ABSTRACT
In humans, gain-of-function (GOF) mutations in RHBDF2 cause the skin disease tylosis. We generated a mouse model of human tylosis and show that GOF mutations in RHBDF2 cause tylosis by enhancing the amount of amphiregulin (AREG) secretion. Furthermore, we show that genetic disruption of AREG ameliorates skin pathology in mice carrying the human tylosis disease mutation. Collectively, our data suggest that RHBDF2 plays a critical role in regulating EGFR signaling and its downstream events, including development of tylosis, by facilitating enhanced secretion of AREG. Thus, targeting AREG could have therapeutic benefit in the treatment of tylosis.
KEY WORDS: CRISPR/Cas9, EGFR, RHBDF2, Amphiregulin, Tylosis
Summary: Gain-of-function mutations in RHBDF2 cause tylosis, a skin disease characterized by hyperproliferation of keratinocytes. We generated mice carrying the human tylosis disease mutation p.P189L and show that enhanced amphiregulin secretion underlies tylosis.
INTRODUCTION
The role of RHBDF2 in enhancing amphiregulin (AREG) secretion, and consequently activating the epidermal growth factor receptor (EGFR) pathway, has significance for the skin disease tylosis. In humans, autosomal dominant mutations in RHBDF2 cause tylosis (Blaydon et al., 2012; Saarinen et al., 2012), a form of focal nonepidermolytic palmoplantar keratoderma (PPK) that manifests on the skin of the palms and soles (Stevens et al., 1996). Tylosis patients also present oral leukokeratosis and follicular hyperkeratosis (Field et al., 1997; Hennies et al., 1995). Currently, there is no cure for tylosis. In a study to identify genetic variants underlying tylosis, Blaydon et al. used targeted capture array and next-generation sequencing and identified two heterozygous missense mutations in the RHBDF2 gene that underlie tylosis, p.Ile186Thr and p.Pro189Leu (Blaydon et al., 2012). Further, substantial evidence implicates the involvement of AREG-induced constitutive activation of the EGFR pathway in tylosis (Blaydon et al., 2012; Brooke et al., 2014; Hosur et al., 2014). In addition, skin biopsies of tylosis patients suggest increased EGFR activity (Blaydon et al., 2012). Together, these results, along with the recent findings that mutations in Rhbdf2 in mice result in enhanced EGFR pathway activation (Hosur et al., 2014; Siggs et al., 2014), suggest a possible mechanism for the association between RHBDF2 mutations and tylosis. However, the detailed physiological role of RHBDF2 in facilitating secretion of AREG needs to be established.
Here, we generated mice carrying a human GOF mutation p.P189L (p.P159L in mice) in RHBDF2 to examine whether GOF mutations in human RHBDF2 lead to enhanced AREG secretion and tylosis. We provide evidence that Rhbdf2 GOF mutations enhance AREG secretion to cause hyperplasia and hyperkeratosis. In addition, we show that genetic ablation of AREG attenuates skin disease in the Rhbdf2P159L/P159L mouse model of human tylosis. Together, our data strongly suggest that inhibition of AREG could have potential therapeutic value in the treatment of tylosis.
