Abstract
Objectives/Hypothesis
Determine the effects on hearing of diabetes mellitus (DM) severity.
Study Design
We conducted a cross-sectional study among Veterans to investigate the relationship of diabetes severity and hearing in randomly selected subjects with (165) and without (137) DM and who had no more than a moderate hearing loss.
Methods
Subjects were classified by three age tertiles (<50, 50–56, and 57+ years). Diabetes severity was classified as insulin-dependent (IDDM), noninsulin-dependent (NIDDM), or no DM. Other DM measures included concurrent serum glucose, serum HbA1c, duration of disease, and several measures of DM-related complications. Pure-tone thresholds were measured in both ears of each subject at frequencies from 250 Hz through 14,000 Hz. Outcome measures were adjusted for age and frequency and analyzed for differences between subject groups using analysis of variance. Contrasts of the mean NIDDM and IDDM thresholds at each frequency to the DM group, and controlled for the effects of frequency, age, and interactions were modeled.
Results
There was greater hearing loss in younger tertile DM subjects compared to those without DM. Significant hearing differences were at all frequencies for NIDDM subjects, but for IDDM subjects, differences were at 1,000 Hz and below, and 10,000 Hz and above. Over age 50 years, there were significant associations between hearing at low frequencies and IDDM only. Self-report of prior noise exposure did not explain observed differences.
Conclusions
Diabetes is associated with an increased risk of hearing loss, and this difference is manifest particularly in adults <50 years old.
Keywords: Audiologic monitoring, hearing loss, Veterans
INTRODUCTION
Diabetes mellitus (DM) is a metabolic disease that produces complications of vascular and neurologic malfunction in those with the disorder. We have reported that Veterans with diabetes have more difficulty understanding speech than age-matched Veterans without diabetes, noted primarily among younger Veterans.1 There have also been reports that patients with diabetes have hearing loss greater than those without.2–4 The most convincing of these reports is a recent study by Bainbridge et al., who found that among 5,742 participants of the National Health and Nutrition Examination survey, those with diabetes evidenced greater hearing loss than those without.5 Participants aged 20 to 49 years had the greatest prevalence disparity of high-frequency impairment of mild or greater severity in the better ear.
In a comparison of electronic medical records of over 12,000 DM patients and over 53,000 age-matched controls from a Veterans Affairs database, sensorineural hearing loss was more common in patients with DM and seemed to correlate with the degree of elevated serum creatinine.6 In addition, it has been suggested that the severity of diabetes or the serum glucose level may be related to hearing loss.7 Few studies have examined the potential link between cochlear dysfunction and severity of diabetes, as assessed by treatment required for patients to manage their diabetes (oral hypoglycemic agents vs. insulin), or through measures of DM complications (neuropathy, retinal angiopathy, nephropathy), or of acute or intermediate measures of control (serum glucose, glycosylated hemoglobin, or HbA1c).
A number of studies have attempted to identify the source of hearing loss in those with DM, but to date the location of the lesions and the mechanism of deficit is not established. Suggested pathogenesis for this DM-associated sensorineural hearing loss has included cochlear microangiopathy, hyperglycemia of the cerebrospinal fluid or perilymph, auditory neuropathy, and diabetic encephalopathy. Fukushima et al.8 have described DM-associated pathology changes within the cochlea that include thickened vessels of the stria vascularis, atrophy of the stria vascularis, and loss of outer hair cells, but there was no loss of spiral ganglion cells compared to controls. The cochlear changes were more marked among those with insulin-dependent DM (IDDM) than those of the less severe noninsulin-dependent DM (NIDDM). Changes in central auditory and cognitive processing have also been documented in subjects with diabetes.9
Most studies of diabetes and hearing have employed rather small numbers of participants. Some previous studies have been primarily of older subjects, whereas those of relatively young subjects have been of type I DM patients almost exclusively. Studies with large numbers, which have included DM patients of all ages, have not included tests of the auditory system beyond establishing pure tone thresholds for the usual clinical audiogram frequencies. Furthermore, age-adjusted effects of diabetes severity have not been examined.
We undertook a cross-sectional study of Veterans with and without DM, to assess possible differences in the auditory system between the two groups, including peripheral and central auditory function and cognitive measures. We planned the current study to include DM patients primarily of type II, to include younger adults, to include tests of the entire auditory system, to include measures of DM severity, and to have a sample size sufficient to be able to distinguish clinically significant differences in hearing and speech understanding between those with and without DM. We hypothesized that DM of greater severity or longer duration, or both, adjusted for noise exposure and patient age, would be associated with higher thresholds for pure tones, and that non-noise hearing loss among DM patients would be associated with some other DM-related complication. This report addresses only the assessment of the hearing thresholds at 12 different frequencies among those participants.
