Abstract
Ontak® is a FDA-approved diphtheria toxin-based recombinant fusion toxin for treatment of human CD25+ cutaneous T cell lymphoma (CTCL). However, it has been discontinued clinically due to the production issue related to the bacterial expression system with difficult purification. Recently we have developed monovalent and bivalent human IL-2 fusion toxins targeting human CD25+ cells using advanced unique diphtheria toxin resistant yeast Pichia Pastoris expression system. In vitro efficacy characterization using human CD25+ HUT102/6TG cells demonstrated that both monovalent and bivalent isoforms are potent and the bivalent isoform is approximately two logs more potent than the monovalent isoform. In this study, we further assessed the in vivo efficacy of the human IL-2 fusion toxins using human CD25+ HUT102/6TG tumor-bearing NSG mouse model. The data demonstrated that both monovalent and bivalent human IL-2 fusion toxins significantly prolonged the survival of the human CD25+ tumor-bearing NSG mice in a dose-dependent manner. Then we further assessed the residual tumor cells from the HUT102/6TG tumor-bearing NSG mice using the residual tumor cell bearing NSG mouse model. The results demonstrated that the residual tumor cells were still sensitive to the continual treatment with the human IL-2 fusion toxin. This yeast-expressed human IL-2 fusion toxin will be a promising candidate to replace the clinically discontinued Ontak®.
Keywords: Ontak, IL-2 fusion toxin, CTCL, CD25, diphtheria toxin, Pichia Pastoris
1. Introduction
Cutaneous T-cell lymphomas (CTCL) are extranodal non-Hodgkin’s lymphomas characterized by skin lesions infiltrated with malignant CD4+ or CD8+ T lymphocytes. Mycosis fungoides and Sezary syndrome are the two most common forms of CTCL (Prince et al., 2010). Overexpression of the IL-2 receptor, CD25, is a prominent molecular characteristic of CTCL. Ontak® (denileukin diftitox, DAB389IL-2, Eisai Medical Research, Inc.) is a genetically engineered monovalent human IL-2 fusion toxin expressed in Escherichia coli which has been approved by the Food and Drug Administration (FDA) for clinical treatment of patients with persistent and recurrent human CD25+ CTCL. The overall response rates of Ontak® range from 30% to 50%, and are even higher (63% and 48%, for early and late stage CTCL, respectively) in patients treated less heavily with other modalities (Prince et al., 2010). Of particular note is the large prolongation (>2 years) of progression-free survival by Ontak®. In addition, treatment significantly improves the patient quality of life (Duvic et al., 2002). However, Ontak® was discontinued clinically due to the production issues related to the E. coli expression system with difficult purification. Recently we have developed monovalent and bivalent human IL-2 fusion toxins using an advanced unique diphtheria toxin resistant yeast Pichia Pastoris expression system (Fig. 1) and characterized their efficacy in vitro (Peraino et al., 2014). In this study, we further assessed the in vivo efficacy of the yeast-produced human IL-2 fusion toxins in human CD25+ CTCL tumor-bearing NSG mouse model. We also assessed the residual tumor cells from the HUT102/6TG tumor-bearing NSG mice using the residual tumor cell bearing NSG mouse model.
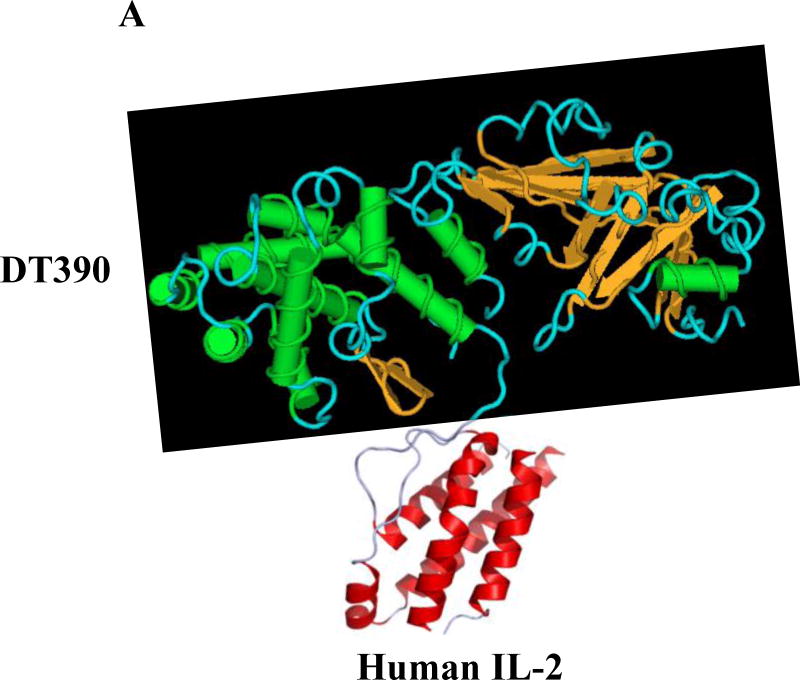
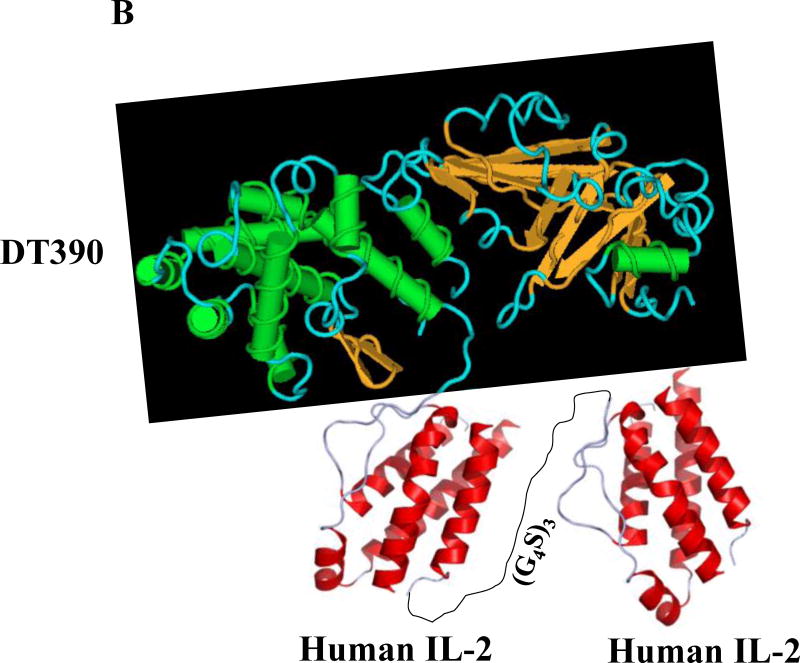
Fig. 1.
