Abstract
Non-alcoholic fatty liver disease (NAFLD) is a spectrum of liver disorders. It is defined by the presence of steatosis in more than 5 % of hepatocytes with little or no alcohol consumption. Insulin resistance, the metabolic syndrome or type 2 diabetes and genetic variants of PNPLA3 or TM6SF2 seem to play a role in the pathogenesis of NAFLD. The pathological progression of NAFLD follows tentatively a ‘three-hit’ process namely steatosis, lipotoxicity and inflammation. The presence of steatosis, oxidative stress and inflammatory mediators like TNF-α and IL-6 have been implicated in the alterations of nuclear factors such as CAR, PXR, PPAR-α in NAFLD. These factors may results in altered expression and activity of drug metabolizing enzymes (DMEs) or transporters.
Existing evidence suggests that the effect of NAFLD on CYP3A4, CYP2E1 and MRP3 are more consistent across rodent and human studies. CYP3A4 activity is down-regulated in NASH whereas the activity of CYP2E1 and the efflux transporter MRP3 are up-regulated. However, it is not clear how the majority of CYPs, UGTs, SULTs and transporters are influenced by NAFLD either in vivo or in vitro. The alterations associated with NAFLD could be a potential source of drug variability in patients and could have serious implications for the safety and efficacy of xenobiotics. In this review, we summarize the effects of NAFLD on the regulation, expression and activity of major drug metabolizing enzymes and transporters. We also discuss the potential mechanisms underlying these alterations.
Keywords: Non-alcoholic fatty liver disease, steatosis, non-alcoholic steatohepatitis, diabetes, drug metabolizing enzymes, transporters, cytochrome P450
INTRODUCTION
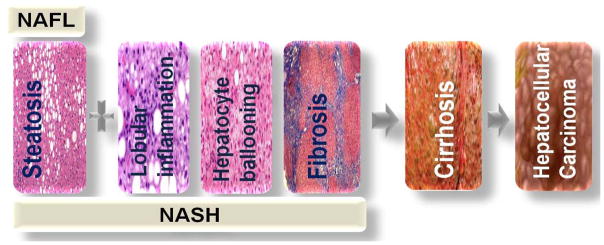
Non-alcoholic fatty liver disease (NAFLD) is a spectrum of liver disorders (Figure 1). It is a condition defined by the presence of steatosis in more than 5 % of hepatocytes (Sanyal et al., 2011) with little or no alcohol consumption. NAFLD consists of the benign non-alcoholic fatty liver (NAFL), and the more severe non-alcoholic steatohepatitis (NASH). NASH is a more progressive form of NAFLD and is characterized by steatosis, hepatocellular ballooning, lobular inflammation and almost always fibrosis (Kleiner and Makhlouf, 2016). In an effort to regenerate new cells, NASH progresses (Argo and Caldwell, 2009, Starley et al., 2010) to cirrhosis with the hepatocytes replaced by scar tissues of type I collagen produced by stellate cells. Cirrhosis is an end-stage organ failure that require liver transplantation or may lead to hepatocellular carcinoma (Sorensen et al., 2003, Yasui et al., 2011). With progression of NASH to full-blown cirrhosis, some of the histological characteristics of NASH might be lost (Yoshioka et al., 2004).
Figure 1.
The progressive stages of NAFLD (non-alcoholic fatty liver disease). The benign form of NAFLD, NAFL (non-alcoholic fatty liver), progresses to NASH (non-alcoholic steatohepatitis) with or without fibrosis. Subsequently, NASH leads to cirrhosis and eventually hepatocellular carcinoma (HCC). NASH may progress to HCC without cirrhosis.
The metabolic syndrome, formerly known as Syndrome X, underlies both non-alcoholic fatty liver disease (NAFLD) and diabetes. It is defined by the presence of at least three of the following (Figure 2): abdominal obesity, increased triglycerides, reduced high density lipoprotein (HDL) cholesterol, increased blood pressure and hyperglycemia (Alberti et al., 2009). Insulin resistance appears to explain almost all situations of metabolic syndrome (Eckel et al., 2010); and hence diabetes (Groop, 1999) and NAFLD (Marchesini et al., 1999).
Figure 2.
