Abstract
We conducted a Bayesian analysis of the association between family-level socioeconomic status and smoking and the prevalence of dental caries among siblings (children from infant to 14 y) among children living in rural and urban Northern Appalachia using data from the Center for Oral Health Research in Appalachia (COHRA). The observed proportion of siblings sharing caries was significantly different from predicted assuming siblings’ caries status was independent. Using a Bayesian hierarchical model, we found the inclusion of a household factor significantly improved the goodness of fit. Other findings showed an inverse association between parental education and siblings’ caries and a positive association between households with smokers and siblings’ caries. Our study strengthens existing evidence suggesting that increased parental education and decreased parental cigarette smoking are associated with reduced childhood caries in the household. Our results also demonstrate the value of a Bayesian approach, which allows us to include household as a random effect, thereby providing more accurate estimates than obtained using generalized linear mixed models.
Knowledge Transfer Statement: Siblings living in the same household tend to share caries status, and selected household factors, such as parental education and smoking, are strongly associated with caries development among siblings. These high-risk households might be targeted for appropriate educational and other interventions to reduce caries risk.
Keywords: Appalachia, oral health, smoking, child, family characteristics, decay probability
Introduction
Childhood dental caries is associated with household characteristics, such as socioeconomic status (SES) and parental smoking (Harris et al. 2004; Tanaka et al. 2009; Paula et al. 2012). Tests of household effects are stronger if siblings living in the same household are studied simultaneously, as that study design allows us to test if the same environment acts equally on individuals with shared genetic traits associated with dental decay: tooth morphology, salivary flow, and immune responses (Wendell et al. 2010; Werneck et al. 2010). However, even though it is commonly believed that rates of dental decay in children from the same household are highly correlated, we found no previous cross-sectional studies that directly tested this belief. We thus designed an analysis to measure childhood caries and its association with SES and smoking factors while using a household variable to measure the level of nonindependence of childhood caries among siblings.
Similar studies often use generalized linear mixed models (GLMMs) to model childhood caries and its associations with SES or environmental factors. GLMMs have several strengths but also limitations. A primary limitation is that they estimate the effect of household factors as one value using data from all of the households. By contrast, the Bayesian approach we took includes household as a random effect and generates an estimate for each household, providing a more direct and accurate estimate of household factors. Therefore, applying a Bayesian method to evaluate the association of household characteristics on siblings’ dental decay will provide a more accurate estimate for the strength of any observed associations between household variables and dental decay.
We demonstrate the value of using the Bayesian approach by examining the association of household income, education, and smoking on risk of dental decay among siblings living in the same household. Parental income and education are two of the best-documented family-level SES factors related to dental decay (Oliveira et al. 2008; Nunn et al. 2009; Li et al. 2010; Wigen et al. 2011; Bernabé et al. 2012; Paula et al. 2012). Parental smoking during and after pregnancy is associated with an increased prevalence of childhood caries (Tanaka et al. 2009; Hanioka et al. 2011; although also see Leader 2014). Smoking has a direct effect on the oral microbiome of smokers, reducing the diversity of the microbiota (Thomas et al. 2014), increasing colonization with cariogenic bacteria (Hanioka et al. 2011), and leading to the production of an unstable dental biofilm (Kumar et al. 2011).
We used data collected by the Center for Oral Health Research in Appalachia (COHRA) that enrolled families (cohort COHRA1). Appalachia has high rates of poverty and unemployment with less educational attainment than the country as a whole (McNeil et al. 2012; Polk et al. 2015). We limited our analysis to households where at least 2 children (each 14 y or younger) and 1 or more adults enrolled. We hypothesized that the prevalence of dental caries among siblings is not independent and that including a household effect would improve model fit in predicting childhood caries. We also hypothesized that after taking the household concordance into account, the household’s income, parent’s education, and the presence of smokers would be associated with the prevalence of childhood caries.