Schematic diagram of the human IL-2 fusion toxins. A) Monovalent human IL-2 fusion toxin. B) Bivalent human IL-2 fusion toxin. The two human IL-2 were linked by a (G4S)3 linker.
2. Materials and Methods
2.1. Tumor cells and antibodies
Human CD25+ T-cell lymphoma cell line HUT 102/6TG (William et al., 1990) was kindly provided by Dr. Robert Harrison, Anjin Group, Inc., Boston, MA. The HUT 102/6TG tumor cells were cultured in RPMI 1640 media supplemented with 12% fetal bovine serum, 10 mM hepes (N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid), 1x nonessential amino acids, 1mM sodium pyruvate, 2 mM glutamine, and 2.5 × 10−5 M 2-mercaptoethanol. The HUT 102/6TG tumor cells were washed twice with 50 mL of the above RPMI1640 media by centrifugation at 558 × g, 25°C for 5 minutes. The HUT 102/6TG cells were then diluted to 5 × 107 cells/mL for injection. FITC-anti-human CD4 mAb (clone# SK-3, cat# 344604, BioLegend) and APC-anti-human CD25 mAb (clone# M-A251, cat# 555434, BD) were used for the flow cytometry analysis. Flow cytometry binding analysis, KD analysis, and tumor cell viability analysis following the incubation with the human IL-2 fusion toxin were performed as described previously (Wang et al., 2015; Peraino et al., 2013a).
2.2. In vitro efficacy analysis
In vitro efficacy of the human IL-2 fusion toxin to the residual tumor cells was assessed using CellTiter-Glo® Luminescent Cell Viability Assay (Promega, cat# G7571) as described previously (Zheng et al., 2017).
2.3. In vivo efficacy study
NOD/SCID human IL-2 receptor γ−/− (NSG) mice were purchased from Jackson laboratories and bred in Massachusetts General Hospital (MGH) rodent barrier facilities. The in vivo experiments were approved by the Institutional Animal Care and Use Committee (IACUC) of MGH. MGH is an Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) recognized institution.
The immune deficient NSG mice were randomly divided into following groups: 1) vehicle (n=7); 2) monovalent human IL-2 fusion toxin at 3 μg/kg (n=7); 3) monovalent human IL-2 fusion toxin at 30 μg/kg (n=7); 4) monovalent human IL-2 fusion toxin at 100 μg/kg (n=7); 5) bivalent human IL-2 fusion toxin at 3 μg/kg (n=7); 6) bivalent human IL-2 fusion toxin at 30 μg/kg (n=7); 7) bivalent human IL-2 fusion toxin at 100 μg/kg (n=7). All animals were injected IV with 10 million of human CD25+ CTCL HUT102/6TG cells via the tail vein. Human IL-2 fusion toxin was injected IP daily for 10 consecutive days starting on day 0 of tumor injection. The injected animals were observed daily for signs and symptoms of illness and scored at least twice weekly based on the parameters as previously reported by our lab (Peraino et al., 2013b). To assess the non-specific toxicity of the human IL-2 fusion toxin, two mice (n=2) injected with the bivalent human IL-2 fusion toxin alone at 100 μg/kg were also included as controls. The in vivo efficacy analysis using the residual tumor cell bearing NSG mouse model was performed as using the parent HUT102/6TG tumor-bearing NSG mouse model.
C21 imunotoxin is a nonrelated truncated diphtheria toxin DT390 based immunotoxin produced in our lab. The DT390 domain of C21 immunotoxin is exactly same with that of human IL-2 fusion toxin. In this study, C21 immunotoxin was used as a negative control for the in vivo efficacy analysis of the human IL-2 fusion toxin using the residual tumor cell bearing NSG mouse model.
2.4. Collection of the residual tumor cells from Hut102/6TG tumor-bearing NSG mouse liver
Liver was removed from the sacrificed Hut102/6TG tumor-bearing mice and cut into small pieces with a #10 surgical blade in a 100 mm petri dish in sterile manner. A 40 μm cell strainer was set onto a 50 ml conical tube. The small liver pieces were mashed through the cell strainer using the back of a 3 ml syringe. The collected cells in the conical tube were washed with HBSS solution and centrifuged at 558 × g for 10 min. The supernatant was discarded and the pellet was re-suspended with 40 ml of HBSS. The cell suspension was underlaid with 10 ml of Histopaque-1077 (Sigma) and followed by gradient centrifugation at 872 × g for 30 min, brake off. The cell suspension resting above the histopaque-1077 layer was transferred into a new 50 ml conical tube and washed twice with HBSS. The cells were cultured in the same media for the parent tumor cell line Hut102/6TG at 37°C and 5% CO2.
2.5. Statistical analysis
All P values were calculated using Log-rank (Mantel-Cox) Test using the GraphPad Prism software. P < 0.05 was considered significant.
3. Results
Recently we have developed monovalent and bivalent human IL-2 fusion toxins targeting human CD25+ cells using unique diphtheria toxin resistant yeast Pichia Pastoris expression system (Fig. 1, Peraino et al., 2014). With this expression system, the expressed human IL-2 fusion toxins were secreted into the culture medium which facilitated the down-stream purification. The production level and quality were significantly improved comparing with the Ontak®-production system E.coli (Peraino et al., 2014). In vitro efficacy analysis demonstrated that both monovalent and bivalent human IL-2 fusion toxins were potent and the bivalent isoform is approximately two logs more potent than the monovalent isoform using human CD25+ HUT102/6TG cells (Peraino et al., 2014).