MATERIALS AND METHODS
Subjects
Diabetes subjects
All 1,291 patients younger than 71 years old with diagnosed diabetes mellitus who received care for any reason through the Portland VA Medical Center (PVAMC) were eligible for the study. We selected three random samples without replacement, with the maximum age for the three samples of 70, 60, or 50 years, respectively, and we prioritized younger patients as the project progressed. Thus some patients in older eligible ages were randomly excluded. Those initially selected numbered 1,130. Eligible subjects were sent a recruitment letter, and those interested in participating responded by phone. Interested respondents were initially screened by phone for eligibility. A priori exclusions included those wearing a hearing aid, and those undergoing treatment for cancer, multiple sclerosis or other neurologic diseases, or with dementia or communication difficulties, leaving 184 patients eligible for in-person screening. We elected to exclude subjects with a hearing loss in both ears exceeding 40 dBHL at 2 kHz or 70 dBHL at 4 kHz so as to optimize chances of obtaining otoacoustic emissions and auditory brainstem responses. Subjects demonstrating tympanometry results outside standard clinical norms or air-bone gaps of greater than 10 dB were also excluded. Eighteen subjects were excluded because of this criterion leaving a total of 166 diabetes patients participating. All but one DM subject had type II DM.
Comparison subjects
We randomly selected three patient samples without replacement totaling 1,862 without diabetes from among 17,685 such patients receiving care at the PVAMC. We attempted to balance the age distribution of comparison subjects to be similar to that of the recruited diabetes group by using either 70 or 50 years as the maximum age. The age selection criterion truncated recruitment above age 70 years, but one 70-year-old subject had a birthday between recruitment and conduct of the test measures, and was allowed to participate. We applied the same prescreening and screening exclusion criteria as for those with diabetes. Of 154 willing participants, 16 were excluded on the basis of the hearing loss criterion, leaving 138 participants.
Demographic questionnaire data for two subjects (one with and one without diabetes) were missing. They were excluded from the analysis, leaving 302 subjects for study. All subjects were consented to participate in the study following the guidelines of the Portland VA Medical Center’s Institutional Review Board (IRB), signed an IRB-approved consent form, and were compensated for their time. No subjects with significant otitis or air-bone gap were included.
Data Collection
Subjects completed an initial questionnaire on demographic and selected medical items, which included questions associated with diabetes duration, treatment history, and complications, such as poor circulation, numbness, tingling, or burning sensation in hands or feet. An audiometric test of both ears was conducted including pure tone thresholds from 250 to 14,000 Hz, followed by stimulus frequency otoacoustic emissions (SFOAEs) recorded in the better hearing ear. For most subjects, immediately following the SFOAE tests, blood glucose and glycosylated hemoglobin (HbA1c) levels were determined. Blood glucose was determined using an AccuCheck Advantge glucometer (Roche, Basel, Switzerland) and HbA1c levels were determined using a Bayer DCA 2000+ Analyzer (Bayer Health-Care, Osaka, Japan). Each test required one drop of blood, obtained via finger stick. Peripheral neuropathy tests were conducted by the research audiologist trained in the standard method as performed in an annual diabetes exam. A standard clinical test of peripheral sensation was performed at specified locations on the feet using a 10-g nylon monofilament. Monofilament test data were recorded by hand and entered into an Microsoft Excel (Microsoft Inc., Redmond, WA) file by the examiner. These data were entered separately for 10 test spots (five on each foot). We reviewed participants’ medical charts to capture certain recent values for laboratory tests, including urinary microalbumin, and results of clinical exams, such as retinal findings. Retinopathy in this institution is described in standard lexicon, which we captured from the chart and scored on a six-point scale (0 = no retinopathy, 1 = mild nonproliferative-background, 2 = moderate nonproliferative-background, 3 = severe nonproliferative-background, 4 = quiescent proliferative, and 5 = proliferative retinopathy), which we used as an ordinal variable.
We also conducted additional tests, not used in this analysis, including speech in noise, time-compressed speech, several cognitive tests, a mood test, and tests of central auditory function performed at the same or a subsequent visit.
Pure tone threshold testing
Pure-tone air conduction thresholds were obtained for pulsed tones using a modified Hughson-Westlake procedure (Carhart and Jerger, 1959).10 Stimuli were presented by a Grason-Stadler GSI Clinical Audiometer (Grason-Stadler, Inc., Madison, WI) through Etymotic Research (ER3A) (Etymotic Research, Inc., Elk Grove Village, IL) insert earphones. Frequencies tested were standard audiometric frequencies from 0.25 to 8 kHz, and the interoctave frequencies 1.5, 3, and 6 kHz. Subjects also underwent extended high-frequency testing (10 kHz, 12.5 kHz, and 14 kHz) using Sennheiser HD-200 headphones (Sennheiser Electronic Corp., Old Lyme, CT). When no response was obtained for pure tone thresholds, the value recorded was arbitrarily set to the maximum output by the equipment at the test frequency and that imputed value was included in analyses.
Data Management
Data from the demographic questionnaire were captured by optically scanning the questionnaires. Data from all other sources were entered into Microsoft Excel files, then converted and combined into an analytic file using SPSS version 13 (SPSS, Inc., Chicago, IL). Initial data cleaning, variable conversions, data reduction, and preliminary analyses were done using SPSS 13.0. Definitive analyses were performed using SAS statistical software version 9.1.3 (SAS, Cary, NC).
Diabetes severity groups
Study participants were categorized into groups according to whether they had DM and whether they used insulin. This constituted the diabetes severity measure with 137 (45.4%) in the non-DM group, and 88 (29.1%) in the noninsulin-dependent (NIDDM) group, and 77 (25.5%) in the insulin dependent (IDDM) group. To confirm the measure’s ability to correctly classify severity, other items related to DM severity were compared across the DM severity groups. One such item was a composite scale computed to measure foot neuropathy. Subjects were asked, “Are you bothered by any of these sensations in your feet?” Choices included feelings of numbness, tingling, or burning sensations in their feet. Subjects were instructed to circle yes or no for each choice. The foot index score ranged from 0, “not bothered by sensations” to 3, “bothered by all three sensations.”
