Abstract
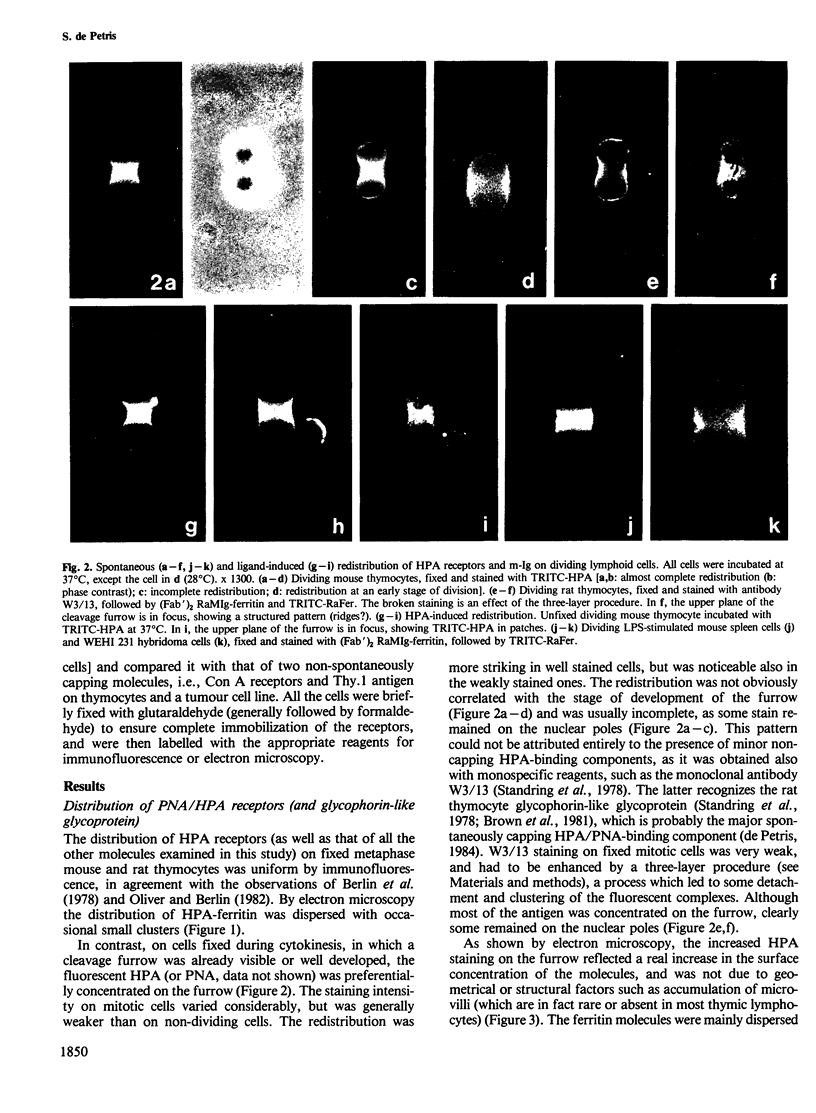
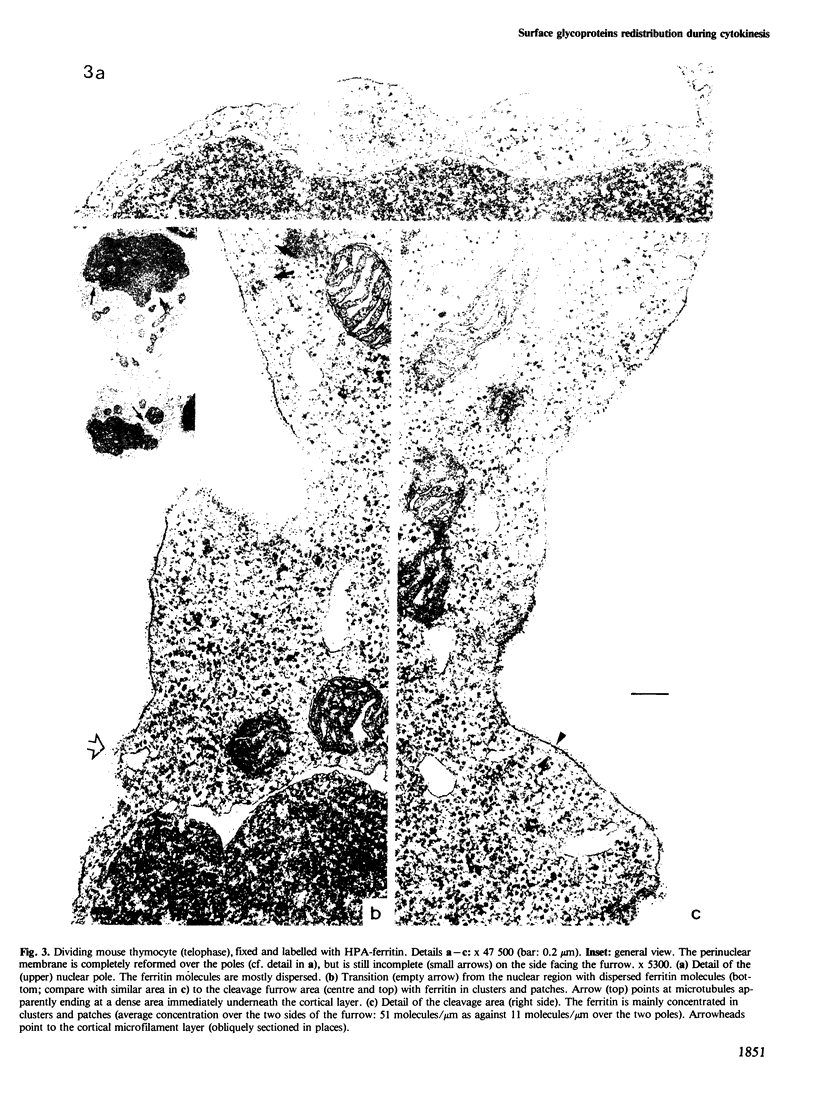
The 'unperturbed' distribution of plasma membrane glycoproteins during cytokinesis has been examined by immunofluorescence and electron microscopy on dividing mouse and rat lymphoid cells fixed before being labelled with the appropriate reagents. Two groups of molecules which cap 'spontaneously' to the uropod of non-dividing cells, i.e., the common receptors for Helix pomatia (HPA) and peanut agglutinin (PNA) (and in particular the thymocyte glycophorin-like glycoprotein) and membrane immunoglobulins, redistribute spontaneously to the cleavage furrow during cytokinesis. By electron microscopy, the redistributed molecules (HPA receptors) appear to be aggregated in clusters. Other glycoproteins, such as Concanavalin A receptors and Thy.1 antigens, which do not cap spontaneously on interphase cells, remain uniformly distributed or are somewhat depleted over the cleavage furrow. The results suggest that a spontaneous 'transport' of certain membrane molecules from the nuclear pole to the cleavage furrow occurs normally during cytokinesis by a mechanism analogous to that of uropod formation and spontaneous capping in interphase cells. The existence of redistribution phenomena in dividing cells imposes some restrictions on the possible mechanisms of redistribution and on certain aspects of the cleavage process.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgartner U. Spontaneous segregation of receptors for peanut and Helix pomatia agglutinins to the uropod region of polarized lymphocytes. J Ultrastruct Res. 1982 Sep;80(3):323–338. doi: 10.1016/s0022-5320(82)80045-2. [DOI] [PubMed] [Google Scholar]
- Berlin R. D., Oliver J. M. Surface functions during mitosis. II. Quantitation of pinocytosis and kinetic characterization of the mitotic cycle with a new fluorescence technique. J Cell Biol. 1980 Jun;85(3):660–671. doi: 10.1083/jcb.85.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlin R. D., Oliver J. M., Walter R. J. Surface functions during Mitosis I: phagocytosis, pinocytosis and mobility of surface-bound Con A. Cell. 1978 Oct;15(2):327–341. doi: 10.1016/0092-8674(78)90002-8. [DOI] [PubMed] [Google Scholar]