Methods
Recruitment and Data Collection
Participants were recruited and examined by the COHRA (cohort designation COHRA1); for details on the study protocol, see Polk et al. (2008). Briefly, recruitment began in 2003 in 4 West Virginia and Pennsylvania rural counties and an urban site in Pittsburgh, Pennsylvania. Eligible households had at least 1 parent-child pair, in which the participating child was the biological child of a participating parent. Everyone living in an eligible household was invited to participate in the study, regardless of biological or legal relationship. A total of 3,074 participants from 862 nuclear families were enrolled, among whom 1,400 were children and adolescents (age ≤14 y). Participants received standard periodontal and caries screenings by a licensed and calibrated dentist or dental hygienist, and sound and carious teeth were identified to calculate the dft (decayed and filled) and DMFT (decayed, missing, and filled) score for primary and permanent teeth, respectively (for detailed enrollment and examination procedure, see Polk et al. 2008). Because white spot lesions can remineralize, they were not included in the calculation of dft or DMFT. The study protocol was reviewed by the University of Pittsburgh Institutional Review Board (coordinating center approval #0207073, Pennsylvania site approval #0506048) and West Virginia University Institutional Review Board (approval #15620B).
For this analysis, we chose the 333 COHRA households with at least 2 participating children aged 14 y or younger. This included 837 children and adolescents from 110 families in Pennsylvania and 223 families in West Virginia. We excluded families that had missing data (child age, child sex, SES, and smoking variables). Participants were classified as having dental caries if the dft or DMFT score was at least 1. At the time of examination, adults and adolescents >14 years completed self-report questionnaires regarding individual income, education, and smoking habits. On average, 1.68 ± 0.58 adults per household completed the questionnaire.
We included all enrolled adults from each household regardless of relationship to participating children on the grounds that their income and behaviors inevitably have an influence on the household’s children. Of the 560 participating adults, 72 were not identified as parents of the participating children. As not all adults from the same household were enrolled, we estimated the household income using the average income/person based on the self-reported personal income of enrolled adults and older siblings (>14 y) (see Table 1 footnote for details). Parental education was estimated as the average of self-reported years of education of enrolled adults in each household. The presence/absence of smokers in each household was determined based on response to the question, “Do you currently smoke cigarettes?” We included answers from adults and adolescents in determining if there was household cigarette exposure.
Table 1.
Sociodemographic Characteristics of Study Participants.
| Characteristic | Value |
|---|---|
| Number of participants (age ≤14 y) | 837 |
| Age, mean ± SD | 7.26 ± 3.7 |
| Male | 424 (50.7) |
| Female | 413 (49.3) |
| Without caries | 411 (49.1) |
| With caries | 426 (50.9) |
| Number of families | 333 |
| Pennsylvania | 110 (33.0) |
| West Virginia | 223 (67.0) |
| Family with only healthy children | 80 (24.0) |
| Family with only carious children | 88 (26.4) |
| Family with carious and healthy children | 165 (49.6) |
| Average number of children/family | 2.51 ± 0.84 |
| Family with 2 children | 210 (63.1) |
| Family with 3 children | 91 (27.3) |
| Family with more than 3 children | 32 (9.6) |
| Average family per person incomea | 2.74 ± 1.72 |
| Family with per person income scale 1 to 3 | 229 |
| Family with per person income scale 4 to 6 | 93 |
| Family with per person income scale 7 to 10 | 11 |
| Average adult education (y)b | 13.12 ± 2.16 |
| Adult with less than high school education | 67 (20.1) |
| Adult with high school education | 91 (27.3) |
| Adult with some college education | 124 (37.2) |
| Adult with college education | 28 (8.4) |
| Adult with more than college education | 23 (6.9) |
| Number of families with smokers | 151 (45.3) |
| Average number of smokers per familyc | 1.37 ± 0.56 |
| Family with 1 smoker | 100 (30.0) |
| Family with 2 smokers | 47 (14.1) |
| Family with more than 2 smokers | 4 (1.2) |
Children aged 14 and younger enrolled in the Center for Oral Health Research in Appalachia (COHRA, cohort COHRA1) from the 333 households where 2 or more children aged 14 y or younger participated. Values are presented as mean ± SD or number (%).
Family’s average annual income/person is calculated as the average income per individual based on scaled personal income from enrolled adults and older children (>14 y) who reported income. The scale was 1 = less than $10,000, 2 = $10,000 to $14,999, 3 = $15,000 to $24,999, 4 = $25,000 to $34,999, 5 = $35,000 to $49,999, 6 = $50,000 to $74,999, 7 = $75,000 to $99,999, 8 = $100,000 to $149,999, 9 = $150,000 to $199,999, and 10 = $200,000 or more.
Average adult education is calculated as the average number of years of education among enrolled adults in the family.
Calculated only among families with smokers.