Age groups
We divided the total participants into nearly equal age tertiles. The youngest tertile (26–49 years) contained 35.8% of the participants; the middle (50–56 years) and oldest tertiles (57–71 years) contained 27.7% and 36%, respectively. When a pure tone threshold was found to be associated with diabetes severity, we examined tertile subgroups to determine if the association was distributed equally in our population or was centered in an age group.
Noise exposure
We computed five noise exposure scales using eight items from the initial questionnaire regarding noise exposure history and use of hearing protection. Subjects were asked, “How often did or does your military service cause you to be exposed to loud noises?” Then they were subsequently asked, “Were you wearing hearing protection when this exposure occurred?” Similar questions were asked for nonmilitary occupational exposures, recreational exposures, and sudden intense noise exposures. Responses for each of the noise exposure items ranged from 1, “never” to 5, “always.” Reponses for hearing protection items ranged from 1, “always” to 5, “never.” A composite scale was computed for each noise exposure category ranging from 2, “no noise exposure” to 10, “frequent noise exposure without hearing protection.” Finally, index values from the four queried sources of noise were combined into a total noise exposure scale ranging from 8, “no noise exposure” to 40, “frequent noise exposure without hearing protection” in all exposure categories.
Analysis
The main focus of this analysis was to examine measures of hearing (pure tone thresholds) by diabetes severity, while adjusting for age and stimulus frequency. Pure tone thresholds as an outcome measure were thus modeled, first in both ears, and for subsequent comparisons, in the better hearing ear used for SFOAE measures (n = 254). Pure tone threshold data were available for all subjects (n = 302) in both ears at all frequencies, though some thresholds (typically at frequencies of 10 kHz or greater) were assigned values as described above.
Diabetes severity (no diabetes, NIDDM, and IDDM) constituted the primary independent variable. Age was treated as a categorical value using the tertile groups. Stimulus frequency was also treated as a categorical predictor, with tests at 250, 500, 1,000, 1,500, 2,000, 3,000, 4,000, 6,000, 8,000, 10,000, 12,500, 14,000 Hz for the pure tone threshold model.
We used repeated measures analysis of variance (ANOVA) to test the effects of diabetes on pure tone thresholds. More specifically, the true mean outcome was modeled using a linear mixed model, with diabetes severity, age tertile, and test frequency as categorical main effects along with all 2-way and 3-way interactions.11 All 2- and 3-way interactions were also tested and reduced by backward elimination using a 0.1 P-value threshold. A subject-specific random effect was included to allow some subjects to have overall better (or worse) hearing. This is the general linear mixed model representation of the repeated measures ANOVA.
The accuracy of model tests and contrasts depends on the fit of the linear mixed model. Normal probability plots and histograms of the transformed residuals were generated for each of the fitted models, as well as boxplots of the transformed residuals by test frequency, age, and diabetes severity group. Results showed no gross deviations of the fitted model from the data, but that the variance of the residuals varied considerably among test frequencies (heteroscedasticity) for each outcome measure. This heteroscedasticity was resolved by including a log-linear model of the variance in the general linear model with test frequency as a predictor.12 This method stabilized the variance in the residuals so that they were constant across test frequencies.
We examined contrasts (differences) between mean pure tone thresholds in subjects with and without DM. Contrasts were derived directly from linear combinations of the estimated parameters in the model. In the absence of the random effects, the model reduces to a standard ANOVA, and tests of the age- and frequency-specific differences in pure tone thresholds between those with and without diabetes are identical to a two-sample t test conducted independently at each frequency. However, this approach ignores correlation among outcomes taken on the same subjects. Some people hear better than others, and thus have, on average, better outcomes than the sample average. Accordingly, linear mixed model-based contrasts are more appropriate and more consistent with the study design since the standard errors of the contrasts are a function of the correlation structure in the data, which is not the case when conducting independent t tests. In addition, the denominator degrees of freedom in each contrast is based on a Satterthwaite approximation.
A subset of subjects (n = 194) had blood glucose and HbA1c determinations immediately following SFOAE measures and within about 1 hour of pure tone tests. We examined those glucose values in conjunction with hearing results, and by tertile level.
RESULTS
Subject Characteristics
Characteristics of the 302 participants are shown in Table I. Male participants predominated (88.7%) and did not differ significantly among the three diabetes severity categories. Age was similarly distributed among the three groups, but education was higher in the IDDM group. Mean blood glucose and glycosylated hemoglobin, measures that reflect how well patients are able to manage their DM, differed significantly according to diabetes severity for all age groups. Self-reported noise exposure was high in this sample of Veterans patients, but did not differ significantly by diabetes severity, and so no adjustment was necessary in multivariate analyses.
TABLE I.