- Bourguignon L. Y., Singer S. J. Transmembrane interactions and the mechanism of capping of surface receptors by their specific ligands. Proc Natl Acad Sci U S A. 1977 Nov;74(11):5031–5035. doi: 10.1073/pnas.74.11.5031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretscher M. S. Directed lipid flow in cell membranes. Nature. 1976 Mar 4;260(5546):21–23. doi: 10.1038/260021a0. [DOI] [PubMed] [Google Scholar]
- Brown W. R., Barclay A. N., Sunderland C. A., Williams A. F. Identification of a glycophorin-like molecule at the cell surface of rat thymocytes. Nature. 1981 Feb 5;289(5797):456–460. doi: 10.1038/289456a0. [DOI] [PubMed] [Google Scholar]
- De Petris S., Raff M. C. Ultrastructural distribution and redistribution of alloantigens and concanavalin A receptors on the surface of mouse lymphocytes. Eur J Immunol. 1974 Feb;4(2):130–137. doi: 10.1002/eji.1830040213. [DOI] [PubMed] [Google Scholar]
- De Petris S., Takacs B. Relationship between mouse lymphocyte receptors for peanut agglutinin (PNA) and Helix pomatia agglutinin (HPA). Eur J Immunol. 1983 Oct;13(10):831–840. doi: 10.1002/eji.1830131010. [DOI] [PubMed] [Google Scholar]
- Hewitt J. A. Surf-riding model for cell capping. J Theor Biol. 1979 Sep 7;80(1):115–127. doi: 10.1016/0022-5193(79)90183-8. [DOI] [PubMed] [Google Scholar]
- Koppel D. E., Oliver J. M., Berlin R. D. Surface functions during mitosis. III. Quantitative analysis of ligand-receptor movement into the cleavage furrow: diffusion vs. flow. J Cell Biol. 1982 Jun;93(3):950–960. doi: 10.1083/jcb.93.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake P., Clark E. A., Khorshidi M., Sunshine G. H. Production and characterization of cytotoxic Thy-1 antibody-secreting hybrid cell lines. Detection of T cell subsets. Eur J Immunol. 1979 Nov;9(11):875–886. doi: 10.1002/eji.1830091109. [DOI] [PubMed] [Google Scholar]
- Marshak-Rothstein A., Fink P., Gridley T., Raulet D. H., Bevan M. J., Gefter M. L. Properties and applications of monoclonal antibodies directed against determinants of the Thy-1 locus. J Immunol. 1979 Jun;122(6):2491–2497. [PubMed] [Google Scholar]
- Nunnally M. H., D'Angelo J. M., Craig S. W. Filamin concentration in cleavage furrow and midbody region: frequency of occurrence compared with that of alpha-actinin and myosin. J Cell Biol. 1980 Oct;87(1):219–226. doi: 10.1083/jcb.87.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver J. M., Berlin R. D. Mechanisms that regulate the structural and functional architecture of cell surfaces. Int Rev Cytol. 1982;74:55–94. doi: 10.1016/s0074-7696(08)61169-9. [DOI] [PubMed] [Google Scholar]