3.1. Off-target analysis of the human IL-2 fusion toxin
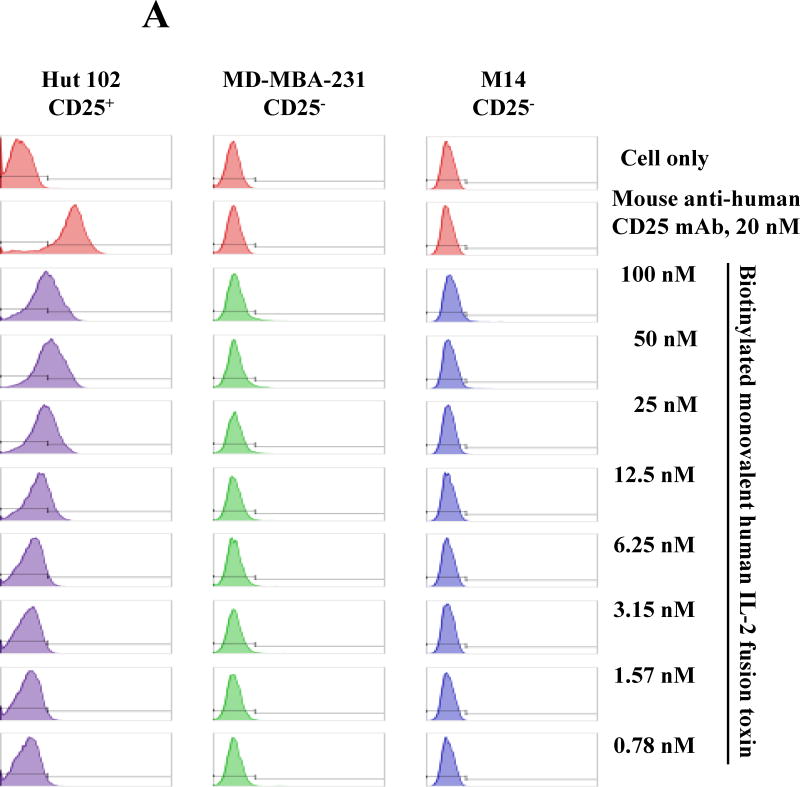
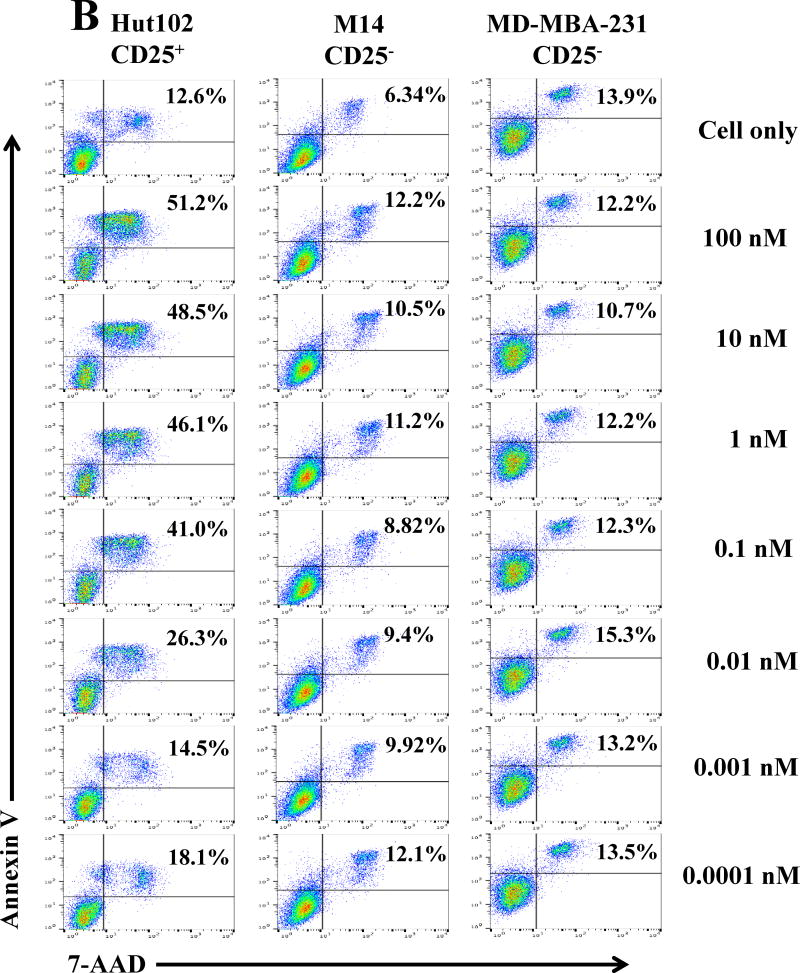
To continually examine the specificity of the human IL-2 fusion toxin, flow cytometry binding analysis was performed using the biotinylated monovalent human IL-2 fusion toxin to two human CD25− tumor cell lines: 1) MD-MBA-231 and 2) M14. As shown in Figure 2A, there was no binding for the biotinylated human IL-2 fusion toxin to the two human CD25− tumor cell lines. In contrast, the biotinylated human IL-2 fusion toxin dose-dependently bound to the human CD25+ control cell line HUT102/6TG. The data indicates that the human IL-2 fusion toxin only specifically bound to human CD25+ cells. We have also analyzed the viability of the two human CD25− tumor cell lines by flow cytometry using 7-AAD and Annexin V staining following incubation with the unlabeled monovalent human IL-2 fusion toxin for 18 h. Their viabilities were not or minimally affected (Figure 2B).
Fig. 2.
Off-target analysis of the human IL-2 immunotoxin. A) Flow cytometry binding analysis of the biotinylated monovalent human IL-2 fusion toxin to two human CD25− tumor cells (M14 and MD-MBA-231). Mouse anti-human CD25 mAb was included as positive antibody control. B) Cell viability analysis of the two human CD25− tumor cell lines by staining with 7-ADD and Annexin V following incubation with the monovalent human IL-2 fusion toxin for 18 h. The data are representative of three individual experiments.
