Abstract
The Middle East Respiratory Syndrome-coronavirus (MERS-CoV) causes a highly lethal pneumonia. MERS was recently identified as a candidate for vaccine development but most efforts focus on antibody responses, which are often transient after CoV infections. CoV-specific T cells are generally long-lived but the virus-specific T cell response has not been addressed in MERS patients. Here, we obtained PBMCs and/or sera from 21 MERS survivors. We detected MERS-CoV-specific CD4 and CD8 T cell responses in all MERS survivors and demonstrated functionality by measuring cytokine expression after peptide stimulation. Neutralizing (PRNT50) antibody titers measured in vitro predicted serum protective ability in infected mice and correlated with CD4 but not CD8 T cell responses; patients with higher PRNT50 and CD4 T cell responses had longer ICU stays and prolonged virus shedding and required ventilation. Survivors with undetectable MERS-CoV-specific antibody responses mounted CD8 T cell responses comparable to those of the whole cohort. There were no correlations between age, disease severity, co-morbidities and virus-specific CD8 T cell responses. In conclusion, measurements of MERS-CoV-specific T cell responses may be useful for predicting prognosis, monitoring vaccine efficacy and identifying MERS patients with mild disease in epidemiological studies and will complement virus-specific antibody measurements.
Introduction
The Middle East Respiratory syndrome-coronavirus (MERS-CoV), recently emerged from zoonotic sources, causes severe pneumonia in patients in the Middle East and in travelers from this region (1). As of 27 April, 2017, 1936 cases with 690 deaths (35.6% case fatality rate) had been reported to the WHO. MERS-CoV, like the coronavirus that caused the Severe Acute Respiratory Syndrome (SARS-CoV), has the potential to cause widespread outbreaks, as occurred in 2015 in South Korea (2). In this instance, a single patient with MERS entered the country, resulting in 186 secondary and tertiary cases and quarantining of approximately 16,000 individuals (2). Further, unlike SARS-CoV, MERS-CoV continues to be introduced from infected intermediates, most importantly dromedary camels, to human populations (3). These observations indicate the need for understanding the human immune response to the virus in order to guide immunotherapy of severely ill patients and vaccine development, and to develop additional tools for determining the prevalence of the infection.
While clinical MERS has been well described, materials from autopsy specimens are available only for a single patient (4). Additionally, the MERS-CoV-specific immune response is not well characterized. In particular, it is known that virus-specific antibody responses can be identified in many but not all infected patients and is only transiently detected in some patients with pneumonia (5–7). In contrast, nothing is known about the T cell response to the virus, about how disease severity impacts this response and about the correlation of anti-virus antibody with T cell responses. In SARS survivors, virus-specific antibody responses could no longer be detected at 6 years after infection, while T cell responses could be detected as long as 11 years after infection (8). Further, administration of convalescent sera is considered a potential therapeutic option (9), but nothing is known about levels of virus-specific antibody that are protective.
We report the first analysis of the MERS-CoV-specific T cell responses in patients and show that CD8 T cell responses can be detected in some patients with undetectable antibody responses. Our results also provide the first correlation between neutralizing antibody titers measured in vitro and protective levels in vivo. We provide information about the correlation of virus-specific antibody and T cell responses with clinical parameters and identify T cell epitopes recognized in some patients. These results have implications for prediction of patient outcomes, for epidemiological studies of the infection and for therapeutic use of convalescent sera in patients.
Results
We obtained PBMCs and sera from 18 MERS survivors and sera from an additional 3 subjects. Samples were obtained at 6 and 24 months after infection from 14, 4 and 3 patients in Riyadh, Jeddah and Mekkah, respectively. Patient demographics and laboratory values including the cellular composition of PBMCs are shown in Tables S1 and S2 (gating strategy is shown in Fig. S1). Patients required hospitalization at approximately seven days after the development of symptoms. These patients were tested serially and remained positive for MERS-CoV RNA for periods ranging from 7 to 45 days. Clinical severity ranged from asymptomatic/subclinical to severe, with most patients with severe disease requiring ICU care and ventilation. Of the 18 patients who provided PBMCs, three patients were asymptomatic, six patients had pneumonia and nine patients had severe pneumonia, requiring intubation and ventilation. Patients remained in the ICU for 2 to 74 days. All patients were discharged from the hospital by 174 days after admission. We also measured hepatic and renal function and found that, in general, renal and hepatic abnormalities were more common in patients with more severe respiratory disease (Table S1).