RESULTS AND DISCUSSION
The human tylosis disease mutation enhances AREG secretion
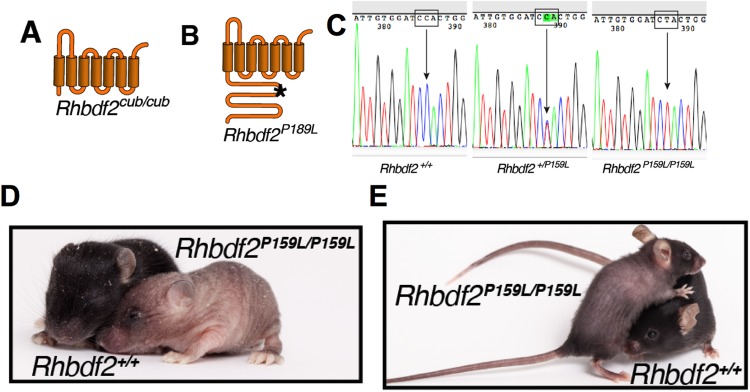
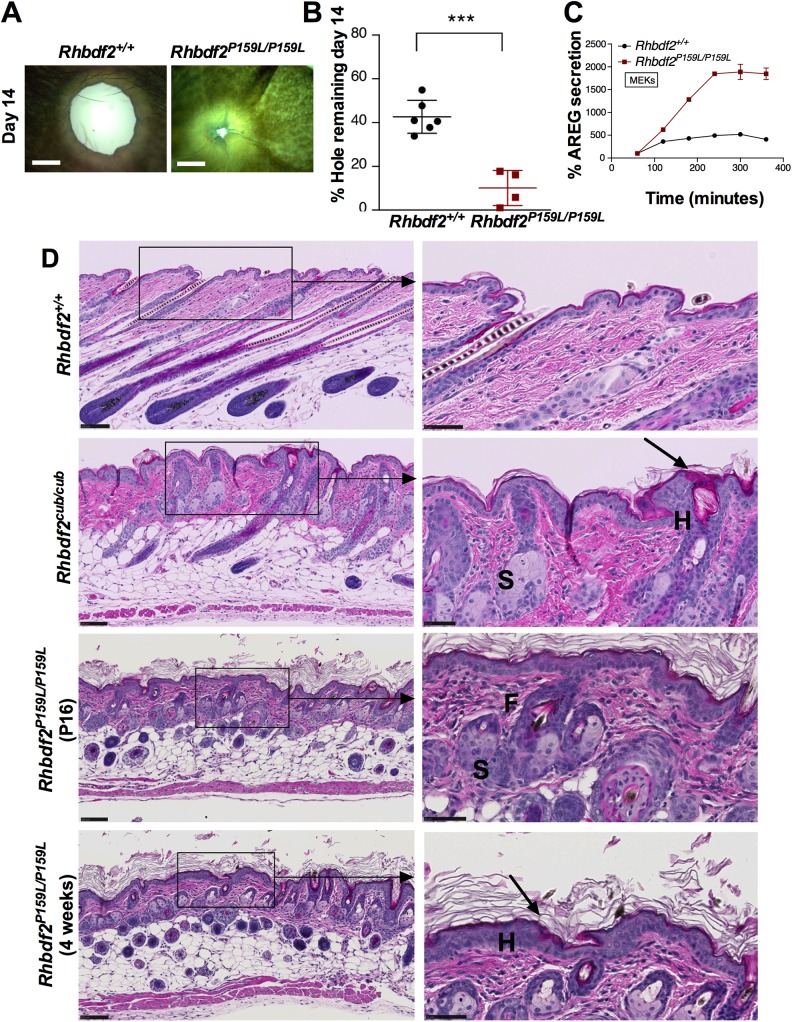
Autosomal dominant mutations in the human RHBDF2 gene cause tylosis (Blaydon et al., 2012; Saarinen et al., 2012). Substantial evidence implicates the involvement of AREG-induced constitutive activation of the EGFR pathway (Blaydon et al., 2012; Brooke et al., 2014; Hosur et al., 2014) in human tylosis. We recently showed that similar to GOF mutations in the mouse Rhbdf2 gene (Rhbdf2cub/cub), GOF mutations in human RHBDF2 (tylosis mutation p.I186T) promote increased secretion of AREG in vitro (Hosur et al., 2014). To determine in vivo whether the Rhbdf2cub mutation (Fig. 1A, loss of the cytosolic N-terminal domain of RHBDF2) phenocopies human tylosis, and whether the tylosis p.P189L mutation (Fig. 1B, missense mutation in the cytosolic N-terminal domain of RHBDF2) in RHBDF2 increases AREG secretion, we generated mice carrying the human tylosis mutation p.P189L (p.P159L in mice) using CRISPR/Cas9-mediated targeting and homology-directed repair in C57BL/6J zygotes (Fig. 1C). We characterized the resulting Rhbdf2P159L/P159L mice and observed that similarly to Rhbdf2cub/cub mice (Hosur et al., 2014), mice carrying the human tylosis mutation present with complete hair loss (Fig. 1D). However, unlike Rhbdf2cub/cub mice (Hosur et al., 2014; Siggs et al., 2014), which present complete hair loss throughout their lives, Rhbdf2P159L/P159L mice developed a thin layer of curly hair coat that was noticeable in adult mice (Fig. 1E), indicating that the Rhbdf2cub phenotype is more severe than that of Rhbdf2P159L. Nevertheless, Rhbdf2P159L/P159L mice elicited significantly accelerated wound healing, as determined by ear punch closure (Fig. 2A,B). Additionally, accelerated wound healing was associated with an increase in stimulated secretion of AREG in Rhbdf2P159L/P159L MEKs compared with Rhbdf2+/+ MEKs (Fig. 2C).