3.2. In vivo efficacy analysis of the human IL-2 fusion toxins
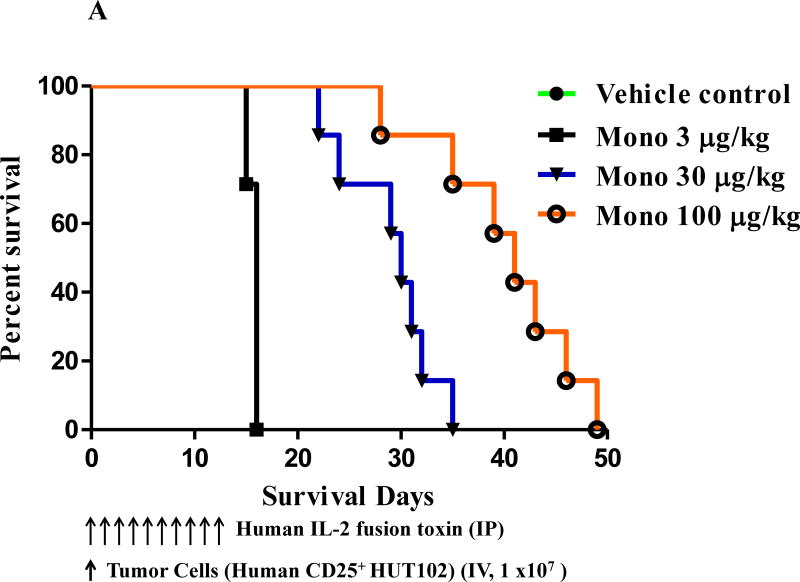
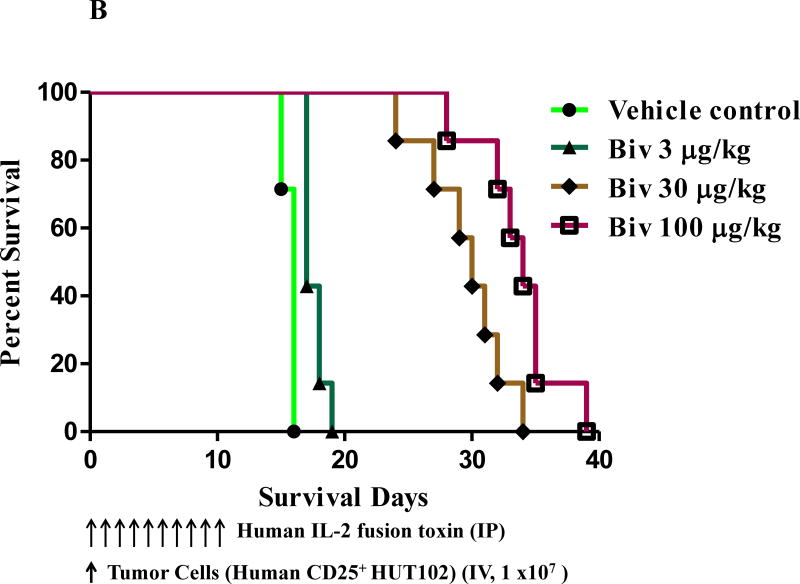
To assess the in vivo efficacy of the human IL-2 fusion toxins, 49 NSG mice at age 6–8 weeks were randomly divided into 7 groups (Table 1): 1) vehicle (n=7); 2) monovalent at 3 μg/kg (n=7); 3) monovalent at 30 μg/kg (n=7); 4) monovalent at 100 μg/kg (n=7); 5) bivalent at 3 μg/kg (n=7); 6) bivalent at 30 μg/kg (n=7); 7) bivalent at 100 μg/kg (n=7). 1 × 107 human CD25+ Hut102/6TG CTCL cells were injected IV via the mouse tail vein on day 0 and the human IL-2 fusion toxins were injected IP daily from day 0 on for consecutive 10 days with the indicated dose. The data demonstrated that both monovalent and bivalent human IL-2 fusion toxins significantly prolonged the survival of the human CD25+ tumor-bearing NSG mice in a dose-dependent manner compared to the vehicle only control. As shown in Fig. 3A and Table 1, the survival was prolonged by the monovalent isoform from median 16 days (vehicle and at 3 μg/kg) to 30 days (at 30 μg/kg) and 41 days (at 100 μg/kg), respectively. As shown in Fig. 3B and Table 1, the survival was prolonged by the bivalent isoform from median 16 days (vehicle) to 17 days (at 3 μg/kg), 30 days (at 30 μg/kg) and 34 days (at 100 μg/kg), respectively. The data also demonstrated that the bivalent isoform at the 3μg/kg dose had statistically-significant increase in survival over the monovalent isoform (median 17 vs 16 days, p = 0.0004). However, the monovalent isoform prolonged the survival comparably with the bivalent isoform at 30 μg/kg (median 30 vs 30 days). The monovalent isoform showed statistically-significant increase in survival over the bivalent isoform at the 100 μg/kg dose (median 41 vs 34 days, p = 0.0169). The in vivo efficacy assessment was repeated once with the same dose schedules (without the 100 μg/kg dose group) and similar survival prolongation was observed (data not shown).
Table 1.
In vivo efficacy analysis of the human IL-2 fusion toxins using human CD25+ HUT102/6TG tumor-bearing NSG mice
| Dose | Median Survival Days
|
|
|---|---|---|
| Monovalent Bivalent | ||
| Vehicle (n=7) | 16 | 16 |
| 3 μg/kg (n=7) | 16 | 17 |
| 30 μg/kg (n=7) | 30 | 30 |
| 100 μg/kg (n=7) | 41 | 34 |
Fig. 3.
In vivo efficacy assessment of the human IL-2 fusion toxins. NSG mice were injected IV with 1.0 ×107 human CD25+ CTCL HUT102/6TG cells on day 0 and treated from day 0 on with the monovalent or bivalent human IL-2 fusion toxin at indicated dose daily for 10 consecutive days. The schedule of drug and tumor cell injection is pictured in the schematic below the survival curve. The vertical arrows indicate the days on which the tumor cells or human IL-2 fusion toxins were injected. A) Monovalent human IL-2 fusion toxin: 1) Vehicle control (n=7, green curve) with a median survival time of 16 days; 2) monovalent human IL-2 fusion toxin at 3 μg/kg (n=7, black curve) with a median survival time of 16 days; 3) monovalent human IL-2 fusion toxin at 30 μg/kg (n=7, blue curve) with a median survival time of 30 days; 4) monovalent human IL-2 fusion toxin at 100 μg/kg (n=7, orange curve) with a median survival time of 41 days. B) Bivalent human IL-2 fusion toxin: 1) Vehicle control (same vehicle control as in Figure 3A); 2) bivalent human IL-2 fusion toxin at 3 μg/kg (n=7, dark green curve) with a median survival time of 17 days; 3) bivalent human IL-2 fusion toxin at 30 μg/kg (n=7, brown curve) with a median survival time of 30 days; 4) bivalent human IL-2 fusion toxin at 100 μg/kg (n=7, purple curve) with a median survival time of 34 days.