Characteristics of Study Participants.
| Diabetes Mellitus | |||||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| No Diabetes | NIDDM | IDDM | |||||
|
|
|
|
|||||
| Characteristic | No. | % | No. | % | No. | % | Significance |
| Number | 137 | 100 | 88 | 100 | 77 | 100 | |
| Age tertile, yr | .172* | ||||||
| 26–49, n = 115 | 49 | 35.8 | 35 | 39.8 | 31 | 40.3 | |
| 50–56, n = 95 | 38 | 27.7 | 27 | 30.7 | 30 | 39.0 | |
| 57–71, n = 92 | 50 | 36.5 | 26 | 29.5 | 16 | 20.8 | |
| Gender | .125* | ||||||
| Male | 116 | 84.7 | 81 | 92.0 | 71 | 92.2 | |
| Female | 21 | 15.3 | 7 | 8.0 | 6 | 7.8 | |
| Mean blood glucose, mg/dl | 107.0 | 176.0 | 223.0 | <.001† | |||
| SD | 18.8 | 92.0 | 117.7 | ||||
| Missing | 3 | 2.2 | 1 | 1.1 | 3 | 3.4 | |
| Mean HbA1c | 5.35 | 7.12 | 7.94 | <.001† | |||
| SD | 0.48 | 1.78 | 2.03 | ||||
| Missing | 6 | 4.4 | 1 | 1.1 | 3 | 3.4 | |
| Mean duration of diabetes, yr | NA | 5.45 | 11.83 | <.001† | |||
| SD | NA | 3.83 | 6.64 | ||||
| Missing | NA | 3 | 3.4 | 5 | 6.5 | ||
| Education | .03* | ||||||
| Some high school or less | 7 | 5.1 | 6 | 6.8 | 5 | 6.5 | |
| Completed high school | 23 | 16.8 | 22 | 25.0 | 8 | 10.4 | |
| Post high school | 59 | 43.0 | 39 | 44.3 | 47 | 61.0 | |
| Completed college | 48 | 35.0 | 19 | 21.6 | 17 | 22.1 | |
| Missing | 0 | 0.0 | 2 | 2.3 | 0 | 0.0 | |
| Noise exposure history | |||||||
| Military noise index mean | 6.84 | 6.99 | 7.11 | .483† | |||
| Civil noise index mean | 6.26 | 6.39 | 6.42 | .735† | |||
| Recreational noise index mean | 6.12 | 6.33 | 6.16 | .563† | |||
| Sudden noise index mean | 6.42 | 6.40 | 6.56 | .692† | |||
| Total indices mean | 25.7 | 26.27 | 26.32 | .504† | |||
| Missing | 9 | 6.6 | 6 | 6.8 | 6 | 8.9 | |
Significance of distribution differences calculated by summary chi-square.
Significance of mean differences calculated by one-way ANOVA.
NIDDM = noninsulin-dependent diabetes mellitus; IDDM = insulin-dependent diabetes mellitus; SD = standard deviation; NA = nonapplicable.
To confirm the validity of the DM severity measure, we compared other items related to diabetes severity across the three DM severity groups for each age tertile (Table II). The mean values did not show a trend by age group. Retinopathy, and peripheral neuropathy were not common in any of the groups; nevertheless the mean scores for all these complications were significantly higher in the IDDM group than in the non-DM group. Serum creatinine follows the same pattern, but was significant only for the middle and oldest tertiles (not shown). There was a statistically significant association between the prevalence of nephropathy, as judged by the presence of microalbuminuria above normal limits, and DM within each age category. However, the prevalence of nephropathy was not consistently higher among IDDM than NIDDM across age categories. Duration of diabetes was significantly longer for the IDDM group in each age tertile (data not shown).
TABLE II.
Comparison of Mean Diabetes Mellitus Complications by Diabetes Status and Insulin Dependence.
| Complication by Tertile | No Diabetes | NIDDM | IDDM |
|---|---|---|---|
| Retinopathy score right eye; range, 0–5* | |||
| Youngest | 0.00 | 0.00 | 0.71† |
| Middle | 0.00 | 0.19 | 0.73‡ |
| Oldest | 0.00 | 0.08 | 0.56‡ |
| Retinopathy score left eye; range, 0–5* | |||
| Youngest | 0.00 | 0.00 | 0.71‡ |
| Middle | 0.00 | 0.19 | 0.77‡ |
| Oldest | 0.00 | 0.04 | 0.44‡ |
| Microalbuminuria level high, % high§ | |||
| Youngest‡ | 0.0 | 29.0 | 44.0 |
| Middle‡ | 2.6 | 34.8 | 20.7 |
| Oldest‡ | 0.0 | 45.8 | 57.1 |
| Feet numb, tingling, or burning index; range, 0–3* | |||
| Youngest | 0.68 | 1.03 | 1.48† |
| Middle | 0.92 | 1.44 | 1.93† |
| Oldest | 0.60 | 0.63 | 1.00‖ |
Significance of analysis of variance comparison to No Diabetes group (Bonferroni correction for multiple comparisons within tertile).
P ≤ .01.
P ≤ .001.
Significance of chi-square test of independence among diabetes groups.
P ≤ .05.
NIDDM = noninsulin-dependent diabetes mellitus; IDDM = insulin-dependent diabetes mellitus.