Data Analysis
To estimate the probability of developing caries by age, we plotted Kaplan-Meier survival curves. For this analysis, we treated birth as the first observation (when all children should be caries free) and estimated time to survival without caries at the age of our examination. We repeated this analysis, stratifying by parents’ education level (with or without high school degrees) and presence of smokers in their household. Each child was treated independently to generate the survival curves without considering household effects.
We next examined the level of dental decay/health concordance among siblings in participating families. We calculated the probability of all children in the household showing concordance (i.e., all decayed or all healthy) under the null condition, where each child’s decay probability was independent from his or her siblings. (For example, in a population where the probability of developing caries is 0.5, in households with 2 children that are independent, the probability that both children are decayed or healthy is 0.5 * 0.5 + 0.5 * 0.5 = 0.5; in households with 3 children, the probability is 0.5 * 0.5 * 0.5 + 0.5 * 0.5 * 0.5 = 0.25.) We then counted the number of households showing concordance and divided it by the total number of households to estimate the observed probability of households with concordance. Last, we assessed whether the observed probabilities were statistically different from the expected using a χ2 test. A significant test indicates household concordance, which means children from the same family are more likely to all have caries or all have healthy teeth.
Next, we constructed a Bernoulli trial to model the binomial probability of caries development (i.e., use “success” or “failure” in Bernoulli trial to model “caries” or “no caries”) of the participants. The probability of the Bernoulli trial was formulated as a logistic linear function. We then used a GLMM to model the association between predictable variables and the probability of dental caries (Javali and Pandit 2007). As shown in Figure 1, among the participants, age was a strong predictor of caries development. Therefore, in the most basic model (“Base model” in Table 2), the probability in the Bernoulli trial was modeled as a response variable with the participant’s age as the explanatory variable. We used a probability-based Bayesian inference with a noninformative prior (normal distributions with small precision) to estimate the posterior distribution of β age.
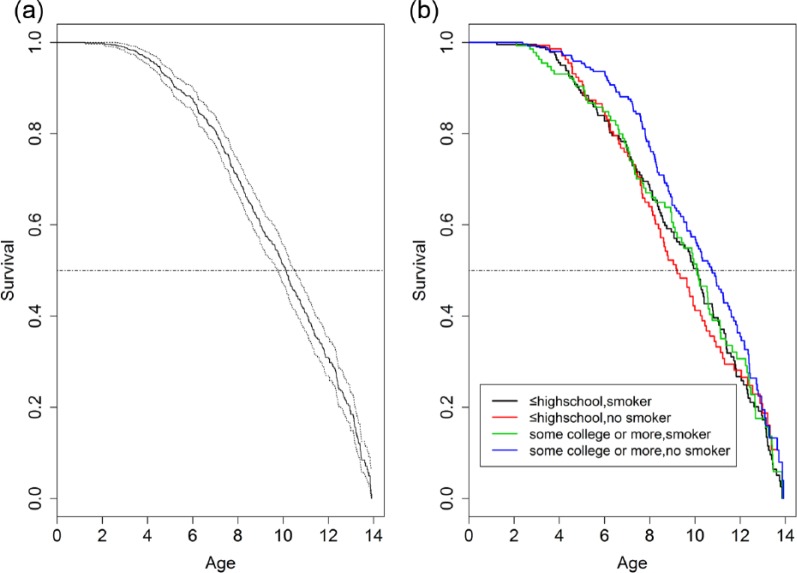
Figure 1.
Kaplan-Meier prediction showing time to the first caries development among 837 children younger than 14 y participating in Center for Oral Health Research in Appalachia (COHRA, cohort COHRA1). (a) Overall survival; dotted lines indicate 95% confidence intervals. (b) Stratified by parental education and presence of a smoker in the household. Parents with ≤12 years of education are categorized as “≤ high school”; parents with >12 years of education are categorized as “some college or more.”
Table 2.
Predictions of Individual Caries Risk among Children Younger Than 14 y Participating in Center for Oral Health Research in Appalachia (COHRA, Cohort 1): Candidate Models, Model Parameters, and Deviance Information Criterion (DIC) for Individual and Household Factors.
| Model | Parameters | DIC | ΔDIC | |
|---|---|---|---|---|
| Fixed | Random | |||
| Base | Age | NA | 996.789 | 29.889 |
| 1 | Age, sex | NA | 998.661 | 31.761 |
| 2 | Age | Household | 983.532 | 16.632 |
| 3 | Age | Household (depending on annual income) | 977.664 | 10.764 |
| 4 | Age | Household (depending on parent education) | 969.646 | 2.746 |
| 5 | Age | Household (depending on smoker status) | 975.418 | 8.518 |
| 6 (top model) | Age | Household (depending on parent education and smoking status) | 966.9 | 0 |
| 7 | Age | Household (depending on annual income and smoking status) | 972 | 5.1 |
| 8 | Age | Household (depending on annual income and parent education) | 969.5 | 2.6 |
NA, random effect was not included in the base model or Model 1.