- Rappaport R. Geometrical relations of the cleavage stimulus in invertebrate eggs. J Theor Biol. 1965 Jul;9(1):51–66. doi: 10.1016/0022-5193(65)90056-1. [DOI] [PubMed] [Google Scholar]
- Schreiner G. F., Braun J., Unanue E. R. Spontaneous redistribution of surface immunoglobulin in the motile B lymphocyte. J Exp Med. 1976 Dec 1;144(6):1683–1688. doi: 10.1084/jem.144.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standring R., McMaster W. R., Sunderland C. A., Williams A. F. The predominant heavily glycosylated glycoproteins at the surface of rat lymphoid cells are differentiation antigens. Eur J Immunol. 1978 Dec;8(12):832–839. doi: 10.1002/eji.1830081203. [DOI] [PubMed] [Google Scholar]
- Warren G., Featherstone C., Griffiths G., Burke B. Newly synthesized G protein of vesicular stomatitis virus is not transported to the cell surface during mitosis. J Cell Biol. 1983 Nov;97(5 Pt 1):1623–1628. doi: 10.1083/jcb.97.5.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. G., Borisy G. G. On the mechanisms of cytokinesis in animal cells. J Theor Biol. 1983 Mar 21;101(2):289–316. doi: 10.1016/0022-5193(83)90342-9. [DOI] [PubMed] [Google Scholar]
- de Petris S. Concanavalin A receptors, immunoglobulins, and theta antigen of the lymphocyte surface. Interactions with concanavalin A and with Cytoplasmic structures. J Cell Biol. 1975 Apr;65(1):123–146. doi: 10.1083/jcb.65.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Petris S. Lectin-binding and spontaneous capping characteristics of the thymocyte glycophorin-like glycoprotein. Exp Cell Res. 1984 Jun;152(2):510–519. doi: 10.1016/0014-4827(84)90653-0. [DOI] [PubMed] [Google Scholar]
- de Petris S. Nonuniform distribution of concanavalin-A receptors and surface antigens on uropod-forming thymocytes. J Cell Biol. 1978 Oct;79(1):235–251. doi: 10.1083/jcb.79.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Petris S. Preferential distribution of surface immunoglobulins on microvilli. Nature. 1978 Mar 2;272(5648):66–68. doi: 10.1038/272066a0. [DOI] [PubMed] [Google Scholar]
- de Petris S., Raff M. C. Distribution of immunoglobulin on the surface of mouse lymphoid cells as determined by immunoferritin electron microscopy. Antibody-induced, temperature-dependent redistribution and its implications for membrane structure. Eur J Immunol. 1972 Dec;2(6):523–535. doi: 10.1002/eji.1830020611. [DOI] [PubMed] [Google Scholar]