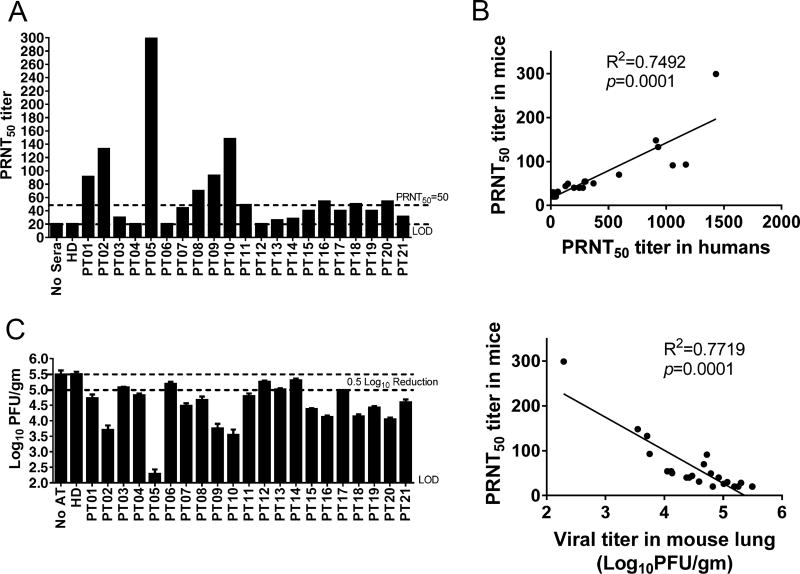
Next, we analyzed sera for bulk MERS-CoV-specific antibodies, using ELISA and IFA, and for neutralizing antibodies, using infectious MERS-CoV in microneutralization, and plaque reduction (PRNT50) assays (Table 1). Titers measured by the four different methods were generally consistent within individual patients. MERS-CoV specific antibodies were undetectable or very low in three asymptomatic patients (Patients 12–14) and in four patients with pneumonia or severe pneumonia (Patients 3,4,7,21). This relationship between low antibody responses and less severe clinical disease was also observed in previous studies (5, 6). To further assess the physiological significance of the magnitude of the neutralizing antibody titers, we transferred 75 µL of antibody from individual patients to mice sensitized for MERS-CoV infection using non-replicating adenovirus vectors expressing the human receptor (human dipeptidyl peptidase, Ad5-hDPP4) (10, 11). As shown in Fig. 1A–B and Table 1, PRNT50 titers in the sera of recipient mice correlated well with titers in the human sera. More importantly, mouse PRNT50 titers in the sera at the time of challenge correlated inversely with virus titers in the lungs, confirming the importance of neutralizing antibody assessed in vitro in virus clearance in vivo (Fig. 1C). These results also suggest that a PRNT50 of >1:50 was required to reduce virus titers by 0.5 log in infected mice. Since transfer of 75 µL of sera to a 25 gm mouse is equivalent to transferring 210 ml sera to a 70 kg patient (calculated on a per kg basis), these data provide a framework for its use in clinical settings.
Table 1.
Serological testing
| PT ID | ELISA result | ELISA | IFA | IFA titer | MicroNT titer | PRNT50 |
|---|---|---|---|---|---|---|
| PT01 | Positive | 12.3 | Positive | 100 | 63.5 | 1057 |
| PT03 | Negative | 0.23 | Negative | <1:10 | ≤10 | ≤20 |
| PT05 | Positive | 6.1 | Positive | 100 | 226.3 | 1432 |
| PT08 | Positive | 4.1 | Positive | 100 | 100.8 | 592 |
| PT09 | Positive | 4.93 | Positive | 100 | 226.3 | 1170 |
| PT10 | Positive | 5.1 | Positive | 100 | 201.6 | 912 |
| PT11 | Positive | 2.3 | Positive | 1:10 | 25.2 | 148 |
| PT18 | Positive | 2.2 | Positive | 100 | 50.4 | 370 |
| PT19 | Positive | 2.98 | Positive | 100 | 40 | 278 |
| PT02 | Positive | 2.02 | Positive | 1:10 | 80 | 930 |
| PT04 | Borderline | 0.87 | Positive | 1:10 | ≤10 | 31 |
| PT06 | Positive | 1.34 | Positive | 100 | 15.9 | 43.5 |
| PT07 | Borderline | 0.97 | Negative | <1:10 | ≤10 | 128 |
| PT20 | Positive | 4.4 | Positive | 1:10 weak | 40 | 293 |
| PT21 | Positive | 1.17 | Borderline | 1:10 weak | ≤10 | 61 |
| PT12 | Negative | 0.56 | Negative | <1:10 | ≤10 | ≤20 |
| PT13 | Negative | 0.36 | Negative | <1:10 | ≤10 | ≤20 |
| PT14 | Negative | 0.38 | Negative | <1:10 | ≤10 | ≤20 |
| PT15 | N.D. | N.D. | N.D. | N.D. | 28.3 | 200 |
| PT16 | Positive | 3.4 | Positive | 100 | 100.8 | 301 |
| PT17 | Positive | 1.85 | Positive | 100 | 25.2 | 247 |
N.D.-Not done



PBMCs not available (black)
Figure 1. Convalescent sera transfer protects mice from MERS-CoV infection.
(A) Mice received 75 µl of patient serum intravenously (i.v.) 12 hours before MERS-CoV infection. One hour prior to infection, mice sera were collected and PRNT50 assays were performed as described in Procedures. (B) Relationship between PRNT50 in human sera and in mouse recipients of transferred sera. (C) To obtain virus titers, lungs were homogenized at day 3 p.i. and titered on Vero 81 cells. Titers are expressed as PFU/g tissue. n= 3 mice/group/time point. LOD-limit of detection (Left). Relationship between PRNT50 in mouse sera and viral titers in mouse lungs (Right).