From the base model, we included the participant’s sex as a fixed effect in the logistic linear function (model 1). We then calculated and compared the deviance information criterion (DIC, a Bayesian equivalent to the Akaike information criterion [AIC]) to determine whether the additional variable improved the goodness of fit in the models. This is measured by the difference between the 2 models’ DIC value (aka ΔDIC). If ΔDIC is greater than 5, it indicates the model with the smaller DIC is a better fitted model. Adding sex did not improve the model fit (ΔDIC <5) over the base model. Therefore, in the following analyses, sex was not included as a fixed effect.
We then included household as a random effect (model 2) to assess whether the caries development of siblings living in the same household is modified by the effect of household.
(In the above model, µ and σ are the mean and variance of the normal distribution, from which the household random effect was drawn.)
If the household effect significantly increases the model fit, it indicates that caries development among siblings is jointly affected by household factors. We further constructed hierarchical models to provide a more robust examination of how household socioeconomic factors modify the random household effects (models 3 to 8 in Table 2). We then applied the Bayesian model selection criteria (DIC) as described above to compare and choose the most parsimonious model with the best fit. For these models, the mean of the normal distributions from which the random household effects were drawn was constructed as a response variable. The explanatory variables were the household SES and smoking factors and combinations of these factors, including income, parent education, and smoking status (Table 2; see model 3 below as an example).
After using DIC to select the most fitted model, we further examined the posterior expectation of the top model to interpret what factors were most associated with the caries probability among participants.
Last, we fit a logistic regression model to examine whether SES and smoking factors are associated with concordance of caries development in the family. The logistic regression modeled each family’s caries concordance as the dependent variable, in contrast to the Bayesian method that modeled the probability of each individual participant’s dental caries. The logistic regression model included parental education, family income, presence in the household of a cigarette smoker, and an indicator of whether some siblings were ≤4 y as explanatory variables.
Results
Participating children averaged 7 y of age and were evenly distributed by sex (Table 1). The Kaplan-Meier survival analysis predicted that half of the children would have caries (DMFT >0) by age 10, and virtually all children would have caries by age 14 y (Fig. 1a). When stratified by parental education and presence of smokers in the household, children from households whose parents had more than 12 y of education and where no household member smoked cigarettes were least likely to develop caries (Fig. 1b).
Overall, over half (50.9%) of participants had dental caries (Table 1). In 26% of the households (88/333), all children had dental decay, and in 24% of the households (80/333), all children had healthy teeth (Table 1). In families with 2 children, the probability of household dental concordance under the null condition (i.e., if one child’s dental caries development was independent from his or her sibling) was 0.50 (calculated by 0.509 * 0.509 + 0.491 * 0.491 = 0.500162), while the observed proportion was 0.62 (130 of 210 households), a significant difference (χ2 P = 0.0005). Similarly, in families with 3 children, the observed proportion (32 of 91 households) was significantly (χ2 P = 0.02) higher than the null 0.25; in families with 4 children, the observed (6 of 23 households) was higher than the null (χ2 P = 0.04).
As shown in Table 2, when predicting dental caries, including a random household effect significantly improved the goodness of fit compared with the base model that only included age as a fixed effect (i.e., model 2 ΔDIC = 13.26 compared with the base model). This suggests that the probability of dental caries among siblings from the same household is not independent. The household effect was inversely associated with household income and parents’ education (Fig. 2). This suggests that an increased household income and parental education reduces siblings’ caries probabilities. On the other hand, the presence of smokers in the household increased siblings’ probability of dental caries.