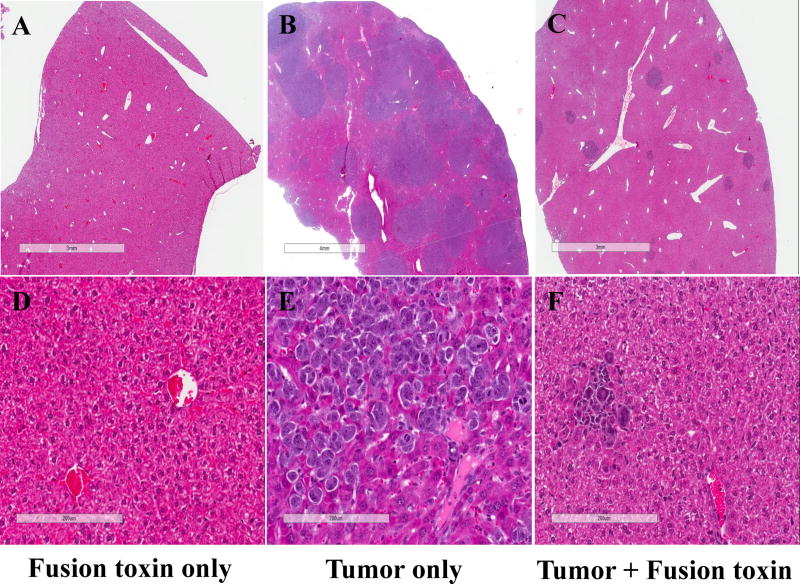
Liver pathology analysis was performed for three representative NSG mice. Animal #1 was injected only with bivalent human IL-2 fusion toxin at 100 μg/kg as control to determine the toxicity of the human IL-2 fusion toxin on host mouse cells. This animal was sacrificed at day 16 and pathology review revealed no effect of the fusion toxin on the host liver (Fig. 4A and 4D). Animal #2 was injected only with HUT102/6TG tumor cells to serve as an untreated control. This animal succumbed to tumors and was sacrificed at day 16. Pathology review showed extensive replacement of the liver parenchyma by a partially necrotic malignant tumor (Fig. 4B and 4E). Animal #3 was injected with both HUT102/6TG tumor cells and bivalent human IL-2 fusion toxin at 100 μg/kg. This animal was sacrificed at day 16 and only some sporadic tumor cell areas were identified in examined section of the liver (Fig. 4C and 4F). This pathology analysis demonstrated that the bivalent human IL-2 fusion toxin effectively depleted the human CD25+ tumor cells in vivo, as the untreated control animal developed visible malignancy (Fig. 4B and 4E) following the tumor cell injection.
Fig. 4.
Pathology analysis: A and D) Liver from an NSG mouse injected with bivalent human IL-2 fusion toxin at 100 μg/kg shows unremarkable hepatic parenchyma with no increase in hepatocyte necrosis or inflammation. B and E) Liver from a mouse injected with HUT102/6TG tumor cells shows replacement of liver parenchyma by sheets of malignant cells with condensed chromatin, prominent nucleoli, an elevated nuclear: cytoplasmic ratio, and geographic necrosis. C and F) Liver from a mouse injected with both HUT102/6TG tumor cells and bivalent human IL-2 fusion toxin at 100 μg/kg shows normal hepatic parenchyma in most of the examined section and a few sporadic malignant cells.