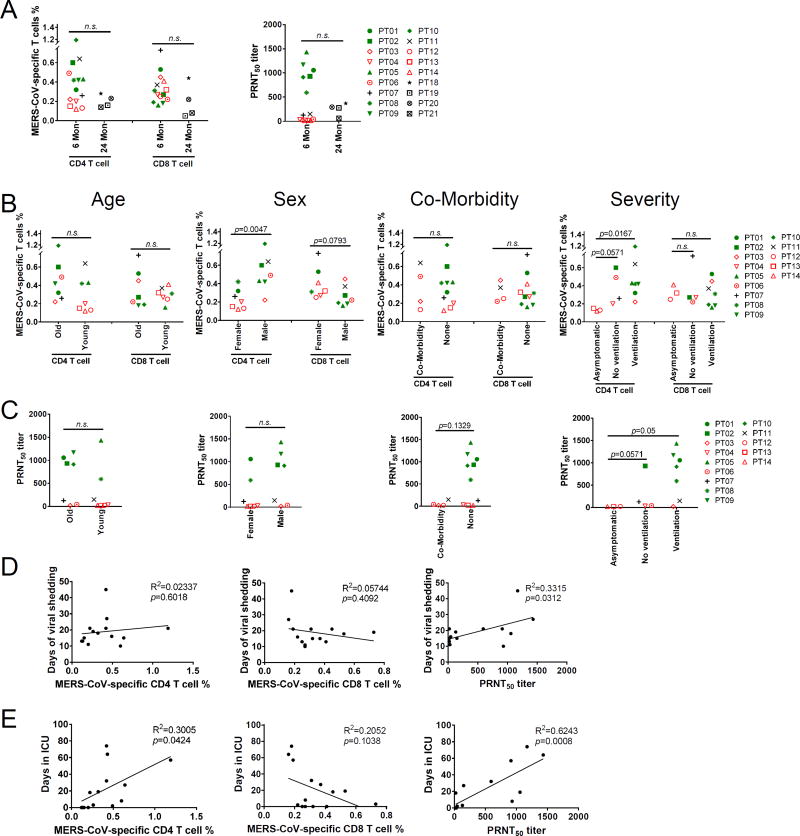
To assess T cell responses, we synthesized a set of 20-mer peptides overlapping by 10 amino acids, encompassing the four MERS-CoV structural proteins (Table S3) and used these peptides in a series of intracellular cytokine (IFN-γ) staining assays with PBMCs from healthy donors and MERS survivors. We used peptides instead of infectious virus for these assays because MERS-CoV has been shown to induce apoptosis in activated T cells, which in these assays would be virus-specific CD4 and CD8 T cells(12). Initially we created 4 pools of peptides (S1, S2, N, ME encompassing the N and C terminal portions of the spike (S) glycoprotein, the nucleocapsid (N) protein and the transmembrane (M) and envelope (E) proteins, respectively). No virus-specific CD4 and CD8 T cell responses were detected in the 4 healthy donors after peptide stimulation (Fig. 2A, C). In contrast, nearly all patients contained CD4 and CD8 T cells that responded to all four peptide pools. Some patients mounted a five to tenfold higher response to the peptide pools, especially to those encompassing the N (CD4) and M (CD8) proteins, compared to the average (Fig. 2A–D). A summary of total CD4 and CD8 T cell responses to all four peptide pools is shown in Fig. 2E. Notably, for individual patients, the percentage of virus-specific CD4 T cells was higher in patients with greater PRNT50 neutralizing titers (green symbols in Fig. 2B, D, E, F) while there was no relationship between the percentage of CD8 T cells responding to MERS-CoV peptides and the PRNT50 response. The virus-specific CD4 and CD8 T cells were highly functional, since a substantial fraction (CD4 T cells) or majority (CD8 T cells) expressed two cytokines (IFN-γ and TNF) (Fig. 3A, C). The CD4 T cells were phenotypically effector memory (CD45RA−CCR7−) cells (Fig. 3B) while the virus-specific CD8 T cell populations also included effector (CD45RA+CCR7−) cells (Fig. 3D). Thus, these cells are multifunctional and are expected to rapidly and efficiently respond to subsequent infection with MERS-CoV. Further, these data demonstrate that virus-specific CD8 T cells were detectable in patients with undetectable antibody responses, suggesting that measurement of the CD8 T cell response might be useful in longitudinal and prevalence studies.
Figure 2. Virus-specific T cell responses are detected in all MERS survivors.
PBMCs from healthy donors and MERS patients were stimulated with MERS-CoV structural protein-specific peptide pools for 12 hours in the presence of brefeldin A. Frequencies of MERS-CoV-specific CD4 (A, B) and CD8 (C, D) T cells (determined by IFN-γ intracellular staining) are shown. (E) Summary of total T cell responses against all four peptide pools is shown. (F) Relationship between T cell and neutralizing antibody responses is shown.
Figure 3. Human PBMC-derived MERS-CoV-specific T cells are highly functional.
(A, C) PBMCs were stimulated with MERS-CoV structural protein-specific peptide pools. Frequency and percentage of cells expressing IFN-γ and TNF are shown. (B, D) PBMCs were stimulated with the N (B) or ME (D) peptide pools. CD4 (B) or CD8 (D) T cells were then analyzed for the indicated phenotypic markers.