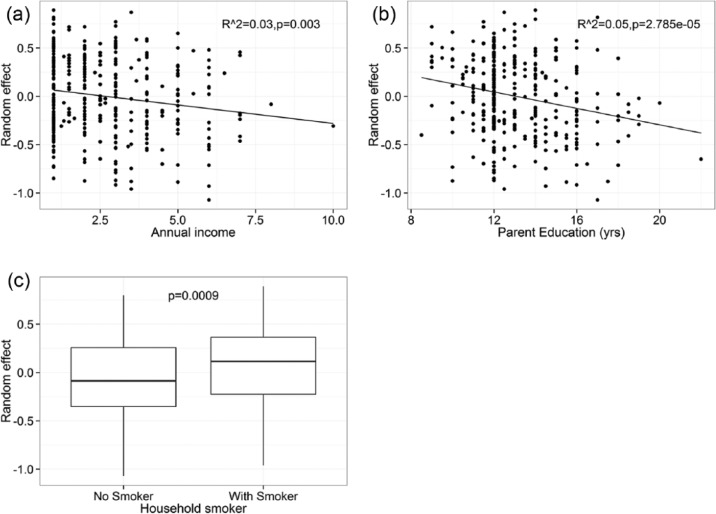
Figure 2.
Associations of a random household effect and (a) income, (b) parental education, and (c) presence of a smoker in the household. Income is measured as scaled (1–10) personal income (see Table 1 legend for details). Results from predictions of individual caries risk among children younger than 14 y participating in the Center for Oral Health Research in Appalachia (COHRA, cohort COHRA1), including age as a fixed effect and a random household effect (model 2 in Table 2).
An analysis of the goodness-of-fit measurements of candidate hierarchical models suggests that the strongest contributor to random household effects is the level of parental education (Table 2). The presence of smokers in the household was also a strong contributor to siblings’ caries development. Model 6 (including parental education and smoker presence) had the lowest DIC among all candidate models, while model 4 (only including parent education) and model 8 (parent education and household income) were of equally good fit (ΔDIC <5).
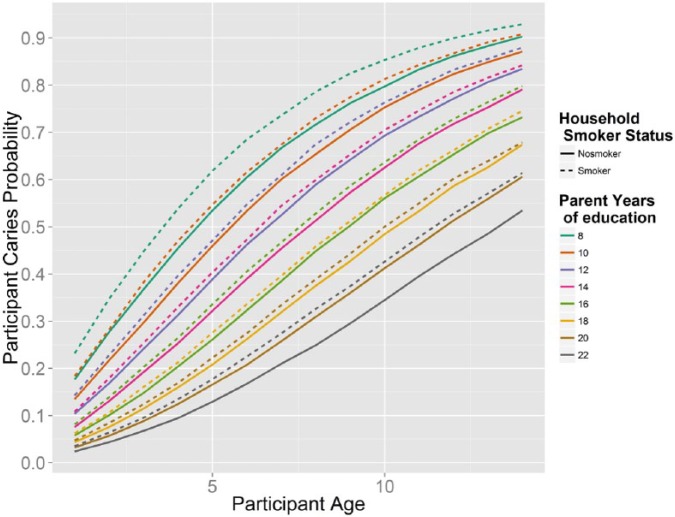
The posterior expectation of the top model (model 6) shows that children’s caries probability increases with age and is affected by their parents’ educational history and the presence of smokers in the household (Fig. 3). The probability of caries decreased when children’s parents had more education, while the presence of cigarette smokers in the household increased the chance that siblings had tooth decay.
Figure 3.
Change in children’s caries probability by age, conditioned on parental education and presence of a smoker in the household. Model predictions of individual caries risk among children younger than 14 y participating in the Center for Oral Health Research in Appalachia (COHRA, cohort1).
Results of a logistic regression model using household’s caries concordance as a dependent variable gave some additional insights: when all children were older than 4 y, the probability that all children had caries was significantly higher (odds ratio [OR], 6.58; 95% confidence interval [95% CI], 3.82–11.72) than in households with children younger than 4 y. Consistent with the Bayesian models, after controlling for siblings’ age, the presence of smokers significantly increased the probability that all children in the household had caries (OR, 2.21; 95% CI, 1.29–3.82), and increased parental education (OR, 0.83; 95% CI, 0.72–0.95) and higher household income (OR, 0.78; 95% CI, 0.65–0.93) were associated with decreased caries concordance in siblings.
Discussion
We used a Bayesian approach to specifically model the association of household factors on caries status of individual siblings living in a household and used a logistic regression model to model siblings’ caries concordance as the dependent variable. The results were complementary, showing an association between household variables, specifically parental education and presence of a cigarette smoker, and concordance of dental decay among siblings.