3.3. In vitro binding and efficacy analysis of the IL-2 fusion toxin to the residual tumor cells
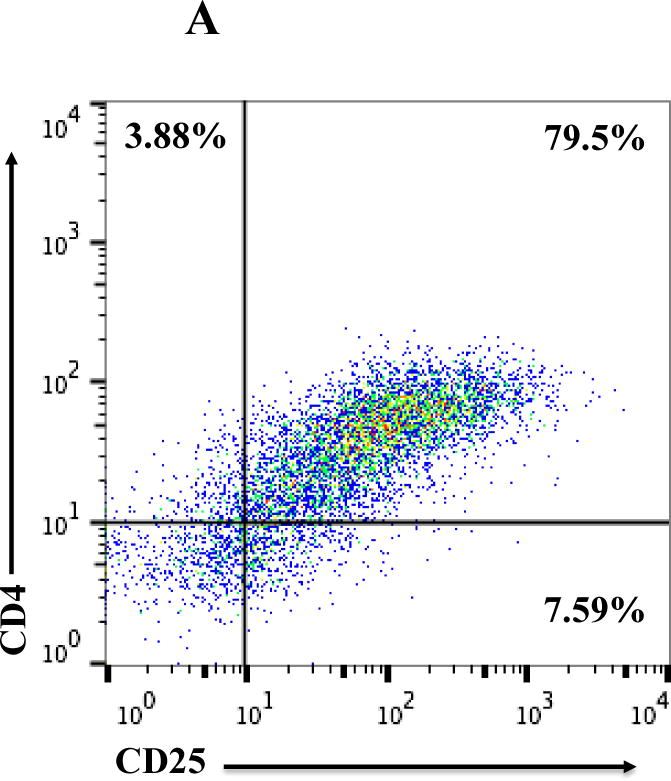
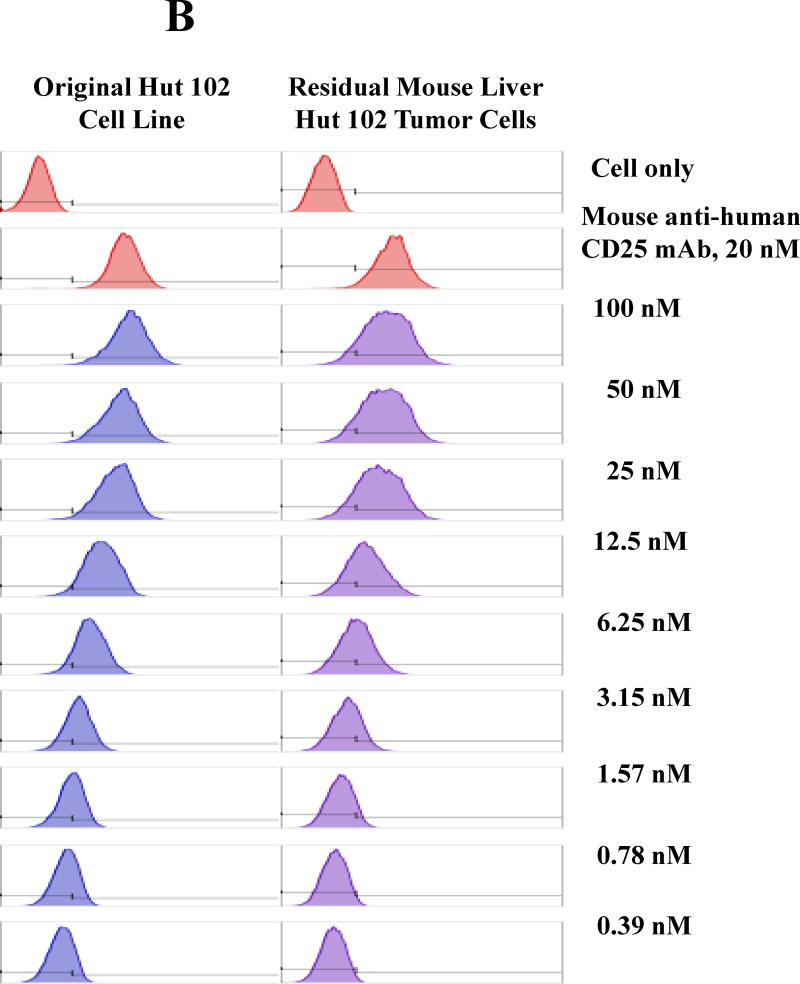
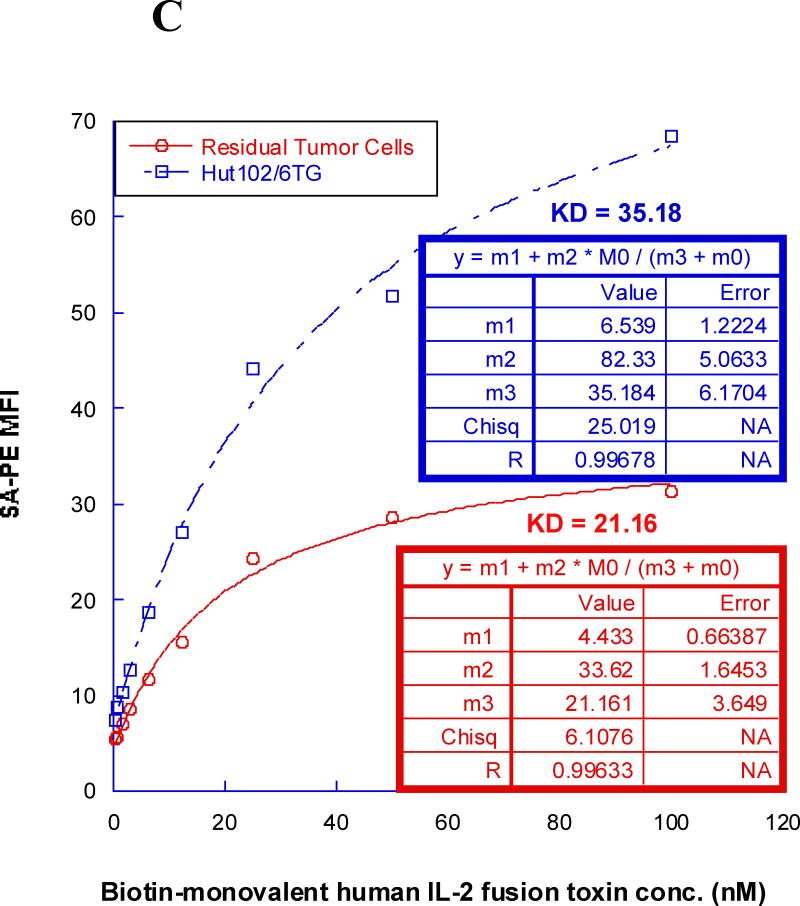
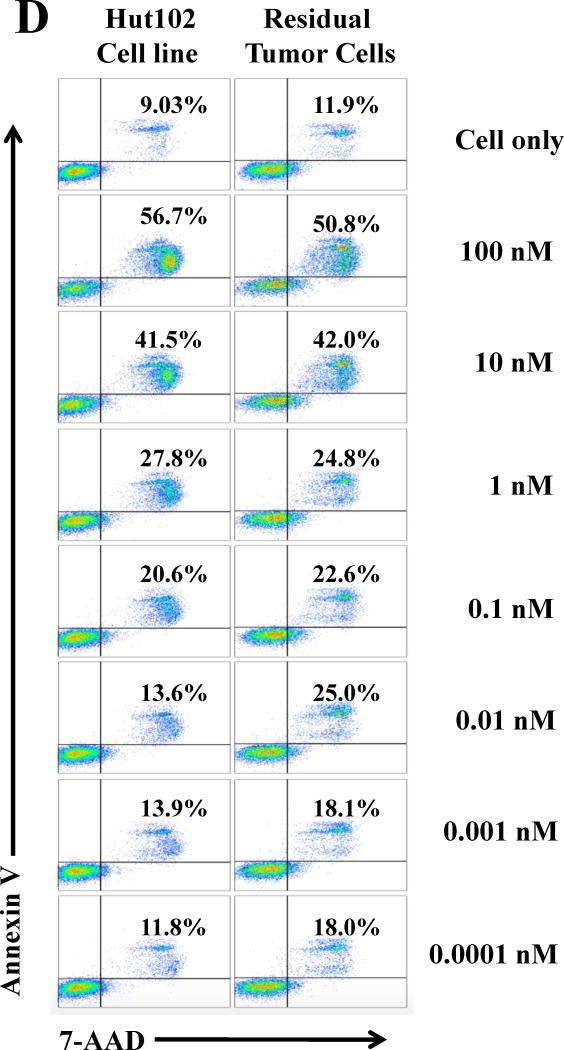
All of the HUT102/6TG tumor-bearing NSG mice eventually succumbed. We hypothesized that the residual tumor cells were still sensitive to the IL-2 fusion toxin treatment. To confirm this hypothesis, the residual tumor cells were collected from HUT102/6TG tumor-bearing mice livers at the study endpoint (day 35) following treatment with the bivalent human IL-2 fusion toxin at 100 μg/kg. Flow cytometry analysis demonstrated that the residual tumor cells were still CD25 positive (Fig. 5A). The residual tumor cells could be easily cultured with the same RPMI 1640-based medium for the parent tumor cell line HUT102/6TG (data not shown). The cultured residual tumor cells were further analyzed using biotinylated monovalent human IL-2 fusion toxin. As shown in Figure 5B, the biotinylated monovalent human IL-2 fusion toxin bound to the residual tumor cells in a dose-dependent fashion comparable to the binding to the parent human CD25+ HUT102/6TG cell line. The KD value (Figure 5C) for the residual tumor cells is even better than that of the parent HUT102/6TG cell line (21 nM for the residual tumor cells vs 35 nM for the HUT102/6TG cell line). The apoptosis flow was performed for the residual tumor cells using 7-AAD and Annexin V staining following incubation with the monovalent human IL-2 fusion toxin for 18 h. As shown in Figure 5D, the viability of the residual tumor cells is comparable with that of the parent HUT102/6TG tumor cell line following the human IL-2 fusion toxin treatment indicating that the residual tumor cells were still sensitive to the human IL-2 fusion toxin treatment.