Blood glucose levels ranged from 39 to 600 mg/dL and, among all ages, the pure tone threshold at 250 and 500 Hz were significantly correlated (0.153, P = .039; and 0.226, P = .002, respectively) with blood glucose. Also, HbA1c was significantly correlated (0.148, P = .048) with the threshold at 500 Hz. These correlations were entirely due to stronger correlations in the youngest tertile only, and were not seen in the two older tertiles. In the younger tertile, blood glucose level correlations with the two lowest frequency pure tone thresholds (250 and 500 Hz) were 0.234, P = .041 and 0.334, P = .003, respectively. HbA1c was correlated with the 500 Hz threshold (0.253, P = .027).
Pure Tone Threshold Comparisons by DM Severity Groups, Age, and Test Frequencies
Table III provides the pure tone threshold results for only the better hearing ears (n = 254), and for each age tertile, stratified by diabetes severity. For all age groups, mean thresholds in each DM severity group increased (become poorer) with increased test frequency. In the youngest tertile, these unadjusted measures also were consistently higher for both DM groups than for those with no DM, but that was not true for the middle and older tertiles. Threshold variability differs considerably among test frequencies, particularly near the highest audible frequencies. On average, younger subjects had slightly better hearing compared with older subjects.
TABLE III.
Mean Pure Tone Thresholds by Diabetes Severity, Youngest Age Tertile (27–49 Years).
| Right Ear (n = 115) | ||||||
|
|
||||||
| No Diabetes (n = 49) | NIDDM (n = 35) | IDDM (n = 31) | ||||
|
|
|
|
||||
| Pure Tone Threshold | Mean | SD | Mean | SD | Mean | SD |
|
| ||||||
| 250 | 8.57 | 6.46 | 11.43 | 6.25 | 13.71* | 7.41 |
| 500 | 10.20 | 6.29 | 16.29† | 7.61 | 17.26† | 6.69 |
| 1,000 | 10.82 | 5.81 | 16.14† | 6.54 | 14.52‡ | 5.82 |
| 1,500 | 10.82 | 6.48 | 16.14‡ | 7.48 | 13.55 | 6.73 |
| 2,000 | 12.86 | 7.30 | 17.29‡ | 7.70 | 15.16 | 9.08 |
| 3,000 | 13.88 | 6.87 | 21.14* | 13.07 | 18.23 | 11.30 |
| 4,000 | 16.43 | 15.68 | 24.43‡ | 17.14 | 18.87 | 11.95 |
| 6,000 | 19.29 | 15.84 | 24.00 | 18.30 | 20.00 | 13.35 |
| 8,000 | 16.94 | 17.91 | 22.43 | 18.96 | 21.45 | 17.14 |
| 10,000§ | 24.29 | 21.16 | 36.14‡ | 23.67 | 37.90‡ | 20.07 |
| 12,500§ | 31.73 | 22.65 | 45.00‡ | 27.03 | 45.81‡ | 19.07 |
| 14,000§ | 41.73 | 22.58 | 51.42 | 26.89 | 56.13‡ | 17.69 |
| Left Ear (n = 115) | ||||||
|
|
||||||
| No Diabetes (n = 49) | NIDDM (n = 35) | IDDM (n = 31) | ||||
|
|
|
|
||||
| Pure Tone Threshold | Mean | SD | Mean | SD | Mean | SD |
|
| ||||||
| 250 | 9.49 | 6.31 | 14.86† | 5.88 | 14.19* | 7.21 |
| 500 | 10.33 | 6.19 | 17.43† | 6.23 | 16.13* | 7.04 |
| 1,000 | 11.43 | 6.29 | 17.57† | 5.99 | 13.23 | 7.02 |
| 1,500 | 10.31 | 7.67 | 17.57† | 6.68 | 13.23 | 7.02 |
| 2,000 | 13.47 | 7.45 | 19.43* | 8.02 | 15.00 | 7.42 |
| 3,000 | 16.53 | 11.65 | 24.00‡ | 13.33 | 17.90 | 11.46 |
| 4,000 | 21.22 | 15.80 | 31.00‡ | 18.46 | 20.32 | 11.90 |
| 6,000 | 21.73 | 15.76 | 29.09 | 19.02 | 20.65 | 12.02 |
| 8,000 | 18.78 | 17.06 | 25.00 | 17.86 | 21.29 | 16.78 |
| 10,000§ | 24.90 | 21.47 | 37.29‡ | 24.17 | 34.52 | 21.38 |
| 12,500§ | 35.71 | 24.45 | 46.71 | 26.79 | 46.13 | 23.12 |
| 14,000§ | 42.55 | 24.77 | 53.14 | 23.17 | 53.71 | 17.93 |
P ≤ .01 compared to no diabetes.
P ≤ .001 compared to no diabetes.
P ≤ .05 compared to no diabetes.
Thresholds placed at limits of equipment +5 dB.
NIDDM = noninsulin-dependent diabetes mellitus; IDDM = insulin-dependent diabetes mellitus; SD = standard deviation.
Since degradation of pure tone thresholds was expected (and found) with increases in the test frequency and the age of the subject, we examined the relationship between pure tone thresholds and DM, adjusting for these factors. A 3-way repeated measures ANOVA was performed for pure tone thresholds obtained in the three DM severity groups, the three age tertiles, and the 12 test frequencies as categorical main effects along with all 2-way and 3-way interactions.