Since one of our ultimate goals was to identify CD4 and CD8 T cell epitopes that predict rapid recovery from primary infection and protection from subsequent challenge, we next used our peptide pools to identify individual target peptides. First, we performed HLA typing for all 18 patients from whom we obtained PBMCs (Table S4). Second, since DR2 and DR3 alleles are common in Saudi Arabian populations, recognized in 18–20% and 25–29% of patients, respectively (13, 14), we obtained mice transgenic for expression of these alleles and infected them with MERS-CoV. We harvested lung cells and stimulated them with individual MERS-CoV peptides. We identified several immunodominant peptides using these mice and then validated their identification in patients expressing DR2 or DR3 alleles (Table S3, Fig. 4A–D). While nearly all of these peptides were recognized in patients, a few were more immunodominant (e.g. DR2, S45; DR3, S106) and might be useful for monitoring CD4 T cell responses in future studies (Fig. 4B, C). For monitoring CD8 T cell responses, we were unable to identify putative epitopes using commercially available humanized HLA-expressing mice. As an alternative approach, since the M/E proteins were prominent targets for the CD8 T cell response in five patients (Fig. 2C, D) and the M and E proteins are small (219 and 82 amino acids, respectively), we screened PBMCs using individual peptides (Fig. 4E). At least three M-specific peptides were recognized in these five patients. Of note, two of these patients (patients 1 and 5) shared HLA-A11 and HLA-C*07 suggesting that peptide M19 is restricted by one of these alleles while patients 8 and 18 both expressed HLA-B40/41/44 and HLA-C*07 suggesting that M13 is restricted by one of these molecules (Fig. 4E, S3).
Figure 4. Identification of MERS-CoV-specific T cell epitopes in mice and patients.
(A) Single cell suspensions were prepared from the lungs of MERS-CoV infected DR2 and DR3 transgenic mice, and stimulated with peptides for 5–6 hours in the presence of brefeldin A. (B, C, D) DR2 or DR3-restricted patient PBMCs were stimulated with peptide pools or individual peptides for 12 hours in the presence of brefeldin A. (E) Patient PBMCs were stimulated with the ME peptide pool or individual peptides for 12 hours in the presence of brefeldin A. Frequencies of MERS-CoV specific T cells (determined by IFN-γ intracellular staining) are shown.
Next, we compared the levels of virus-specific antibody and T cell responses over several variables including patient age, sex, ventilation status, presence of co-morbidities, length of viral shedding and time in ICU. T cell and antibody responses tended to be lower (although not significantly different) at 24 compared to 6 months after infection (Fig. 5A), probably reflecting decay of the response with increased time after infection. Therefore, we confined our analyses to the 14 patients in the former group. There were no differences in the MERS-CoV-specific CD4 and CD8 T cell and PRNT50 responses between patients younger and older than 50 years (Fig. 5B, C). Males and females mounted similar CD8 T cell and PRNT50 responses but males exhibited greater CD4 T cell responses (Fig. 5B, C). We found no relationship between the height of the PRNT50 and T cell responses and the presence of co-morbidities (Fig. 5B, C). Patients with severe disease requiring ICU admission and ventilation had higher PRNT50 and CD4 T cell but not CD8 T cell responses compared to asymptomatic patients (Fig. 5B, C). Furthermore, patients with prolonged viral shedding had significantly higher antibody, but not T cell responses compared to patients with more transient virus shedding (Fig. 5D). Most strikingly, virus-specific CD4 T cell and PRNT50 correlated with length of stay in the ICU (R2 = 0.3005 and 0.6243, p = 0.04 and 0.0008, AICc = 6.78 and 208.78, respectively), while CD8 T cell responses were negatively correlated, although this did not reach statistical significance (R2 = 0.2052, p=0.10) (Fig. 5E). No bivariate models showed improvement over the univariate models for PRNT50 and CD8 T cells. In contrast, for CD4 T cells, the addition of viral shedding to length of stay in the ICU improved the model (AICc decreased from 6.78 to 3.20 in the bivariate model). Holding viral shedding constant, a 10 day increase in length of ICU stay would result in a 0.15% increase (p = 0.0010) in CD4 T cells. Holding length of ICU stay constant, an increase in viral shedding by 10 days would result in a 0.31% decrease (p = 0.0087) in CD4 T cells.
Figure 5. Relationship between MERS-CoV-specific T cell and neutralizing antibody responses and disease variables and severity.
(A) Relationship between T cell and PRNT50 responses and time p.i. when samples were obtained. (B, C) Relationship between T cell (B) and PRNT50 (C) responses and co-morbidity (Co-Morbidity vs None), ventilator status, sex and age. (D, E) Relationship between T cell and PRNT50 responses and the duration of virus shedding (D) and length of ICU stay (E).
Discussion
While there is no evidence that MERS-CoV has mutated to enhance virulence and transmissibility in humans since it was first identified in 2012(15), it is also apparent that the virus continues to be introduced into human populations, most likely from camels (‘primary cases’). 70 new, mostly primary cases have been diagnosed in Saudi Arabia thus far in 2017 (as of 4/26/17) showing that the disease continues to be a public health threat. MERS was recently identified by WHO and the Coalition for Epidemic Preparedness (CEPI) as a prime candidate for vaccine development (http://www.sciencemag.org/news/2016/09/new-vaccine-coalition-aims-ward-epidemics) since it poses a potential outbreak threat. Targeted vaccination of high risk human populations or vaccination of the likely intermediate host, dromedary camels, are under consideration (16) but no vaccine is presently licensed for human use. Efforts to develop vaccines for use in humans have been hampered by a lack of understanding of protective immune responses. Here, we show that virus-specific T cell responses can be identified in all MERS survivors, even in those with mild or subclinical infection, in whom serological testing is often negative. We also identified specific HLA-restricted CD4 and CD8 T cell epitopes, which is the first step in ascertaining protective and possibly pathogenic responses to individual T cell epitopes in MERS patients.