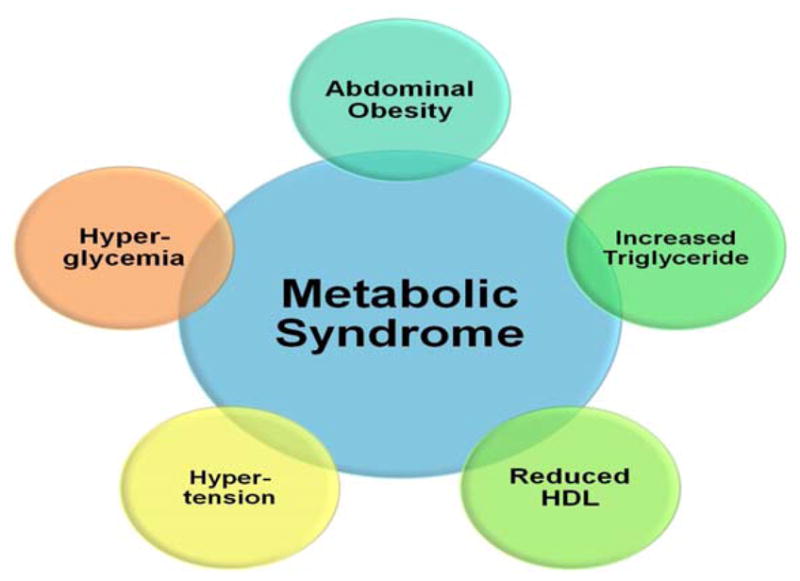
Major components of the metabolic syndrome. The presence of at least three of these components define the presence of metabolic syndrome.
Though NAFLD is more prevalent in obese and diabetic patients, it is also present in lean and non-diabetic individuals (Vos et al., 2011, Younossi et al., 2012). It is the most common cause of cryptogenic cirrhosis (Clark and Diehl, 2003) and approximately 30–50 % of NASH patients may progress to cirrhosis within 10 years (Jou et al., 2008). NAFLD is not only common in industrialized countries, but also developing ones. Global prevalence of NAFLD has been reviewed and ranges from 6 – 35 % (Fazel et al., 2016, Sayiner et al., 2016, Bellentani, 2017); and approximately 30% of the population of the United States (90 million persons) are estimated to be affected by NAFLD (Fazel et al., 2016). Eighteen out of 25 million Americans with diagnosed type 2 diabetes are believed to have NAFLD while 63–87% of patients having both diabetes and NAFLD may have NASH (Bazick et al., 2015, Corey et al., 2016). The economic burden of NAFLD in four European countries (Germany, France, Italy and the United Kingdom) was projected to be ~35 billion US dollars compared to the approximately 103 billion dollars in the United States (Younossi et al., 2016).
Pharmacotherapy of NAFLD or NASH is an unmet clinical need. To date, no drug has received FDA approval for NASH (Sanyal et al., 2015), thus a clinical or regulatory pathway has not yet been established. Current therapies like vitamin E (Rinella and Sanyal, 2016), pentoxifylline (Zein et al., 2011) and insulin sensitizers such as pioglitazone in patients with diabetes (Cusi, 2016) have been used. Therapies in development include obeticholic acid, a semi-synthetic bile acid analogue undergoing development by Intercept Pharmaceuticals, and elafibranor (formerly GFT505) a Peroxisome proliferator-activated receptor (PPAR) alpha and a gamma agonist (Rinella and Sanyal, 2016). In view of the lack of standard therapy, international guidelines on NAFLD (European Association for the Study of the Liver (EASL), 2016) recommend lifestyle modifications particularly diet and exercise as viable treatment options. Recently, a role for Mediterranean diet in the prevention and treatment of NAFLD has been proposed (Abenavoli et al., 2014, Godos et al., 2017).
The main clearance mechanisms of xenobiotics from the body are hepatic, renal and biliary. It has been reported that more than 60 % of commonly prescribed drugs in the United States are cleared hepatically (Williams et al., 2004), indicating the crucial role of the liver in drug metabolism. Hepatic clearance of drugs is achieved through the activities of drug metabolizing enzymes (DMEs) and transporters and hence factors that affect their regulation and activities eventually alter drug disposition.
In this review, we summarize the effects of NAFLD on the regulation, expression and activity of major drug metabolizing enzymes and transporters. In addition, we discuss the various classification systems of NAFLD and the potential mechanisms underlying these alterations. Our review however does not include a discussion on models of NAFLD and most findings published before 2011 since these have been reviewed by other groups (Merrell and Cherrington, 2011, Naik et al., 2013).