Fig. 5.
In vitro flow cytometry binding analysis of the residual tumor cells. A) Flow cytometry binding analysis of the residual tumor cells with mAbs against human CD4 and CD25. The residual tumor cells were collected from the human CD25+ HUT102/6TG CTCL tumor-bearing NSG mouse liver at the study endpoint (day 35) following 10-day course treatment with the bivalent human IL-2 fusion toxin at 100 μg/kg. B) Flow cytometry binding analysis of the residual tumor cells (right panel) using the biotinylated monovalent human IL-2 fusion toxin. Mouse anti-human CD25 mAb was included as positive control. The parent human CD25+ HUT102/6TG cells were included as positive control cell line (left panel). C) KD determination using flow cytometry and nonlinear least squares fit. MFI was plotted over a wide range of concentrations of biotinylated monovalent human IL-2 fusion toxin. The accompanying least-squares fits are shown based on the hyperbolic equation y = m1 + m2 * m0/(m3 + m0) where y = MFI at the given biotinylated monovalent human IL-2 fusion toxin concentration, m0 = biotinylated human IL-2 fusion toxin concentration, m1 = MFI of zero biotinylated human IL-2 fusion toxin control, m2 = MFI at saturation and m3 = KD. D) Apoptosis flow of the residual tumor cells by staining with 7-ADD and Annexin V following incubation with the monovalent human IL-2 fusion toxin for 18 h. 1) HUT102/6TG cell line as control (left panel); 2) Residual tumor cells (right panel). The data are representative of two individual experiments.
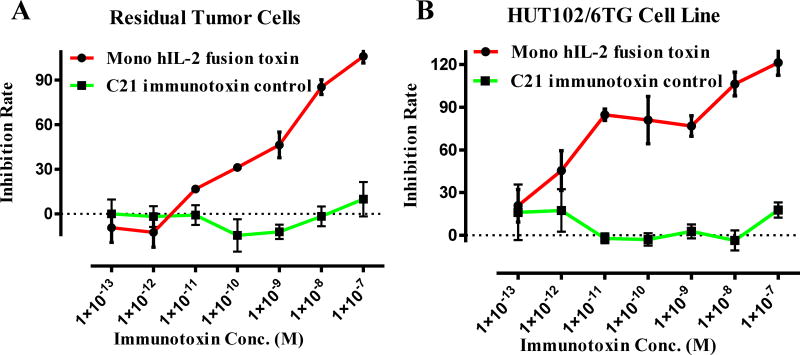
In vitro efficacy analysis of the IL-2 fusion toxin to the residual tumor cells was performed using Promega CellTiter-Glo® Luminescent Cell Viability Assay. As shown in Figure 6, the residual tumor cells were still sensitive to the human IL-2 fusion toxin.
Fig. 6.
In vitro efficacy analysis of the monovalent human IL-2 fusion toxin to the residual tumor cells using CellTiter-Glo® Luminescent Cell Viability Assay (Promega, cat# G7571). A) To the residual tumor cells: 1) monovalent human IL-2 fusion toxin (red line); 2) C21 immunotoxin as negative control (black line). B) To the HUT102/6TG cell line: 1) monovalent human IL-2 fusion toxin (red line); 2) C21 immunotoxin as negative control (black line). Y-axis: inhibition rate of the cell viability by determining the number of viable cells based on the quantification of the ATP present. X-axis: plated human IL-2 fusion toxin concentration. Cycloheximide (1.25 mg/mL) was used as a positive control. The negative control contained cells without immunotoxin. P < 0.0001 by Log-rank (Mantel-Cox) Test of Prism (n=3). Error bars indicate ±SD. Data are representative of three assays.
3.4. In vivo efficacy analysis to the residual tumor cells
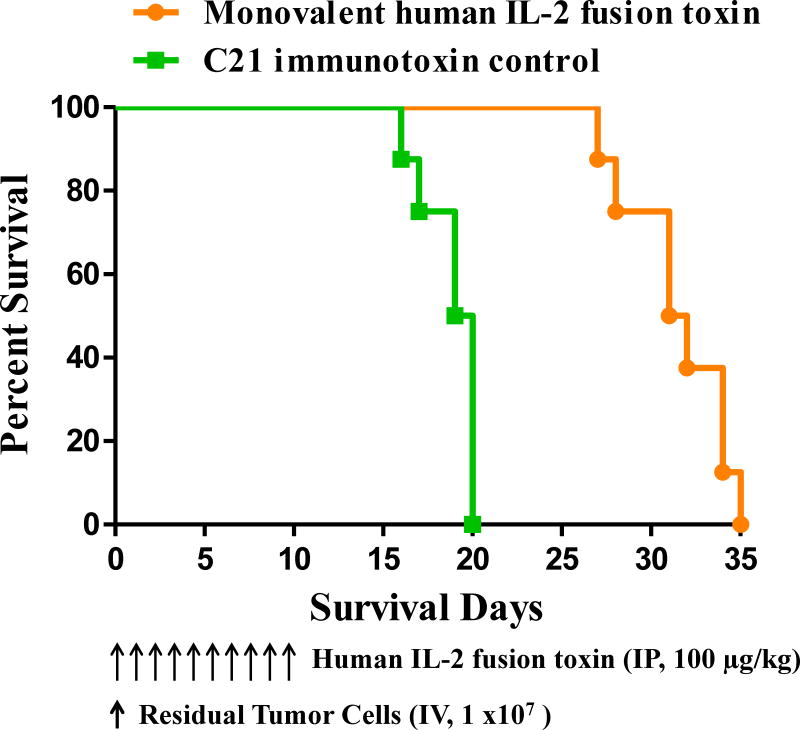
To confirm the in vitro binding and efficacy data of the IL-2 fusion toxin to the residual tumor cells, in vivo efficacy analysis was performed using the residual tumor cell bearing NSG mice as performed using the parent human CD25+ HUT102/6TG tumor bearing NSG mice. The dosing schedule was 100 μg/kg, daily for consecutive 10 days. As shown in Figure 7, the monovalent human IL-2 fusion toxin significantly prolonged the survival of the residual tumor cell bearing NSG mice from median survival of 19.5 days of the negative control C21 immunotoxin (a non-related DT390 based immunotoxin) group to 31.5 days of the monovalent human IL-2 fusion toxin group. The data demonstrated that the residual tumor cells were still sensitive to the human IL-2 fusion toxin in vivo. We only chose the monovalent human IL-2 fusion toxin for this in vivo analysis and some in vitro assays in this study because the in vivo efficacy of the monovalent isoform was comparable with that of the bivalent isoform using the HUT102/6TG bearing NSG mouse model. The production level of the monovalent isoform was much higher than that of the bivalent isoform.