Previous studies of MERS prevalence have been based on virus-specific antibody measurements (5). Our results, as well as those that show that antibody titers are often transient or low in magnitude (5, 6), suggest that the true incidence of the infection is much greater than is now recognized and that a more accurate estimation could be determined if T cell responses were also measured. This approach might also provide information about the true prevalence of the infection in Africa, where a high percentage of camels are seropositive for MERS-CoV antibodies but where only a few patients with detectable antibody and no patients with clinical disease have been identified (17). We observed that the virus-specific CD8 T cell and antibody responses were not correlated, indicating that the CD8 T cell response would be most useful in determining the true incidence of infection. Low or transient MERS-CoV-specific antibody responses also raised the concern that patients with mild disease would be susceptible to re-infection and the development of clinical disease on subsequent virus exposure. However, the presence of a virus-specific CD8 T cell response in all survivors partly alleviates this concern, because memory CD8 T cells, especially if they are at site of infection (the respiratory tract), would be expected to initiate an early and protective host immune response (18).
Virus-specific PRNT50 and memory CD4 T cell but not CD8 T cell responses correlated with severe disease, using days in the ICU as a marker for severity. These findings suggest that higher virus-specific antibody responses in severely ill patients reflect prolonged exposure to virus antigen or higher viral load. Higher levels of MERS-CoV were detected in nasopharyngeal samples obtained from patients with more severe disease or death compared to survivors (19). Conversely, patients with more robust virus-specific CD8 T cells may clear infectious virus and viral antigen more rapidly, resulting in lower CD4 T cell and antibody responses. Deciphering the relationship between virus-specific T cell and antibody responses and disease severity will help in the management of patients during the acute phase of the illness. No information is yet available about T cell responses in patients who succumbed to the infection during the acute phase. However, based on the magnitude of MERS-CoV-specific CD8 T cell responses in survivors, their measurement might provide information relevant to prognosis while patients are still hospitalized: patients with detectable virus-specific CD8 T cell responses at earlier times after infection might be expected to have more favorable outcomes.
Our observations and analyses will need to be confirmed with larger numbers of patients. We have thus far obtained PBMCs from 18 and sera from 21 previously infected individuals, which represents 2–3% of all reported MERS survivors in Saudi Arabia (http://www.moh.gov.sa/en/CCC/PressReleases/Pages/default.aspx). Longitudinal studies of previously infected patients will also be required to more precisely compare the longevity of the virus-specific T cell versus antibody responses. A potential limitation of our study is that MERS-CoV-specific T cell epitopes may cross-react with epitopes present in common upper respiratory tract infection-associated CoV, especially since some of the epitopes are present on conserved proteins, such as the N protein. Use of pools of immunogenic peptides mitigates this concern to a large extent, since several epitopes are immunogenic and it is unlikely that most would be cross-reacting. Notably, none of these epitopes were recognized by T cells from any of the healthy donors that we tested. Also, similar levels of T cell responses were detected to epitopes on conserved proteins and on ones that are less conserved such as the surface glycoprotein, especially the S1 part, which is highly divergent between different CoV.
In summary, we found that all MERS survivors that we analyzed developed CD4 and CD8 T cell responses. We also defined a titer of neutralizing antibody that was able to effect virus clearance in an animal model and is predicted to be useful in clinical settings. Patients with mild or subclinical illness develop prominent virus-specific CD8 T cell responses, which may be useful in predicting prognosis of hospitalized patients and will be useful in studies of transmission patterns and prevalence by identifying previously infected patients with undetectable antibody responses to MERS-CoV.
Materials and Methods
Study design and participants
Four tertiary care hospitals in Saudi Arabia participated in this study, one from Riyadh, two from Jeddah and one from Makkah. All hospitals had infection control departments, critical care units and access to sub-specialty consultant services. During the MERS outbreak in 2015 at the National Guard Hospital in Riyadh, 94 patients were identified as infected using a real-time reverse transcription PCR (RT-qPCR) assay with specimens obtained by nasopharyngeal swab or bronchoalveolar lavage. 54 patients survived and were contacted about providing blood samples for immune analyses. 14 patients agreed to participate. Similarly, 40 MERS patients were identified during the 2014 MERS outbreak in King Faisal Specialist Hospital and Research Center in Jeddah. Of the 29 survivors, 2 agreed to provide blood for further analysis. In King Fahad General Hospital in Jeddah, 61 cases were identified and 19 died. Of the 42 survivors, 2 agreed to participate. In Al Nour Specialist Hospital in Makkah, 30 cases were identified and 9 died. 3 survivors provided blood for serological testing but not for T cell analyses. Control samples of PBMCs were obtained from 4 anonymous donors at the University of Iowa. In total, the patient cohort for this study consisted of 21 patients and 4 controls.
Study approval
The Institutional Review Boards of all of the centers approved the study. Written informed consent was obtained from all study participants.
Clinical information and serological testing
Patients’ medical records were reviewed for information on demographic characteristics, comorbidities, clinical presentation, intensive care unit admission, radiographic findings, duration of viral shedding, hematological parameters, renal profile, hepatic profile, development of acute kidney injury, requirement for dialysis, treatments received and outcome. Blood from the Riyadh and Jeddah patients were fractionated into sera and PBMCs. Anti-MERS-CoV antibody titers were initially quantified by ELISA and immunofluorescence assay performed in Jeddah and Riyadh as previously described (5). The ELISA for MERS-CoV S-specific antibody was read as positive (>1.1), negative (<0.8), or borderline (0.8 and 1.1). Sera were then analyzed for neutralizing antibody titer as described below.