Of the household factors we examined, parents’ educational level was most strongly associated with siblings having caries. This is consistent with other studies that document a strong association between child’s dental health and parental education (Finlayson et al. 2007; Wigen et al. 2011; Paula et al. 2012; Kumar et al. 2014). Parental education may be associated with other known dental caries risk factors such as diet and oral hygiene habit of the children, which change children’s oral microbial community and consequently change the probability of dental decay. Furthermore, parents with greater education—even if of lower income—may be more likely to be aware of and take advantage of dental health services. Moreover, individuals of higher educational attainment are less likely to smoke cigarettes (Centers for Disease Control and Prevention 2013).
However, even among households with higher parental education, the presence of smokers was associated with a 2-fold increase in the probability that the children would have caries. Postnatal exposure to environmental tobacco usage may increase the probability of dental caries by suppressing immune system function (Kum-Nji et al. 2006), increasing growth of cariogenic bacteria (Zonuz et al. 2008), or be a marker of poorer hygiene, dietary habits, or health habits that contribute to caries development (Tanaka et al. 2009; Nakayama and Mori 2015). Parental tobacco usage can significantly decrease bacterial species richness and modify the stability and variability of oral biofilm of smokers and other household members (Thomas et al. 2014). Transmission of bacteria from mother and other family members to infants is essential to the initial establishment of an oral microbial community in children (Li et al. 2007) and therefore may also partially explain why the presence of a smoker in the household influenced the probability of dental caries among children living in the household.
Smoking was measured by self-report of parents and adolescents older than 14 y. For almost half of the households (45.3%), we had a report of smoking by at least 1 parent or by children older than 14 y. However, we had no direct measures of the amount that household children were exposed to cigarette smoke. An analysis that attempted to take into account the number of cigarettes smoked by parents and older children produced a similar but more complicated picture: while lower income participants were more likely to smoke, they smoked fewer cigarettes per day, probably because of the cost. This is consistent with previous studies showing that intensity of cigarette consumption is sensitive to price (Cavazos-Rehg et al. 2014; MacLean et al. 2016). Our results would be strengthened if we could have included a more accurate measure of exposure to cigarette smoking. Nonetheless, our findings support the existence of an association between exposure to cigarette smoke and caries risk.
The association of household variables and childhood caries was not independent: there was interaction between the presence of smokers in the household and parental education. Note that in Figure 1b, among families with lower parental education (high school or less), the presence or absence of smokers did not change the survival curve as significantly as in families with higher parental education (some college or more); nonetheless, the posterior output from the top model (model 6) suggests that including smoker presence can improve the goodness of fit. However, the survival curves in Figure 1b categorized parental education only into 2 categories (“high school or less” and “some college and more”), while in the model, education was a continuous variable. Thus, the latter has more power to examine the effect of smoker presence across all educational levels, and the posterior output is an average effect across all levels.
Use of hierarchical Bayesian modeling is relatively rare in the dental literature, although the Bayesian approach has been applied for studies modeling DMFT using zero inflated Poisson and negative binomial models (Matranga et al. 2014). Bayesian analysis has the advantage of easier interpretation of estimated parameters and probability, reduced bias associated with small samples compared with maximum likelihood procedures, and the ability to explicitly incorporate prior information (Matranga et al. 2014). Meanwhile, the tendency of data misinterpretation of the traditional hypothesis testing has been noticed (Kagereki et al. 2016), as well as the advantage and strength of Bayesian methods in medical science (Gurrin et al. 2000). For comparison, we ran a GLMM, using each participant’s caries status as the dependent variable, as well as parents’ education and the presence of smoker as explanatory variables, and included a random variable to account for each household (note this is different from the logistic regression model using the household’s caries concordance as the dependent variable). The GLMM yielded similar results to our Bayesian analysis, showing that increased parent education significantly reduced (OR, 0.87; CI, 0.7–0.93) while the presence of smokers significantly increased (OR, 1.44; CI, 1.02–2.14) the probability of caries development in the participants. However, unlike the Bayesian hierarchical method, which drew different random effects for each household, GLMM yielded only 1 random household effect for all the households to fit the linear regression. The Bayesian approach allowed us to maximize the inferences we drew from the observations about which household factors are most strongly associated with participant’s dental caries.