Fig. 1.
The human tylosis mutation enhances secretion of AREG. (A) Schematic of the mouse Rhbdf2cub/cub mutation, which results in the loss of the cytosolic N-terminus domain of RHBDF2 (ΔN-RHBDF2). (B) Schematic of the human RHBDF2 (p.P189L) mutation in the cytosolic N-terminus domain. (C) Sequencing chromatograms of Rhbdf2+/+, Rhbdf2+/ P159L and Rhbdf2 P159L/P159L mice. Arrows indicate a heterozygous single nucleotide polymorphism (SNP) (CCA/CTA) or homozygous SNP (CTA). (D) Images of Rhbdf2+/+ and Rhbdf2P159L/P159L mice at postnatal day 11. Significant hair loss is visible in Rhbdf2 P159L/ P159L mice as early as day 11. (E) Representative image of an eight-week-old female Rhbdf2P159L/P159L mouse presenting a thin hair coat.
Fig. 2.
Rapid wound healing and proliferative skin phenotype in Rhbdf2P159L/P159L mice. (A) Representative images of regenerating ear tissue in eight-week-old female Rhbdf2+/+ and Rhbdf2P159L/ P159L mice at 14 days postwounding. Magnification, 4×; scale bars: 1 mm. (B) Quantification of ear hole closures shown in A. Rhbdf2+/+ (n=6) and Rhbdf2P159L/P159L (n=4). (C) ELISA quantitation of AREG levels in the supernatants of cultured Rhbdf2+/+ and Rhbdf2P159L/P159L MEKs in response to stimulation with 100 nM PMA for the indicated times. (D) H&E-stained skin sections of female Rhbdf2+/+, Rhbdf2cub/cub and Rhbdf2P159L/P159L mice. Both the Rhbdf2cub/cub and Rhbdf2P159L/P159L mice present with significant hyperplasia (H), hyperkeratosis (arrow), enlarged sebaceous glands (S), and follicular dystrophy (F) compared with the Rhbdf2+/+ mice. Scale bars: 100 μm (low magnification); 50 μm (high magnification).
Next, to examine whether mice carrying the human tylosis mutation phenocopy the Rhbdf2cub mutation (Hosur et al., 2014), we performed histological analysis of skin sections from Rhbdf2+/+ and Rhbdf2P159L/P159L mice. Rhbdf2P159L/P159L mice presented with follicular dystrophy (F), enlarged sebaceous glands (S), hyperplasia (H), and hyperkeratosis (arrow) of skin (Fig. 2D). To assess enhanced EGFR signaling in the skin of Rhbdf2P159L/P159L mice, we performed immunohistochemical analyses of skin sections from Rhbdf2+/+ and Rhbdf2P159L/P159L mice. We observed a considerable increase in epidermal EGFR signaling pathway downstream effectors phospho-ERK1/2 and phospho-mTOR (Fig. 3A). Similarly, immunoblot analyses of mouse embryonic fibroblasts (MEFs) isolated from Rhbdf2+/+ and Rhbdf2P159L/P159L mice also revealed enhanced EGFR signaling as demonstrated by reduced EGFR protein levels (due to downregulation of sustained EGFR receptor activation), and by enhanced protein levels of phospho-AKT, phospho-ERK1/2 and phospho-S6 in Rhbdf2P159L/P159L MEFs compared with those of Rhbdf2+/+ mice (Fig. 3B). Also, there was a two- to threefold increase in the levels of the proliferation marker Ki-67 in the skin of Rhbdf2P159L/P159L mice compared with that of Rhbdf2+/+ mice (Fig. 3C). Collectively, these data suggest that Rhbdf2cub/cub and Rhbdf2P159L/P159L are GOF mutations in the Rhbdf2 gene, and that these mutations enhance secretion of AREG to cause tylosis.