Mice, virus and cells
Specific pathogen-free 6 week-old BALB/c mice were purchased from the National Cancer Institute and Charles River Laboratories International. HLA-DR2 (DRB1*1501) and HLA-DR3 (DRB1*0301) transgenic mice were produced as previously described (20, 21). Mice were maintained in the Animal Care Facility at the University of Iowa. All protocols were approved by the University of Iowa Institutional Animal Care and Use Committee. The EMC/2012 strain of MERS-CoV (passage 8, designated MERS-CoV) was provided by Drs. Bart Haagmans and Ron Fouchier (Erasmus Medical Center). All work with infectious MERS-CoV was conducted in the University of Iowa Biosafety Level 3 (BSL3) Laboratory.
Antibody treatment and MERS-CoV infection of mice
Since mice do not express a functional receptor for MERS-CoV, six-week-old female BALB/c mice were lightly anesthetized with isoflurane and transduced intranasally with 2.5×108 PFU of Ad5-human DPP4 in 75 µl DMEM as described (10). Five days post transduction, mice were infected intranasally with MERS-CoV (1×105 PFU) in a total volume of 50 µl DMEM. Mice were monitored daily for morbidity (weight loss) and mortality. All work with MERS-CoV was conducted in the University of Iowa Biosafety Level 3 (BSL3) Laboratory. Mice were injected with 75 µl human serum intravenously (i.v.) 12 hours before MERS-CoV infection. Control mice were given an equal volume of healthy donor serum.
Virus titers
To obtain virus titers, lungs were harvested from subgroups of 3 animals at the indicated time points (see Results) and homogenized into 3 ml of phosphate buffered saline (PBS), using a manual homogenizer. Lung homogenates were aliquoted and kept at −80°C. Virus was titered on Vero 81 cells (10). Viral titers are expressed as PFU/g tissue for MERS-CoV.
MERS-CoV microneutralization assays
Serial two-fold dilutions of human sera were prepared and equal volumes of MERS-CoV (EMC/2012) and sera were combined and incubated for one hour at room temperature. The mixture was then added in quadruplicate to Vero81 cells. The neutralization titer is the reciprocal of the highest serum dilution that neutralized the infectivity of 100 TCID50 of virus, read as the absence of cytopathic effect in the cells on day four post infection (p.i.).
MERS-CoV plaque reduction neutralization assay
Serum samples were serially diluted in DMEM and mixed with an equal volume of MERS-CoV (EMC/2012) containing 80 PFU. Following incubation at 37°C for 1 h, aliquots were added to cultures of Vero 81 cells in 48 well plates and incubated at 37°C in 5% CO2 for 1 h. Virus titers (PRNT50) were determined as described(22).
Preparation of cells from mouse lungs
Mice were sacrificed at day 8 p.i. Lungs were removed, cut into small pieces and digested in HBSS buffer containing 2% FCS, 25 mM HEPES, 1 mg/ml collagenase D (Roche) and 0.1 mg/ml DNase (Roche) for 30 minutes at 37°C. Tissues were dispersed using a 70 µM cell strainer and single-cell suspensions were prepared. Live cells were enumerated by 0.2% trypan blue exclusion. Cells were stimulated with peptides for intracellular cytokine expression as described previously (23).
Flow cytometry
The following anti-human monoclonal antibodies were used: CD3 (HIT3a); CD4 (RPA-T4); CD8 (SK1); CD14 (M5E2); CD19 (SJ25C1); CD56 (5.1H11); TCR γδ (B1); IFN-γ (B27); TNF (MAb11); CD45RA (HI100); CD27 (M-T271); CCR7 (G043H7); all antibodies were from BD Bioscience, eBioscience or Biolegend. FC receptor blocking solution was obtained from Biolegend.
PBMCs were prepared from blood samples at the Riyadh and Jeddah sites using Lympholyte-H (Cedarlane) following the product instruction. Cells were stored in liquid nitrogen prior to and during shipping to the University of Iowa where the cells were further analyzed. For surface staining, 105–106 cells were blocked with Fc receptor blocking solution, labeled with LIVE/DEAD Staining dye (ThermoFisher), and then stained with the indicated antibodies at 4°C. For in vitro intracellular cytokine staining, 105–106 cells/well were cultured in 96-well round bottom plates at 37°C for 12 hours in the presence of 2 µM peptide (GenScript) and brefeldin A (BFA, BD Biosciences). Cells were then labeled for cell surface markers, fixed/permeabilized with Cytofix/Cytoperm Solution (BD Biosciences) and labeled with anti-intracellular cytokine/protein antibodies. All flow cytometry data were acquired on a BD FACSVerse and analyzed using FlowJo software (Tree Star, Inc.).
Statistical analysis
The Mann-Whitney test was used for initial analyses comparing the differences between groups, with p-values < 0.05 being considered statistically significant. However, this approach tends to have low power and mostly insignificant results. Therefore, we also performed linear regression analyses to compare the model fits between different predictor sets with the same outcome. Due to the small sample size (14 when doing model comparisons), we determined that the most appropriate measure to use for model comparison was the corrected Akaike information criterion (AICc) (24, 25). This measure is an extension of the Akaike information criterion (AIC) (26, 27) and is more appropriate when the sample size is small. For each outcome, the predictor sets were limited to null, univariate, and bivariate models. By comparing the AICc for all models with the same outcome, we can determine the most favorable model predictor set. A smaller AICc indicates a more favorable model.
Supplementary Material
Figure S1. Gating strategy for determining cellular composition of PBMCs.