Pathogenesis of NAFLD
The mechanisms leading to NAFLD is unclear to date. Several mechanisms have been proposed, but insulin resistance seems to be pivotal in the pathogenesis of both NAFLD and type 2 diabetes (Shulman, 2000, Tarantino and Finelli, 2013). The genetic variant of PNPLA3 (patatin-like phospholipase domain containing 3), an enzyme encoding I148M (rs738409 C/G) and involved in the hydrolysis of triacylglycerols in adipocytes, has been reported to be associated with NAFLD independent of the metabolic syndrome (Romeo et al., 2008, Sookoian and Pirola, 2011). Similarly, the genetic variant of the lipid transporter located on ER (endoplasmic reticulum) and ER-Golgi compartments, TM6SF2 (transmembrane 6 superfamily member 2), encoding E167K (rs58542926 C/T), causes loss of function of the protein and increases hepatic deposition of triglycerides (Dongiovanni et al., 2015). The pathological progression of NAFLD follows tentatively a ‘three-hit’ process (Jou et al., 2008) namely steatosis, lipotoxicity and inflammation.
Steatosis results from the interplay between diet, gut microbiota (Jiang et al., 2015, Kirpich et al., 2015), genetic factors (Romeo et al., 2008), and de novo lipogenesis via up-regulation of lipogenic transcription factors like sterol regulatory binding protein-1c (SREBP1c), carbohydrate-responsive element-binding protein (chREBP), and peroxisome proliferator-activated receptor gamma (PPAR-γ) (Anderson and Borlak, 2008). Primarily, fatty acid (FA) is stored in the adipose tissue as TAG (triacylglycerol). However, in obese subjects, fatty acids seem to be misrouted from their primary storage site to ectopic sites like skeletal and hepatic tissues for re-esterification into diacyl glycerols (DAGs), perhaps through increased adipocyte lipolysis. The uptake of fatty acid by these organs probably is facilitated by fatty acid transport proteins (FATPs) and FAT/CD36 (fatty acid translocase) which have been shown to be elevated in obese subjects and NAFLD patients (Greco et al., 2008, Fabbrini et al., 2009).
Steatosis leads to increased signalling of the transcription factor NF-κβ (nuclear factor – kappaβ) through the upstream activation of IKKβ (inhibitor of nuclear factor kappaB [NF-κB]). The activation of NF-κβ induces the production of pro-inflammatory mediators like TNF-α (tumor necrosis factor - alpha), IL-6 (interleukin-6) and IL-1β (interleukin-1 β). These cytokines contribute to the recruitment and activation of Kupffer cells (resident hepatic macrophages) (Anderson and Borlak, 2008) to mediate inflammation in NASH (Ramadori and Armbrust, 2001, Joshi-Barve et al., 2007). Additionally, TNF- α and IL-6 have been reported to play a role in hepatic insulin resistance through the up-regulation of SOCS3 (suppressor of cytokine signalling 3) (Persico et al., 2007, Torisu et al., 2007).
The excess fat in the liver causes lipotoxicity and leads to organelle failure mainly mitochondrial dysfunction and endoplasmic reticulum stress (Browning and Horton, 2004, Bell et al., 2008 ). A dysfunctional mitochondrion has an elevated capacity to oxidize FA resulting in the production of ROS (reactive oxygen species) and causing oxidative stress due to an imbalance between the production of ROS and protective oxidants. Oxidative stress in NAFLD patients (Sanyal et al., 2001, Tiniakos et al., 2010) is regarded as the third insult that eventually leads to hepatocyte death. The pathogenesis of NAFLD seem to be a vicious cycle of steatosis, lipotoxicity and inflammation resulting in intricate alterations in the histopathological and biochemical features of the liver.
Diagnosis and Classification of NAFLD
The diagnosis of NAFLD is challenging, as the current available routine techniques (serological tests and imaging techniques) are unable to distinguish between steatosis and NASH. Liver biopsy is considered the gold standard in defining NAFLD and is capable of differentiating steatosis and NASH. It is however, not recommended for routine use due to increased risk of bleeding and complications. In the last decades, many diagnostic non-invasive tools have been described (Table 1). Accurate diagnosis of NAFLD is important for its classification. Some of the classification systems available include the scoring systems by Matteoni (Matteoni et al., 1999 ), Brunt (Brunt et al., 1999), NASH CRN (Clinical Research Network) system (Kleiner et al., 2005), and the SAF (steatosis, activity and fibrosis) system (Bedossa et al., 2012). The different classification systems of NAFLD may thus yield different results and hence introduce variability into scientific investigations.
