, Pirastu N, Mohney RP, MacGregor A, Menni C. Food preference patterns in a UK Twin cohort. Twin Research and Human Genetics. 2015;18(6):793–805. doi: 10.1017/thg.2015.69. [DOI] [PubMed] [Google Scholar]
- Peleg H, Noble AC. Effect of viscosity, temperature and pH on astringency in cranberry juice. Food Quality and Preference. 1999;10(4–5):343–34. [Google Scholar]
- Pelletier CA, Lawless HT, Horne J. Sweet–sour mixture suppression in older and young adults. Food Quality and Preference. 2004;15(2):105–116. [Google Scholar]
- Pickering GJ, Simunkova K, DiBattista D. Intensity of taste and astringency sensations elicited by red wines is associated with sensitivity toPROP (6-n-propylthiouracil) Food Quality and Preference. 2004;15:147–154. [Google Scholar]
- Pohjanheimo T, Sandell MA. Explaining the liking for drinking yoghurt: The role of sensory quality, food choice motives, health concern and product information. International Dairy Journal. 2009;19(8):459–466. [Google Scholar]
- Prescott J, Soo J, Campbell H, Roberts C. Responses of PROP taster groups to variations in sensory qualities within foods and beverages. Physiology & Behavior. 2004;82(2–3):459–469. doi: 10.1016/j.physbeh.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Rawal S, Hayes JE, Wallace MR, Bartoshuk LM, Duffy VB. Do polymorphisms in the TAS1R1 gene associate with broader differences in human taste intensity? Chemical Senses. 2013;38(8):719–728. doi: 10.1093/chemse/bjt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawal S, Hoffman HJ, Honda M, Huedo-Medina TB, Duffy VB. The taste and smell protocol in the 2011–2014 U.S. National health and nutrition examination survey (NHANES): Test-Retest reliability and validity testing. Chemosensory Perception. 2015;8(3):138–148. doi: 10.1007/s12078-015-9194-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed DR, Zhu G, Breslin PA, Duke FF, Henders AK, Campbell MJ, Wright MJ. The perception of quinine taste intensity is associated with common genetic variants in a bitter receptor cluster on chromosome 12. Human Molecular Genetics. 2010;19(21):4278–4285. doi: 10.1093/hmg/ddq324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rødbotten M, Martinsen B, Borge G, Mortvedt H, Knutsen S, Lea P, et al. A cross-cultural study of preference for apple juice with different sugar and acid contents. Food Quality and Preference. 2009;20(3):277–284. [Google Scholar]
- Sandell M, Hoppu U, Lundén S, Salminen M, Puolimatka T, Laaksonen O, Hopia A. Consumption of lingonberries by TAS2R38 genotype and sensory quality of texture-designed lingonberry samples. Food Quality and Preference. 2015;45:166–170. [Google Scholar]
- Sandell M, Hoppu U, Mikkila V, Mononen N, Kahonen M, Mannisto S, Raitakari OT. Genetic variation in the hTAS2R38 taste receptor and food consumption among Finnish adults. Genes & Nutrition. 2014;9(6):433. doi: 10.1007/s12263-014-0433-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarmo S, Henebery K, Peracchio H, Cartmel B, Lin H, Ermakov IV, Mayne ST. Skin carotenoid status measured by resonance Raman spectroscopy as a biomarker of fruit and vegetable intake in preschool children. European Journal of Clinical Nutrition. 2012;66(5):555–560. doi: 10.1038/ejcn.2012.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwieterman ML, Colquhoun TA, Jaworski EA, Bartoshuk LM, Gilbert JL, Tieman DM, Clark DG. Strawberry flavor: Diverse chemical compositions, a seasonal influence, and effects on sensory perception. PLoS One. 2014;9(2):e88446. doi: 10.1371/journal.pone.0088446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharafi M, Duffy VB, Miller RJ, Winchester SB, Sullivan MC. Dietary behaviors of adults born prematurely may explain future risk for cardiovascular disease. Appetite. 2016;99:157–167. doi: 10.1016/j.appet.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharafi M, Hayes JE, Duffy VB. Masking vegetable bitterness to improve palatability depends on vegetable type and taste phenotype. Chemo-sensory Perception. 2013;6(1):8–19. doi: 10.1007/s12078-012-9137-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharafi M, Perrachio H, Scarmo S, Huedo-Medina TB, Mayne ST, Cartmel B, et al. Preschool-adapted liking survey (PALS): A brief and valid method to assess dietary quality of preschoolers. Childhood Obesity. 2015;11(5):530–540. doi: 10.1089/chi.2015.0037. [DOI] [PubMed] [Google Scholar]
- Suomela JP, Vaarno J, Sandell M, Lehtonen HM, Tahvonen R, Viikari J, et al. Children’s hedonic response to berry products: Effect of chemical composition of berries and hTAS2R38 genotype on liking. Food Chemistry. 2012;135(3):1210–1219. doi: 10.1016/j.foodchem.2012.05.079. [DOI] [PubMed] [Google Scholar]
- Taheri R, Connolly BA, Brand MH, Bolling BW. Underutilized chokeberry (Aronia melanocarpa, Aronia arbutifolia, prunifolia) accessions are rich sources of anthocyanins, flavonoids, hydroxycinnamic acids, and proanthocyanidins. Journal of Agricultural and Food Chemistry. 2013;61(36):8581–8588. doi: 10.1021/jf402449q. [DOI] [PubMed] [Google Scholar]
- Tornwall O, Silventoinen K, Hiekkalinna T, Perola M, Tuorila H, Kaprio J. Identifying flavor preference subgroups. Genetic basis and related eating behavior traits. Appetite. 2014;75:1–10. doi: 10.1016/j.appet.2013.11.020. [DOI] [PubMed] [Google Scholar]
- Tornwall O, Silventoinen K, Keskitalo-Vuokko K, Perola M, Kaprio J, Tuorila H. Genetic contribution to sour taste preference. Appetite. 2012;58(2):687–694. doi: 10.1016/j.appet.2011.12.020. [DOI] [PubMed] [Google Scholar]
- Tuorila H, Cardello AV. Consumer responses to an off-flavor in juice in the presence of specific health claims. Food Quality and Preference. 2002;13(7–8):561–569. [Google Scholar]
- Tuorila H, Huotilainen A, Lähteenmäki L, Ollila S, Tuomi-Nurmi S, Urala N. Comparison of affective rating scales and their relationship to variables reflecting food consumption. Food Quality and Preference. 2008;19(1):51–61. [Google Scholar]
- Wang X, Ouyang YY, Liu J, Zhao G. Flavonoid intake and risk of CVD: A systematic review and meta-analysis of prospective cohort studies. British Journal of Nutrition. 2014;111(1):1–11. doi: 10.1017/S000711451300278X. [DOI] [PubMed] [Google Scholar]
- Wise PM, Hansen JL, Reed DR, Breslin PA. Twin study of the heritability of recognition thresholds for sour and salty taste. Chemical Senses. 2007;32(8):749–754. doi: 10.1093/chemse/bjm042. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