Table S1 Clinical information including laboratory values
Table S2 PBMC cell composition
Table S3 peptide list
Table S4 HLA Typing
Acknowledgments
We thank Drs. Katherine Kedzierska and Zhongfang Wang for helpful discussions and Drs. Anthony Fehr, Jian Zheng and Rudragouda Channappanavar and D. Lori Wheeler for critical review of the manuscript. We thank all of the patients who took part in this study. Funding: This study was funded by grants from the National Institutes of Health (USA) (PO1 060699), Thousand Talents Plan Award of China 2015, the Municipal Healthcare Joint-Innovation Major Project of Guangzhou (201604020011) and the Division of Intramural Research of NIAID, NIH.
Footnotes
This manuscript has been accepted for publication in Science Immunology. This version has not undergone final editing. Please refer to the complete version of record at www.scienceimmunology.org. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
Author contributions: J.Z, A.A, S.P, J.Z. conceived the study, W.A., S.B., L.L., M.A., M.A.G. were site investigators, I.K., S.A.J., A.D., M.B. acquired and validated the data, J.Z., J.Z., L.V., A.B., A.N. executed the experiments, J.Z., J.Z., S.P., L.V., K.S., P.T.E., C.W. analyzed the data. A.M. provided reagents. J.Z, A.A., S.P, J.Z., P.T.E. wrote the manuscript.
Competing interests: The authors have declared that no conflict of interest exists.
References
- 1.Zumla A, Hui DS, Perlman S. Middle East respiratory syndrome. Lancet. 2015;386:995–1007. doi: 10.1016/S0140-6736(15)60454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cho SY, Kang JM, Ha YE, Park GE, Lee JY, Ko JH, Lee JY, Kim JM, Kang CI, Jo IJ, Ryu JG, Choi JR, Kim S, Huh HJ, Ki CS, Kang ES, Peck KR, Dhong HJ, Song JH, Chung DR, Kim YJ. MERS-CoV outbreak following a single patient exposure in an emergency room in South Korea: an epidemiological outbreak study. Lancet. 2016;388:994–1001. doi: 10.1016/S0140-6736(16)30623-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azhar EI, El-Kafrawy SA, Farraj SA, Hassan AM, Al-Saeed MS, Hashem AM, Madani TA. Evidence for camel-to-human transmission of MERS coronavirus. New Engl J Med. 2014;370:2499–2505. doi: 10.1056/NEJMoa1401505. [DOI] [PubMed] [Google Scholar]
- 4.Ng DL, Al Hosani F, Keating MK, Gerber SI, Jones TL, Metcalfe MG, Tong S, Tao Y, Alami NN, Haynes LM, Mutei MA, Abdel-Wareth L, Uyeki TM, Swerdlow DL, Barakat M, Zaki SR. Clinicopathologic, Immunohistochemical, and Ultrastructural Findings of a Fatal Case of Middle East Respiratory Syndrome Coronavirus Infection in the United Arab Emirates, April 2014. Am J Pathol. 2016;186:652–658. doi: 10.1016/j.ajpath.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alshukairi AN, Khalid I, Ahmed WA, Dada AM, Bayumi DT, Malic LS, Althawadi S, Ignacio K, Alsalmi HS, Al-Abdely HM, Wali GY, Qushmaq IA, Alraddadi BM, Perlman S. Antibody response and disease severity in healthcare worker MERS survivors. Emerg Inf Dis. 2016;22:1113–1115. doi: 10.3201/eid2206.160010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drosten C, Meyer B, Muller MA, Corman VM, Al-Masri M, Hossain R, Madani H, Sieberg A, Bosch BJ, Lattwein E, Alhakeem RF, Assiri AM, Hajomar W, Albarrak AM, Al-Tawfiq JA, Zumla AI, Memish ZA. Transmission of MERS-coronavirus in household contacts. New Engl J Med. 2014;371:828–835. doi: 10.1056/NEJMoa1405858. [DOI] [PubMed] [Google Scholar]
- 7.Payne DC, Iblan I, Rha B, Alqasrawi S, Haddadin A, Al Nsour M, Alsanouri T, Ali SS, Harcourt J, Miao C, Tamin A, Gerber SI, Haynes LM, Al Abdallat MM. Persistence of antibodies against Middle East Respiratory Syndrome Coronavirus. Emerg Inf Dis. 2016;22:1824–1826. doi: 10.3201/eid2210.160706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tang F, Quan Y, Xin ZT, Wrammert J, Ma MJ, Lv H, Wang TB, Yang H, Richardus JH, Liu W, Cao WC. Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. J Immunol. 2011;186:7264–7268. doi: 10.4049/jimmunol.0903490. [DOI] [PubMed] [Google Scholar]
- 9.Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw FM, Lim WS, Makki S, Rooney KD, Beck CR. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Inf Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao J, Li K, Wohlford-Lenane C, Agnihothram SS, Fett C, Gale MJ, Jr, Baric RS, Enjuanes L, Gallagher T, McCray PB, Jr, Perlman S. Rapid generation of a mouse model for Middle East respiratory syndrome. Proc Natl Acad Sci. 2014;111:4970–4975. doi: 10.1073/pnas.1323279111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raj VS, Mou H, Smits SL, Dekkers DH, Muller MA, Dijkman R, Muth D, Demmers JA, Zaki A, Fouchier RA, Thiel V, Drosten C, Rottier PJ, Osterhaus AD, Bosch BJ, Haagmans BL. Dipeptidyl peptidase 4 is a functional receptor for the emerging human coronavirus-EMC. Nature. 