Fig. 3.
Enhanced EGFR signaling in Rhbdf2P159L/P159L mice. (A) Immunohistochemical staining of Rhbdf2+/+ and Rhbdf2P159L/P159L skin sections with phospho-ERK1/2 and phospho-mTOR. Arrows indicate increased levels of phospho-ERK1/2 and phospho-mTOR staining. Scale bars: 100 μm. (B) Immunoblot analysis of cell lysates obtained from Rhbdf2+/+ and Rhbdf2P159L/P159L MEFs. Actin served as a loading control. Band intensities were quantified using ImageJ (https://imagej.nih.gov/ij/). (C) Immunohistochemical staining of Rhbdf2+/+ and Rhbdf2P159L/P159L skin sections with proliferation marker Ki-67. Quantification of proliferating cells was performed as described previously (Almeida et al., 2012). Scale bars: 100 μm.
AREG deficiency restores the normal skin phenotype in Rhbdf2P159L/P159L mice
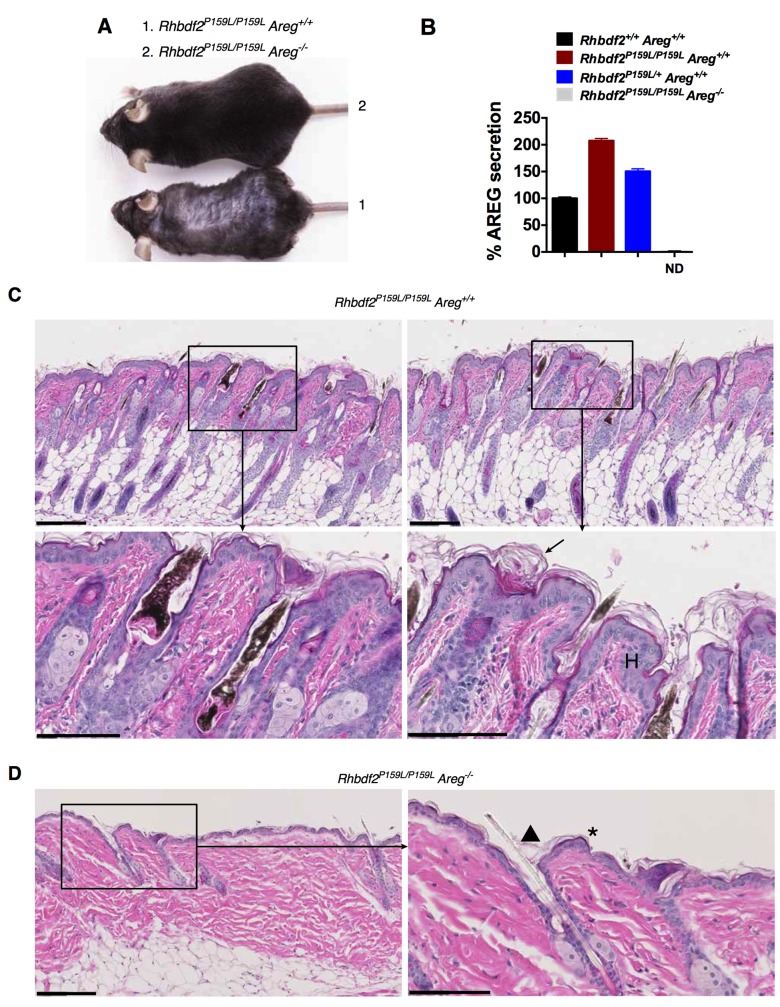
Mice carrying the human tylosis GOF mutation Rhbdf2P159L elicit enhanced AREG secretion (Fig. 2). To test whether AREG drives the skin disease phenotype in Rhbdf2P159L/P159L mice, we genetically deleted AREG in Rhbdf2P159L/P159L mice by crossing Rhbdf2P159L/P159L with AREG-null mice. Rhbdf2P159L/P159L mice lacking AREG presented a full wavy coat rather than a partial hair-loss phenotype of Rhbdf2P159L/P159L mice (Fig. 4A). We confirmed that these mice are null for AREG by measuring serum AREG levels in Rhbdf2+/+, Rhbdf2P159L/+, Rhbdf2P159L/P159L and Rhbdf2P159L/P159L Areg−/− mice. As expected, we found no detectable levels of AREG in Rhbdf2P159L/P159L Areg−/− mice (Fig. 4B). Additionally, whereas homozygous mutant Rhbdf2P159L/P159L mice had various degrees of follicular dystrophy or hair loss, the homozygous mutant mice with no AREG had no follicular dystrophy or hair loss (Fig. 4C). Also, there was no evidence of hyperplasia or hyperkeratosis of skin in Rhbdf2P159L/P159L Areg−/− mice (Fig. 4D). Collectively, these data strongly suggest that AREG mediates the skin disease phenotype of mice carrying the human tylosis mutation Rhbdf2P159L, and that AREG is thus a potential therapeutic target for treating the skin disease tylosis.