Table IV presents type 3 test statistics for the repeated measures ANOVA with pure tones. Results show that the factors included in the model were important predictors of pure tone threshold. DM severity appears to have an important effect on hearing even after adjusting for the age of the patient, but the magnitude of that effect depends on the age of the patient and the test stimulus frequency. Main effects of DM severity (F2,360 = 8.14; P = .0003), age tertile (F2,360 = 61.61; P < .0001), and test frequency (F2,360 = 835.47; P < .0001) were each significant. The interaction of DM severity by test frequency was not significant (F11,956 = 1.44; P > .05). In contrast, the DM severity by age tertile interaction was significant (F4,360 = 4.11; P = .0028), as was the test frequency by age tertile interaction (F22,956 = 23.44; P < .0001), and the 3-way (DM severity by age by test frequency) interaction (F44,956 = 2.22; P < .0001).
TABLE IV.
Type 3 Tests of the Independent Variables Included in the Repeated Measures Analysis of Variance.
| Effect | Numerator Degrees of Freedom |
Denominator Degrees of Freedom |
F Statistics | P Value |
|---|---|---|---|---|
| Diabetes severity | 2 | 360 | 8.14 | .0003 |
| Age tertile | 2 | 360 | 61.61 | <.0001 |
| Pure tone test frequency* | 11 | 956 | 835.47 | <.0001 |
| Pure tone test frequency × age tertile | 22 | 956 | 23.44 | <.0001 |
| Pure tone test frequency × diabetes severity | 22 | 956 | 1.44 | .0854 |
| Diabetes severity × age tertile | 4 | 360 | 4.11 | .0028 |
| Diabetes severity × pure tone test frequency × age tertile | 44 | 956 | 2.22 | <.0001 |
Both ears used were used (n = 604).
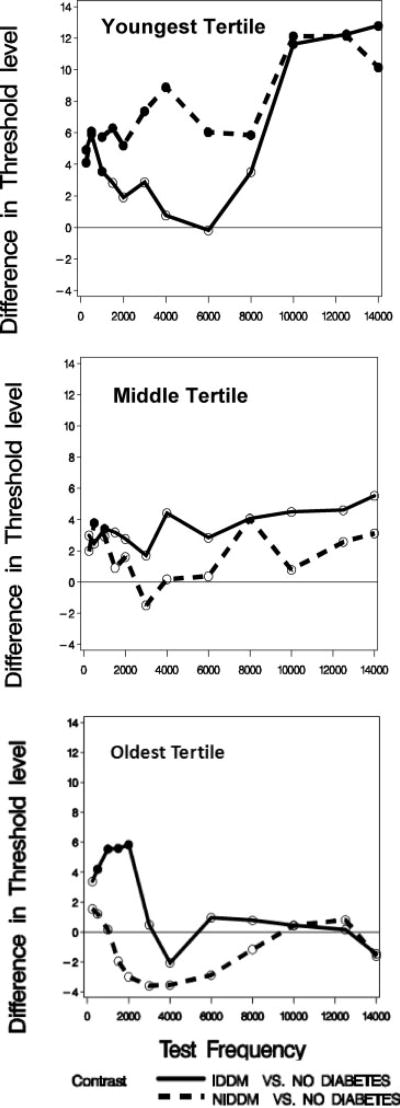
Contrasts comparing mean pure tone threshold between the non-DM and IDDM groups, and between the non-DM and the NIDDM groups, thus depend on the age group under consideration and the stimulus frequency. Figure 1 shows pure tone threshold results of all participants (n = 302) based on both ears (n = 604 ears). Separate panels present data for the (Fig. 1A) youngest, (Fig. 1B) middle, and (Fig. 1C) oldest age tertiles, respectively. The parameter in each panel is DM severity, and plotted values for NIDDM and IDDM represent the contrast (modeled difference of mean thresholds, in dB) between those groups and the non-DM group at that frequency. The solid line in each figure represents the contrasts between IDDM and non-DM groups, and the dashed line shows the contrasts for NIDDM versus non-DM groups. The horizontal line at zero on the vertical axis represents no difference between the two contrasted groups. Positive values correspond to worse hearing among those with DM. Filled circles indicate contrasts that are statistically significantly different from zero at the 0.05 test level. Empty circles indicate contrasts that are not significantly different from zero.
Fig. 1.
Mean pure tone threshold differences between subjects with insulin-dependent diabetes mellitus (IDDM) or noninsulin-dependent diabetes mellitus (NIDDM) and those without diabetes. Differences are plotted by frequency. (A) Youngest age tertile. (B) Middle age tertile. (C) Oldest age tertile.
The general pattern of results is similar to that shown in Table III, in that subjects with DM had significantly greater pure tone thresholds at certain frequencies compared with subjects without DM. However, threshold differences at the lower frequencies were larger for the NIDDM group, and there were statistically significant differences between NIDDM and IDDM groups in this sample. All age groups showed significant differences in mean pure tone thresholds between those with IDDM and those without DM at some of the lowest frequencies. The youngest age group showed additional important contrasts. Those with NIDDM have significantly worse hearing (higher mean pure tone thresholds) than those with no DM across all test frequencies. In addition, in the youngest tertile those with IDDM have significantly worse hearing than do those without DM at higher frequencies (10000 Hz and above).