Fig. 7.
In vivo efficacy analysis of the monovalent human IL-2 fusion toxin to the residual tumor cells. NSG mice were injected IV with 1.0 ×107 residual tumor cells on day 0 and treated from day 0 on with the monovalent human IL-2 fusion toxin at 100 μg/kg daily for 10 consecutive days. The schedule of drug and tumor cell injection is pictured in the schematic below the survival curve. The vertical arrows indicate the days on which the residual tumor cells or human IL-2 fusion toxins were injected. 1) Monovalent human IL-2 fusion toxin at 100 μg/kg (n=7, orange curve) with a median survival time of 31.5 days. 2) C21 immunotoxin as negative control at 100 μg/kg (n=7, green curve) with a median survival time of 19.5 days.
Discussion
In this study, we have demonstrated that the human IL-2 fusion toxins were effective in vivo using human CD25+ HUT102/6TG CTCL tumor bearing NSG mouse model. We also demonstrated that the human IL-2 fusion toxin was still effective to the residual tumor cells from the HUT102/6TG bearing NSG mouse liver. We did not observe any toxicity at the highest dose tested. To eradicate the tumor cells more completely, it might be necessary to administer the fusion toxin with higher dose and/or longer duration such as multiple cycles.
Human IL-2 fusion toxins were developed using advanced unique diphtheria toxin resistant yeast Pichia Pastoris expression system which facilitated large scale production for marketing. Given that the yeast-expressed human IL-2 fusion toxins demonstrated in vivo antitumor efficacy without any toxicity at the doses tested, they represent potential candidates to replace the discontinued Ontak® for research community and in the clinic.
We assumed that the bivalent isoform would be more potent than the monovalent isoform in vivo as we demonstrated in vitro (Peraino et al., 2014). However, the in vivo data demonstrated that the in vivo efficacies for both isoforms are comparable. Given the production level is significantly higher for the monovalent isoform than that of the bivalent isoform, the monovalent isoform will be investigated further in the future. Of course, we cannot rule out the possibility that the used animal model and dosing schedule were not suitable to demonstrate the benefits of the bivalent isoform.
Highlights.
Human IL-2 fusion toxin was developed using yeast Pichia Pastoris expression system.
In vivo efficacy was assessed using human CD25+ tumor-bearing NSG mouse model.
The residual tumor cells are still sensitive to the human IL-2 fusion toxin treatment.
The IL-2 fusion toxin is a promising candidate to replace the discontinued Ontak.
Acknowledgments
This study was supported in part by NIH/NCI SBIR grant (1R43CA192540-01 to Paul Guyre). We would like to thank the Dartmouth Transgenic Shared Resource for their support. We would like to thank Dr. Robert Harrison for generously providing HUT 102/6TG cell line.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Duvic M, Kuzel TM, Olsen EA, Martin AG, Foss FM, Kim YH, Heald PW, Bacha P, Nichols J, Liepa A. Quality-of-life improvements in cutaneous T-cell lymphoma patients treated with denileukin diftitox (ontak) Clin Lymphoma. 2002;2:222. doi: 10.3816/clm.2002.n.003. [DOI] [PubMed] [Google Scholar]
- Peraino JS, Schenk M, Zhang H, Li G, Hermanrud CE, Neville DM, Jr, Sachs DH, Huang CA, Duran-Struuck R, Wang Z. A truncated diphtheria toxin based recombinant porcine CTLA-4 fusion toxin. J Immunol Methods. 2013a;391:103. doi: 10.1016/j.jim.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peraino JS, Schenk M, Li G, Zhang H, Farkash EA, Sachs DH, Huang CA, Duran-Struuck R, Wang Z. Development of a diphtheria toxin-based recombinant porcine IL-2 fusion toxin for depleting porcine CD25+ cells. J Immunol Methods. 2013b:398–399. 33. doi: 10.1016/j.jim.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peraino JS, Zhang H, Rajasekera PV, Wei M, Madsen JC, Sachs DH, Huang CA, Wang Z. Diphtheria toxin-based bivalent human IL-2 fusion toxin with improved efficacy for targeting human CD25+ cells. J Immunol Methods. 2014;405:57. doi: 10.1016/j.jim.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince HM, Duvic M, Martin A, Sterry W, Assaf C, Sun Y, Straus D, Acosta M, Negro-Vilar A. Phase III placebo-controlled trial of denileukin diftitox for patients with cutaneous t-cell lymphoma. J Clin Oncol. 2010;28:1870. doi: 10.1200/JCO.2009.26.2386. [DOI] [PubMed] [Google Scholar]
- Wang Z, Wei M, Zhang H, Chen H, Germana S, Huang CA, Madsen JC, Sachs DH, Wang Z. Diphtheria-toxin based anti-human CCR4 immunotoxin for targeting human CCR4+ cells in vivo. Mol Oncol. 2015;9:1458–70. doi: 10.1016/j.molonc.2015.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DP, Wen Z, Watson RS, Boyd J, Strom TB, Murphy JR. Cellular Processing of the Interleukin-2 Fusion Toxin DAB486-IL-2 and Efficient Delivery of Diphtheria Fragment A to the Cytosol of Target Cells Requires Arg194. J Biol Chem. 1990;265:20673. [PubMed] [Google Scholar]
- Zheng Q, Wang Z, Zhang H, Huang Qi, Madsen JC, Sachs DH, Huang CA, Wang Z. Diphtheria toxin-based anti-human CD19 immunotoxin for targeting human CD19+ tumors. Mol Oncl. 2017;11:584–594. doi: 10.1002/1878-0261.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]