Fig. 4.
Genetic deletion of Areg restores the normal skin phenotype in Rhbdf2P159L/P159L mice. (A) Representative images of age-matched Rhbdf2P159L/P159L and Rhbdf2P159L/P159L Areg−/− male mice. Rhbdf2P159L/ P159L mice show a loss of hair phenotype (1); Rhbdf2P159L/P159L Areg−/− mice show a full hair coat (2). (B) Percentage serum AREG levels in age-matched female Rhbdf2+/+, Rhbdf2P159L/+, Rhbdf2P159L/P159L and Rhbdf2P159L/ P159L Areg−/− mice. AREG was not detected (ND) in the serum of Rhbdf2P159L/P159L Areg−/− mice. Data represent mean±s.d. (n=4 mice per group). (C,D) H&E-stained skin sections of male Rhbdf2P159L/P159L and Rhbdf2P159L/ P159L Areg−/− mice. Follicular dystrophy, hyperplasia (H) and hyperkeratosis (arrow) observed in Rhbdf2P159L/P159L mice are restored in Rhbdf2P159L/P159L Areg−/− mice. We found no evidence of follicular dystrophy (arrowhead), hyperplasia or hyperkeratosis of skin (asterisk) in Rhbdf2P159L/P159L Areg−/− mice. Scale bars: 200 μm (low magnification); 100 μm (high magnification).
MATERIALS AND METHODS
Animals
Mice were bred and maintained under modified barrier conditions at The Jackson Laboratory (JAX). The Animal Care and Use Committee at JAX approved the experimental procedures. For the tylosis mutation, we targeted the Rhbdf2 locus in C57BL/6J zygotes by pronuclear microinjection of Cas9 mRNA (60 ng/μl), single guide RNA (sgRNA) (30 ng/μl), and single-stranded oligonucleotide DNA (ssDNA) (1 ng/μl). A total of 10 pups were born, and five of the 10 pups exhibited partial to complete hair loss. Tail DNA was amplified using PCR and then sequenced, and subsequently founder mice carrying the desired modified alleles were backcrossed to C57BL/6J mice to validate germline transmission. The resulting offspring heterozygous for the Rhbdf2P159L allele were intercrossed to generate homozygous C57BL/6J-Rhbdf2P159L/P159L mice. Similar to the Rhbdf2cub/+ heterozygous mice (Hosur et al., 2014; Johnson et al., 2003; Siggs et al., 2014), the Rhbdf2P159L/+ heterozygous mice present with a normal hair coat, and moreover there is no evidence of epidermal hyperplasia or hyperkeratosis. We recently showed that B6.Cg-AregMcub Rhbdf2cub/J mice carry a T-to-G point mutation that disrupts the coding frame and introduces a premature stop codon in the Areg gene (Hosur et al., 2014). B6.Cg-AregMcub Rhbdf2cub/J do not produce a functional AREG protein; hence, AregMcub/Mcub mice are referred to as Areg−/− in this manuscript.
Ear hole closure and histology
Ear punch closure, histology and histological analyses of hematoxylin and eosin (H&E)-stained sections were performed as previously described (Hosur et al., 2014). Slides were scanned using a Nanozoomer digital slide scanner (2.0-HT, Hamamatsu Photonics, Hamamatsu, Japan) and analyzed with the help of Dr Rosalinda Doty, a board-certified pathologist at JAX.