One study limitation is the quantification of the SES and environmental smoking variables of participating families. We used self-reported smoking behavior, family income, and parental education, which were subject to errors. For almost half the households, we had information for only 1 adult, and thus it is likely that we have incomplete information on income and exposure to smoke. Adults living in the same household likely are correlated with respect to education, so the bias in this variable may be somewhat less. If 2 adults participated, we averaged the values to calculate per person income and average adult education. This might have truncated the distributions and shifted the means downward. However, we did observe wide ranges in income and education (Table 1). Families with extreme values of income and education might potentially skew the analysis if data were analyzed using conventional statistics like linear regression (as shown in Fig. 2a, b, the 1 point on the right edge might be driving the regression). A strength of our use of Bayesian analysis is that it reduces the effect of extreme values compared with conventional analyses.
In summary, we examined the association between household variables and the prevalence of dental caries in siblings in a high-risk population, where an estimated 50% of all children have dental caries by age 10 y. Our results suggest siblings tend to be concordant with respect to dental caries. In addition, by using the prevalence of dental caries among siblings as the outcome, our results control for effects of household behaviors and genetic predisposition, strengthening the evidence that increasing parental education and decreasing parental cigarette smoking might improve oral health.
Author Contributions
A. Wen, B. Foxman, contributed to conception, design, data analysis, and interpretation, drafted and critically revised the manuscript; R. Weyant, contributed to data acquisition and interpretation, critically revised the manuscript; D.W. McNeil, R. Crout, K. Neiswanger, M.L. Marazita, contributed to data acquisition, critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Acknowledgments
Many thanks are due to the study participants, community partners, and also the dedicated research staff at all of the research sites, without whom these studies would be impossible.
Footnotes
Funding for this study came from the Center for Oral Health Research in Appalachia (a collaboration between the University of Pittsburgh, West Virginia University, and University of Michigan; grant from National Institute of Dental and Craniofacial Research R01-DE 014899).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Bernabé E, Delgado-Angulo EK, Murasko JE, Marcenes W. 2012. Family income and tooth decay in US children: does the association change with age? Caries Res. 46(3):221–227. [DOI] [PubMed] [Google Scholar]
- Cavazos-Rehg P, Krauss M, Spitznagel E, Chaloupka F, Luke D, Waterman B, Grucza R, Bierut L. 2014. Differential effect of cigarette price changes on adult smoking behaviors. Tob Control. 23(2):113–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health. 2013. Current cigarette smoking among adults in the United States [accessed 2017 Feb 13]. http://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm#ref
- Finlayson T, Siefert K, Ismail A, Sohn W. 2007. Psychosocial factors and early childhood caries among low-income African-American children in Detroit. Community Dent Oral. 35(6):439–448. [DOI] [PubMed] [Google Scholar]
- Gurrin LC, Kurinczuk JJ, Burton PR. 2000. Bayesian statistics in medical research: an intuitive alternative to conventional data analysis. J Eval Clin Pract. 6(2):193–204. [DOI] [PubMed] [Google Scholar]
- Hanioka T, Ojima M, Tanaka K, Yamamoto M. 2011. Does secondhand smoke affect the development of dental caries in children? A systematic review. Int J Environ Res Public Health. 8(5):1503–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris R, Nicoll AD, Adair PM, Pine CM. 2004. Risk factors for dental caries in young children: a systematic review of the literature. Community Dent Health. 21(1, Suppl):71–85. [PubMed] [Google Scholar]
- Javali SB, Pandit PV. 2007. Use of the generalized linear models in data related to dental caries index. Indian J Dent Res. 18(4):163–167. [DOI] [PubMed] [Google Scholar]
- Kagereki E, Gakonyo J, Simila H. 2016. Significance bias: an empirical evaluation of the oral health literature. BMC Oral Health. 16(1):53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kum-Nji P, Meloy L, Herrod HG. 2006. Environmental tobacco smoke exposure: prevalence and mechanisms of causation of infections in children. Pediatrics. 117(5):1745–1754. [DOI] [PubMed] [Google Scholar]