DISCUSSION
Summary
The overall purpose of our study was to investigate the locations of DM-associated lesions in the auditory system. This report addresses hearing threshold effects of DM severity, controlling for confounding effects of age, and considering subjects’ previous noise exposure. In our study population of Veterans without severe hearing loss, we confirm that those with DM have more pure tone hearing loss than those without. The hearing difference between those with and those without DM was most marked among those under 50 years of age, particularly at the higher frequencies (>10,000 Hz). In the youngest tertile, thresholds for the NIDDM group were also significantly higher than for the IDDM group at several frequencies, but most at 4,000 Hz.
We also found significant associations between DM and hearing loss in those over 50 years, but in older subjects the differences were limited to certain frequencies within the range from 250 to 2,000 Hz. Since the index of noise exposure history did not differ among comparison groups, it could not contribute to the difference observed in hearing thresholds.
Effect of Age Group and DM Severity
Pure tone thresholds
NIDDM subjects under age 50 years, compared to those without DM, had significantly worse hearing at all tested frequencies, whereas for those with IDDM significant losses were limited to frequencies under 2,000 and over 8,000 Hz. For the frequencies of 1,500, 4,000, and 6,000, IDDM and NIDDM thresholds were significantly different from each other. When the hearing thresholds in all age tertiles and DM severity groups were computed (Table IV), diabetes severity, age tertile, and pure tone frequency all had independent effects on the threshold. In addition, among all the possible 2-way and 3-way interactions, only the possible interaction between pure tone frequency and diabetes severity did not evidence a significant separate effect.
Among Veterans aged 50 to 56 years old, there was a modest DM-associated hearing deficit in the lower frequencies (250–500 Hz) for both NIDDM and IDDM. Among those from 57 to 71 years old, a low frequency deficit occurred (500–2,000 Hz) in those with IDDM only, whereas those with NIDDM had nonsignificantly better hearing than those without DM from 1,500 to 8,000 Hz. Thus in the middle and oldest tertiles, only patients with the more severe form of DM (IDDM) had significantly poorer hearing compared with subjects free from DM. Together, these findings suggest that among younger patients, NIDDM has a broader effect than IDDM, but both levels of DM severity have significant effects on low and high frequencies. Among older patients, only the low frequency effects are seen and primarily for the more severe IDDM.
Our measure of DM severity was whether or not patients with DM used insulin. Approximately 47% of our subjects with DM (45% of those under 50 years old) used insulin. The severity of DM was significantly associated with the amount of hearing loss in the younger subjects, but it was not the most severe DM subgroup that had the largest deficit. In fact, in the under 50 years group, the NIDDM group had significantly worse hearing at intermediate frequencies than the non-DM group, whereas those using insulin (IDDM) had hearing nearly identical to those without DM in the range 2,000–8,000 Hz. If IDDM is actually a more severe form of DM, as is clinically accepted, and is suggested by the fact that glucose levels, HbA1c, and other complications of DM (foot neuropathy, retinopathy, and nephropathy) were worse in the IDDM group than in the NIDDM group, then the hearing threshold findings in the youngest tertile are the reverse of the expected DM-associated auditory deficit. The differences between NIDDM and IDDM results for the youngest tertile notwithstanding, subjects with DM generally had poorer hearing in our study compared to those without. Older adults with IDDM had excess hearing loss at the low frequencies.
It seems important to us that DM-associated differences in the lowest tertile were most marked at the higher frequencies, not routinely tested in audiograms. Testing hearing at >8,000 Hz may be a useful measure of preclinical hearing loss among DM subjects under age 50 years.
The finding that in the youngest tertile pure tone thresholds were different between the two DM severity groups, and that those with NIDDM were the more severely affected, was contrary to our original hypothesis. We had hypothesized that with increasing diabetes severity hearing would worsen, at least for Veterans under 50 years.
It is possible that IDDM is not the more severe subgroup of diabetes, or that insulin provides some relative protective effect on hearing threshold not related to its effect on blood sugar or on other DM complications. Another possibility is that the disparity between hearing thresholds in the younger group is either a chance finding or due to some selection bias and will not be confirmed in other studies. Nonetheless, if IDDM is actually a more severe form of DM, as is clinically accepted, and is suggested by the fact that glucose levels, HbA1c, and other complications of DM (foot neuropathy, retinopathy, and nephropathy) were worse in the IDDM group than in the NIDDM group, then the hearing threshold findings are the reverse of the expected DM-associated auditory deficit.
Possible Effects Elevated Blood Glucose
We were able to examine some of the prevailing hypotheses that may account for the DM-associated hearing loss. One hypothesis is that the level of glucose in the endolymph, as a reflection of ambient blood glucose, may be responsible for the findings (Hirose 2008). If so, the pure tone deficit should be directly related to the blood glucose taken in the same session. In our study, blood glucose values ranged from 39 to 600 mg/dL. There was some support for that hypothesis, in that the two lowest frequency pure tone thresholds were significantly related to a glucose level measured about 1 hour earlier. We considered the possibility that repeated acute hyperglycemia episodes could have a chronic effect on cochlear structures or fluid homeostasis over time. If so, we reasoned that the duration of DM would be a strong predictor of the hearing effect.
Another possibility is that the effect is mediated through strial damage and/or outer hair cell damage.