2013;495:251–254. doi: 10.1038/nature12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chu H, Zhou J, Wong BH, Li C, Chan JF, Cheng ZS, Yang D, Wang D, Lee AC, Li C, Yeung ML, Cai JP, Chan IH, Ho WK, To KK, Zheng BJ, Yao Y, Qin C, Yuen KY. Middle East Respiratory Syndrome Coronavirus Efficiently Infects Human Primary T Lymphocytes and Activates the Extrinsic and Intrinsic Apoptosis Pathways. J Inf Dis. 2016;213:904–914. doi: 10.1093/infdis/jiv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sheth KV, Edwards JA, Godwin JT. Study of the HLA gene and antigen frequency from a Saudi Arabian hospital. Tissue Antigens. 1985;25:156–162. doi: 10.1111/j.1399-0039.1985.tb00430.x. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Galarza FF, Takeshita LY, Santos EJ, Kempson F, Maia MH, da Silva AL, Teles e Silva AL, Ghattaoraya GS, Alfirevic A, Jones AR, Middleton D. Allele frequency net 2015 update: new features for HLA epitopes, KIR and disease and HLA adverse drug reaction associations. Nuclei Acids Res. 2015;43:D784–788. doi: 10.1093/nar/gku1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaki AM, van Boheemen S, Bestebroer TM, Osterhaus AD, Fouchier RA. Isolation of a novel coronavirus from a man with pneumonia in Saudi Arabia. New Engl J Med. 2012;367:1814–1820. doi: 10.1056/NEJMoa1211721. [DOI] [PubMed] [Google Scholar]
- 16.Haagmans BL, van den Brand JM, Raj VS, Volz A, Wohlsein P, Smits SL, Schipper D, Bestebroer TM, Okba N, Fux R, Bensaid A, Solanes Foz D, Kuiken T, Baumgartner W, Segales J, Sutter G, Osterhaus AD. An orthopoxvirus-based vaccine reduces virus excretion after MERS-CoV infection in dromedary camels. Science (New York, N.Y) 2016;351:77–81. doi: 10.1126/science.aad1283. [DOI] [PubMed] [Google Scholar]
- 17.Liljander A, Meyer B, Jores J, Muller MA, Lattwein E, Njeru I, Bett B, Drosten C, Corman VM. MERS-CoV antibodies in humans, Africa, 2013–2014. Emerg Inf Dis. 2016;22:1086–1089. doi: 10.3201/eid2206.160064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosato PC, Beura LK, Masopust D. Tissue resident memory T cells and viral immunity. Curr Opin Virol. 2016;22:44–50. doi: 10.1016/j.coviro.2016.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feikin DR, Alraddadi B, Qutub M, Shabouni O, Curns A, Oboho IK, Tomczyk SM, Wolff B, Watson JT, Madani TA. Association of higher MERS-CoV virus load with severe disease and death, Saudi Arabia, 2014. Emerg Infect Dis. 2015;21:2029–2035. doi: 10.3201/eid2111.150764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koehm S, Slavin RG, Hutcheson PS, Trejo T, David CS, Bellone CJ. HLA-DRB1 alleles control allergic bronchopulmonary aspergillosis-like pulmonary responses in humanized transgenic mice. J Allergy Clin Immunol. 2007;120:570–577. doi: 10.1016/j.jaci.2007.04.037. [DOI] [PubMed] [Google Scholar]
- 21.Kong YC, Lomo LC, Motte RW, Giraldo AA, Baisch J, Strauss G, Hammerling GJ, David CS. HLA-DRB1 polymorphism determines susceptibility to autoimmune thyroiditis in transgenic mice: definitive association with HLA-DRB1*0301 (DR3) gene. J Exp Med. 1996;184:1167–1172. doi: 10.1084/jem.184.3.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Y, Wang C, Qiu B, Li C, Wang H, Jin H, Gai W, Zheng X, Wang T, Sun W, Yan F, Gao Y, Wang Q, Yan J, Chen L, Perlman S, Zhong N, Zhao J, Yang S, Xia X. Passive immunotherapy for Middle East Respiratory Syndrome coronavirus infection with equine immunoglobulin or immunoglobulin fragments in a mouse model. Antiviral Res. 2016;137:125–130. doi: 10.1016/j.antiviral.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao J, Zhao J, Legge K, Perlman S. Age-related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J Clin Invest. 2011;121:4921–4930. doi: 10.1172/JCI59777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hurvich CM, Tsai CL. Regression and Time Series Model Selection in Small Samples. Biometrika. 1989;76:297–307. [Google Scholar]
- 25.Burnham KP, Anderson DR. Model Selection and Inference: A Practical Information-Theoretical Approach. New York: Springer-Verlag. 1998 [Google Scholar]
- 26.Akaike H. Information theory and an extension of the maximum likelihood principle. In: Petrov BN, Csaki F, editors. 2nd International Symposium on Information Theory (Akademia Kiado, Budapest); 1973. pp. 267–281. [Google Scholar]
- 27.Akaike H. A new look at the statistical model identification. IEEE Transactions on Automatic Control. 1974;19:716–723. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Gating strategy for determining cellular composition of PBMCs.
Table S1 Clinical information including laboratory values
Table S2 PBMC cell composition
Table S3 peptide list
Table S4 HLA Typing