- Kumar PS, Matthews CR, Joshi V, de Jager M, Aspiras M. 2011. Tobacco smoking affects bacterial acquisition and colonization in oral biofilms. Infect Immun. 79(11):4730–4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Kroon J, Lalloo R. 2014. A systematic review of the impact of parental socio-economic status and home environment characteristics on children’s oral health related quality of life. Health Qual Life Out. 12:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leader D. 2014. Limited evidence shows a possible causal relationship between secondhand smoke and caries in children. J Am Dent Assoc. 145(2):179–181. [DOI] [PubMed] [Google Scholar]
- Li Y, Ismail AI, Ge Y, Tellez M, Sohn W. 2007. Similarity of bacterial populations in saliva from African-American mother-child dyads. J Clin Microbiol. 45(9):3082–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang Y, Yang R, Zhang Q, Zou J, Kang D. 2010. Associations of social and behavioural factors with early childhood caries in Xiamen city in China. Int J Paediatr Dent. 21(2):103–111. [DOI] [PubMed] [Google Scholar]
- MacLean JC, Kessler AS, Kenkel DS. 2016. Cigarette taxes and older adult smoking: evidence from the Health and Retirement Study. Health Econ. 25(4):424–438. [DOI] [PubMed] [Google Scholar]
- Matranga D, Campus G, Castiglia P, Strohmenger L, Solinas G. 2014. Italian deprivation index and dental caries in 12-year-old children: a multilevel Bayesian analysis. Caries Res. 48(6):584–593. [DOI] [PubMed] [Google Scholar]
- McNeil DW, Crout RJ, Marazita ML. 2012. Oral health in Appalachia. In: Ludke RL, Obermiller PJ, editors. Appalachian health and well-being. Lexington: University Press of Kentucky; p. 275–294. [Google Scholar]
- Nakayama Y, Mori M. 2015. Association of environmental tobacco smoke and snacking habits with the risk of early childhood caries among 3-year-old Japanese children. J Public Health Dent. 75(2):157–162. [DOI] [PubMed] [Google Scholar]
- Nunn ME, Dietrich T, Singh HK, Henshaw MM, Kressin NR. 2009. Prevalence of early childhood caries among very young urban Boston children compared with US children. J Public Health Dent. 69(3):156–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira LB, Sheiham A, Bönecker M. 2008. Exploring the association of dental caries with social factors and nutritional status in Brazilian preschool children. Eur J Oral Sci. 116(1):37–43. [DOI] [PubMed] [Google Scholar]
- Paula JS, Leite IC, Almeida AB, Ambrosano GM, Pereira AC, Mialhe FL. 2012. The influence of oral health conditions, socioeconomic status and home environment factors on schoolchildren’s self-perception of quality of life. Health Qual Life Out. 10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk DE, Kim S, Manz M, Weyant RJ. 2015. Is there an Appalachian disparity in dental caries in Pennsylvania schoolchildren? Community Dent Oral. 43(1):24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk DE, Weyant RJ, Crout RJ, McNeil DW, Tarter RE, Thomas JG, Marazita ML. 2008. Study protocol of the Center for Oral Health Research in Appalachia (COHRA) etiology study. BMC Oral Health. 8:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Miyake Y, Sasaki S. 2009. The effect of maternal smoking during pregnancy and postnatal household smoking on dental caries in young children. J Pediatr. 155(3):410–415. [DOI] [PubMed] [Google Scholar]
- Thomas AM, Gleber-Netto FO, Fernandes GR, Amorim M, Barbosa LF, Francisco AL, de Andrade AG, Setubal JC, Kowalski LP, Nunes DN, Dias-Neto E. 2014. Alcohol and tobacco consumption affects bacterial richness in oral cavity mucosa biofilms. BMC Microbiol. 14:250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendell S, Wang X, Brown M, Cooper ME, DeSensi RS, Weyant RJ, Crout R, McNeil DW, Marazita ML. 2010. Taste genes associated with dental caries. J Dent Res. 89(11):1198–1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneck RI, Mira MT, Trevilatto PC. 2010. A critical review: an overview of genetic influence on dental caries. Oral Dis. 16(7):613–623. [DOI] [PubMed] [Google Scholar]
- Wigen TI, Espelid I, Skaare AB, Wang NJ. 2011. Family characteristics and caries experience in preschool children: a longitudinal study from pregnancy to 5 years of age. Community Dent Oral. 39(4):311–317. [DOI] [PubMed] [Google Scholar]
- Zonuz AT, Rahmati A, Mortazavi H, Khashabi E, Farahani RM. 2008. Effect of cigarette smoke exposure on the growth of Streptococcus mutans and Streptococcus sanguis: an in vitro study. Nicotine Tob Res. 10(1):63–67. [DOI] [PubMed] [Google Scholar]