In the present study, there was evidence that the relationship between DM and hearing was different in subjects, depending upon the age tertile and with different severity of DM. In the youngest tertile compared to those without DM, the hearing loss was greater for NIDDM subjects. For subjects in the middle tertile, differences in hearing among subjects with and without DM were minimal, and for the oldest tertile, hearing was better among DM subjects.
Limitations
Our Veterans population is limited to those who have served in the military. This precludes children and also excludes the large majority of those with type I diabetes.
We considered the possibility that noise-related hearing losses might account for the association of hearing thresholds with NIDDM in the youngest tertile, but not in older groups. That possibility is not supported by the finding of no differences in noise exposure, by self-report, among the three DM groups. It is possible that our questionnaire failed to detect noise-related hearing loss differences, but if so, one must hypothesize that subjects with NIDDM in the youngest group had excess noise exposure at all frequencies, whereas those with IDDM in the youngest, middle, and oldest groups had excess noise exposure in the lowest frequencies, in order for differential noise exposures to account for the observed differences in hearing thresholds. That possibility does not seem plausible.
Audiograms cannot distinguish between cochlear and retrocochlear perceptive hearing loss, and our findings may reflect either or both of these possibilities. Whether additional auditory deficits occur in the auditory nerve or may even occur higher up in the auditory pathway, but are not evident in pure tone threshold responses, is not addressed in this analysis.
CONCLUSION
Our results confirm previous reports that DM is associated with an increased risk of hearing loss in younger adults, at least in patients with DM, and no more than a moderate hearing loss. In older adults, in contrast to those under 57 years, DM associated hearing loss was only in the low frequencies and in IDDM subjects. In the youngest age group there was some support for the hypothesis that ambient glucose level within the range 39 to 600 mg/dL was related to hearing, at least in the very low frequency range. HbA1c was less strongly correlated to hearing threshold in this group and for 500 Hz only.
Acknowledgments
The authors acknowledge contributions to the study design and management of this project by Nancy E. Vaughan. We thank Peter G. Jacobs and Aynun Naher, VA RR&D, National Center for Rehabilitative Auditory Research, for programming and data collection assistance.
This work was supported by the United States Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development Rehabilitation Research and Development Service grants C3446R and C4447K.
Footnotes
Presented in part at the Triological Society Southern and Middle Combined Sections Meeting, Bonita Springs, Florida, U.S.A, January 8–11, 2009.
BIBLIOGRAPHY
- 1.Vaughan N, James K, McDermott D, Griest S, Fausti S. A 5-year prospective study of diabetes and hearing loss in a veteran population. Otol Neurotol. 2006;27:37–43. doi: 10.1097/01.mao.0000194812.69556.74. [DOI] [PubMed] [Google Scholar]
- 2.Cullen JR, Cinnamond MJ. Hearing loss in diabetics. J Laryngol Otol. 1993;107:179–182. doi: 10.1017/s0022215100122571. [DOI] [PubMed] [Google Scholar]
- 3.Lisowska G, Namyslowski G, Morawski K, Strojek K. Early identification of hearing impairment in patients with type 1 diabetes mellitus. Otol Neurotol. 2001;22:316–320. doi: 10.1097/00129492-200105000-00008. [DOI] [PubMed] [Google Scholar]
- 4.Frisina ST, Mapes F, Kim S, Frisina DR, Frisina RD. Characterization of hearing loss in aged type II diabetics. Hear Res. 2006;211:103–113. doi: 10.1016/j.heares.2005.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bainbridge KE, Hoffman HJ, Cowie CC. Diabetes and hearing impairment in the United States: audiometric evidence from the National Health and Nutrition Examination Survey, 1999 to 2004. Ann Intern Med. 2008;149:1–10. doi: 10.7326/0003-4819-149-1-200807010-00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kakarlapudi V, Sawyer R, Staecker H. The effect of diabetes on sensorineural hearing loss. Otol Neurotol. 2003;24:382–386. doi: 10.1097/00129492-200305000-00006. [DOI] [PubMed] [Google Scholar]
- 7.Hirose K. Hearing loss and diabetes: You might not know what you’re missing. Ann Intern Med. 2008;149:54–55. doi: 10.7326/0003-4819-149-1-200807010-00232. [DOI] [PubMed] [Google Scholar]
- 8.Fukushima J, Cureolglu S, Schachern PA, Paparella MM, Harada T, Mehet OF. Effects of type 2 diabetes on cochlear structure in humans. Arch Otolaryngol Head Neck Surg. 2006;132:934–938. doi: 10.1001/archotol.132.9.934. [DOI] [PubMed] [Google Scholar]
- 9.Lisowska G, Namyslowski G, Morawski K, Strojek K. Cochlear dysfunction and diabetic microangiopathy. Scand Audiol. 2001;30(suppl 52):199–203. doi: 10.1080/010503901300007524. [DOI] [PubMed] [Google Scholar]
- 10.Carhart R, Jerger J. Preferred method for clinical determination of pure-tone thresholds. J Speech Hear Dis. 1959;16:340–345. [Google Scholar]
- 11.Fitzmaurice GM, Laird NM, Ware JH. Applied Longitudinal Analysis. Hoboken, NJ: John Wiley & Sons; 2004. [Google Scholar]
- 12.Aitkin M. Modeling variance heterogeneity in normal regression using GLIM. J Appl Stat. 1987;36:332–339. [Google Scholar]