Isolation of fibroblasts and keratinocytes
MEFs were isolated from 13.5 days postcoitum mouse embryos using Liberase DL (Sigma-Aldrich). Embryos were dissected in 15 ml sterile serum-free DMEM and minced with a razor blade in a 100 mm petri dish. The DMEM medium was replaced with fresh serum-free DMEM containing Liberase DL (0.5 mg/ml), and the cell suspension was allowed to incubate at 37°C for 75 min. Following incubation, cells were centrifuged, washed three times with sterile PBS, re-suspended in DMEM containing 10% fetal bovine serum and antibiotic/antimycotic (Thermo Fisher Scientific), and plated in collagen-coated T-75 flasks. Mouse embryonic keratinocytes were isolated from the skin of embryonic day 18 mouse embryos using Neutral Protease (Dispase) (Worthington Biochemical Corporation, Lakewood, USA). Briefly, skin was peeled off, washed twice with sterile PBS, and incubated in Keratinocyte Growth Medium-2 (Lonza, Allendale, USA) containing antibiotic/antimycotic (Thermo Fisher Scientific). Following overnight incubation at 4°C, epidermis was carefully separated from dermis and placed in a sterile petri dish containing 2 ml trypsin (TrypLE Select Enzyme, Thermo Fisher Scientific) for 30 min at room temperature. Subsequently, trypsin was neutralized with Defined Trypsin Inhibitor (Thermo Fisher Scientific), the cell suspension was centrifuged, and cells were seeded in collagen-coated six-well plates.
AREG ELISA
MEKs seeded in collagen-coated six-well dishes were stimulated with 100 nM PMA (R&D Systems, Minneapolis, USA) for the indicated times. AREG protein levels in the conditioned medium were measured using a Mouse Amphiregulin DuoSet ELISA Developmental Kit (#DY989, R&D Systems). A spectrophotometer (SpectraMax 190, Molecular Devices, Sunnyvale, USA) was used to determine the optical density.
SDS/PAGE and immunoblotting
SDS/PAGE and immunoblotting assays were performed as described previously (Hosur et al., 2014). Briefly, MEFs grown in six-well dishes were lysed with RIPA buffer (Cell Signaling Technology) and protein concentrations were determined using a Qubit Fluorometer (Life Technologies). After loading 50 μg of protein onto 4-20% (wt/vol) precast gels (Lonza), proteins were transferred to a PVDF membrane, before blocking with 5% milk for 1 h at room temperature (RT). Membranes were then exposed to EGFR (#2232; 1:1000), phospho-ERK1/2 (#4370; 1:1000), phospho-AKT (#4060; 1:1000), phospho-S6 (#4858,; 1:1000 dilution) or actin (#4970; 1:1000) antibodies (Cell Signaling Technology) overnight at 4°C. Subsequently, membranes were washed in TBST for 2 h, exposed to secondary antibodies (Santa Cruz Biotechnology; 1:10,000) for 1 h at RT, and washed for 2 h in TBST. Membranes were then exposed to Luminol reagent (Santa Cruz Biotechnology) for 3 min and visualized using a gel documentation system (G:BOX F3, Syngene, Frederick, USA).
Immunohistochemistry
Immunohistochemical staining of skin sections with specific antibodies [phospho-p44/42 MAPK (ERK1/2) (#4370, Cell Signaling Technology; 1:400), phospho-mTOR (#2976, Cell Signaling Technology; 1:100) and Ki-67 (Thermo Fisher Scientific; prediluted)] was performed on a Ventana Discovery XT automated IHC research slide staining system (Roche, Tucson, USA). For antigen unmasking, deparaffinized slides were pretreated with Cell Conditioning Solution (Ki-67) or citrate buffer (10 mM Sodium Citrate, 0.05% Tween 20, pH 6.0) (phospho-ERK1/2 and phospho-mTOR). Ki-67 proliferation index was determined as described previously (Almeida et al., 2012).
Statistical analysis
GraphPad Prism v6 was used to analyze data. Student's t-test was used to assess the statistical difference between two groups. P<0.05 was considered statistically significant.