Table 1.
Biomarkers and imaging techniques employed in diagnosis of NAFLD.
| Diagnosis Tools | Technique/Principle | Features | References |
|---|---|---|---|
| Serological Tests | Aspartate aminotransferase (AST) | Raised levels not indicative of NAFLD because AST and ALTs are normal in some NAFLD patients. | (Mofrad et al., 2003, Browning et al., 2004, Bugianesi et al., 2004) |
| Alanine aminotransferase (ALT) | |||
| AST/ALT | > 1 is predictive of fibrosis | ||
| Imaging Techniques | Ultrasonography | Sensitive when steatosis is > 30 % of hepatocytes; Does not distinguish between steatosis and NASH | (Wieckowska and Feldstein, 2008) |
| Computerized Tomographic (CT) Scanning Magnetic Resonance Imaging (MRI) |
More sensitive than ultrasonography Cannot distinguish between steatosis and NASH Expensive |
||
| Transient Elastography | Can detect fibrosis but expensive | ||
| Liver Biopsy | Histological evaluation of hepatic tissues. Hepatic lesions like steatosis, inflammation and ballooning are graded; and fibrosis is staged. |
Gold Standard but invasive and may be involved with complications and sampling variability Able to detect steatosis and inflammation |
(Ratziu et al., 2005, Wieckowska and Feldstein, 2008) |
One of the pioneering works with the largest number of patients and longest follow-up for the stratification of NAFLD patients was carried out Matteoni and colleagues (Matteoni et al., 1999 ). The Matteoni’s system was based on fat accumulation, inflammation, ballooning degeneration, Mallory hyaline and fibrosis. NAFLD patients were put into four groups: Type I (simple fatty liver), Type II (steatohepatitis), Type III (steatonecrosis) and Type IV (steatonecrosis plus either Mallory hyaline or fibrosis). Type I was relatively benign whereas the necrotic forms were considered aggressive. The aggressive forms have higher risk of cirrhosis and liver-related death. Though this system helps to identify patients at risk of cirrhosis and liver-related death, it does not take into account NAFLD in children.
The system developed by Brunt (Brunt et al., 1999, Brunt et al., 2004) is semi-quantitative and evaluates the unique lesions of NASH. It unifies steatosis and steatohepatitis into a ‘grade’ and fibrosis into a ‘stage’(Angulo, 2002). Steatosis is graded on a scale of 1 to 3 depending on the percentage of hepatocytes affected (<33 % =1; 33–66% = 2; >66% = 3). Steatohepatitis was similarly graded on a scale of 1 to 3 (1 = mild; 2 = moderate; 3 = severe) but on the basis of the severity and extent of steatosis, ballooning, lobular inflammation and portal inflammation. Fibrosis on the other hand was staged on a scale of 1 to 4. Brunt’s system does not cover the entire spectrum of NAFLD as defined by Matteoni’s system. Additionally, it was not designed to evaluate NAFLD in children (Kleiner et al., 2005).
In 2005, the Pathology Committee of the NASH Clinical Research Network (NASH CRN) of the National Institute of Diabetes & Digestive & Kidney Disease (NIDDK) came up with a scoring system and NAFLD activity score (NAS) for use in clinical trial (Kleiner et al., 2005). The scoring system was intended to address the full spectrum of lesions of NAFLD. The histological features considered were grouped into five broad categories each with a scoring scale. These features, which were independently associated with NASH, included steatosis (0–3), lobular inflammation (0–3), hepatocellular injury (0–2), fibrosis (0–4) and miscellaneous features like Mallory’s hyaline and glycogenated nuclei. The NAS is the unweighted sum of steatosis, lobular inflammation, and hepatocellular ballooning scores. NAS of ≥ 5 was found to correlate with the diagnosis of NASH and biopsies with scores of less than 3 were classified as “not NASH”. Notwithstanding, not all biopsies with NAS ≥ 5 meet the diagnostic criteria of definite NASH and should be used carefully in establishing the presence or absence of NASH (Brunt et al., 2011). In a number of experimental work involving humans and rodents, a NAS score of at least 4 was considered as NASH (Canet et al., 2014, Ferslew et al., 2015).