Acknowledgements
We thank Scientific Services at JAX for assistance with microinjections (Peter M. Kutny, Genetic Engineering Technologies) and histopathology, Rosalinda Doty for histopathological examination of H&E slides, Stephen B. Sampson for critical reading of the manuscript, and Zoë Reifsnyder for help with graphics.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: V.H.; Methodology: V.H.; Investigation: V.H., B.E.L.; Writing - original draft: V.H.; Writing - review & editing: V.H., L.D.S., M.V.W.; Supervision: L.D.S., M.V.W.; Funding acquisition: L.D.S., M.V.W.
Funding
This work was supported by the National Institutes of Health (CA034196 and R21 OD023800-01 to M.V.W.).
References
- Almeida J. S., Iriabho E. E., Gorrepati V. L., Wilkinson S. R., Gruneberg A., Robbins D. E. and Hackney J. R. (2012). ImageJS: personalized, participated, pervasive, and reproducible image bioinformatics in the web browser. J. Pathol. Inform. 3, 25 10.4103/2153-3539.98813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaydon D. C., Etheridge S. L., Risk J. M., Hennies H.-C., Gay L. J., Carroll R., Plagnol V., McRonald F. E., Stevens H. P., Spurr N. K. et al. (2012). RHBDF2 mutations are associated with tylosis, a familial esophageal cancer syndrome. Am. J. Hum. Genet. 90, 340-346. 10.1016/j.ajhg.2011.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke M. A., Etheridge S. L., Kaplan N., Simpson C., O'Toole E. A., Ishida-Yamamoto A., Marches O., Getsios S. and Kelsell D. P. (2014). iRHOM2-dependent regulation of ADAM17 in cutaneous disease and epidermal barrier function. Hum. Mol. Genet. 23, 4064-4076. 10.1093/hmg/ddu120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field E. A., Ellis A., Friedmann P. S., Leigh I. M. and Field J. K. (1997). Oral tylosis: a re-appraisal. Oral Oncol. 33, 55-57. 10.1016/S0964-1955(96)00052-8 [DOI] [PubMed] [Google Scholar]
- Hennies H.-C., Hagedorn M. and Reis A. (1995). Palmoplantar keratoderma in association with carcinoma of the esophagus maps to chromosome 17q distal to the keratin gene cluster. Genomics 29, 537-540. 10.1006/geno.1995.9971 [DOI] [PubMed] [Google Scholar]
- Hosur V., Johnson K. R., Burzenski L. M., Stearns T. M., Maser R. S. and Shultz L. D. (2014). Rhbdf2 mutations increase its protein stability and drive EGFR hyperactivation through enhanced secretion of amphiregulin. Proc. Natl. Acad. Sci. USA 111, E2200-E2209. 10.1073/pnas.1323908111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. R., Lane P. W., Cook S. A., Harris B. S., Ward-Bailey P. F., Bronson R. T., Lyons B. L., Shultz L. D. and Davisson M. T. (2003). Curly bare (cub), a new mouse mutation on chromosome 11 causing skin and hair abnormalities, and a modifier gene (mcub) on chromosome 5. Genomics 81, 6-14. 10.1016/S0888-7543(02)00013-7 [DOI] [PubMed] [Google Scholar]
- Saarinen S., Vahteristo P., Lehtonen R., Aittomäki K., Launonen V., Kiviluoto T. and Aaltonen L. A. (2012). Analysis of a Finnish family confirms RHBDF2 mutations as the underlying factor in tylosis with esophageal cancer. Fam. Cancer 11, 525-528. 10.1007/s10689-012-9532-8 [DOI] [PubMed] [Google Scholar]
- Siggs O. M., Grieve A., Xu H., Bambrough P., Christova Y. and Freeman M. (2014). Genetic interaction implicates iRhom2 in the regulation of EGF receptor signalling in mice. Biol. Open 3, 1151-1157. 10.1242/bio.201410116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens H. P., Kelsell D. P., Bryant S. P., Bishop D. T., Spurr N. K., Weissenbach J., Marger D., Marger R. S. and Leigh I. M. (1996). Linkage of an American pedigree with palmoplantar keratoderma and malignancy (palmoplantar ectodermal dysplasia type III) to 17q24. Literature survey and proposed updated classification of the keratodermas. Arch. Dermatol. 132, 640-651. 10.1001/archderm.1996.03890300056010 [DOI] [PubMed] [Google Scholar]