Recently, the SAF (steatosis, activity and fibrosis) system has been proposed. The SAF considers steatosis, lobular inflammation and ballooning in defining NAFL and NASH. The activity is defined as the sum of the grades of lobular inflammation and ballooning and ranges from 0–4. The presence of NAFLD is defined by steatosis in the presence of any degree of activity. This implies that the definition of either NAFL or NASH requires the presence of steatosis (1–3) and varying degree of activity (NAFL: steatosis (1–3) + lobular inflammation (0) + ballooning (0–2), or steatosis (1–3) + lobular inflammation (1–2) + ballooning (0); and NASH: steatosis (1–3) + lobular inflammation (1) + ballooning (1–2) or steatosis (1–3) + lobular inflammation (2) + ballooning (1–2)) (Bedossa et al., 2012, Kleiner and Makhlouf, 2016).
Clinicobiological scores have also been used in relation to NAFLD for several reasons including selection of patients needing biopsy and prediction of advanced forms of NASH. These clinicobiologial scores make use of indices like body mass index (BMI), Age, AST/ALT ratio, albumin, platelet count, diabetes, hyperglycemia, insulin resistance index, triglycerides, hypertension and others (Angulo et al., 1999, Dixon et al., 2001, Harrison et al., 2003). For instance, ‘BAAT’ scoring (Ratziu et al., 2000 ) uses BMI, age, ALT, and serum triglycerides. The BAAT score is calculated as the sum of categorical variables with a scale of 0 to 4. A score of 0 or 1 on the BAAT scale would indicate absence of septal fibrosis. ‘HAIR’ scoring (Dixon et al., 2001) on the other hand utilizes hypertension, ALT and insulin resistance as an index with a scale of 0 to 3. A score of ≥2 is suggestive of NASH.
Possible mechanisms of the alteration of DMEs and transporters in NAFLD and diabetes
The influence of diseases on DMEs and transporters is complex due to the associated physiological and pathological changes. For instance, inflammatory conditions have been reported to cause the release of circulating pro-inflammatory cytokines like TNF-α, IL-1β, and IL-6 which act as signalling molecules to mediate the down-regulation of drug metabolizing enzymes partly through the suppression of transcription (Aitken et al., 2006, Aitken and Morgan, 2007). The inflammation models, bacteria endotoxemia (lipopolysaccharide (LPS)) and turpentine have been employed in rodents and hepatocytes to gain some insight into the role of cytokines on the regulation of DMEs and transporters. It seems that in majority of cases, inflammation and the associated cytokines down-regulate the expression and activity of DMEs and some transporters as described in these reviews (Aitken et al., 2006, Morgan, 2009).
Oxidative stress in NAFLD and diabetes causes activation of Nrf2 (nuclear factor erythroid 2-related factor 2) in both experimental (Fisher et al., 2008) and clinical studies (Hardwick et al., 2010). Nrf2 is a specific transcription factor that controls the antioxidant response. It is released from keapl (Kelch-like ECH-associated protein 1) and translocates to the nucleus where it binds to antioxidant response element (ARE) within promoters of target genes, and induces expression of DMEs and transporters central to the maintenance of oxidative stress inducing molecules (Jaiswal, 2004, Nakata et al., 2006, Zhang, 2006).
Fatty acids regulate gene expression by controlling the activity or expression of key nuclear receptors. In vitro studies have identified many transcription factors as possible targets for fatty acid regulation, including hepatic nuclear factors (HNF-4α and γ), PPARα, β, γ1, and γ2, SREBP-1c, retinoid X receptor (RXRα), liver X receptor (LXRα), and others. Some nuclear receptors, PPAR, HNF4 (hepatic nuclear factor), RXRα, and LXRα, bind directly to non-esterified fatty acids (NEFA), but others like SREBP-1c and NF-κB are regulated by fatty acids through indirect mechanisms (Jump et al., 2005, Jump, 2008). In rodents, SREBP-1c inhibits PXR (pregnane X receptor) and CAR (constitutive androstane receptor) (Roth et al., 2008), and has been shown to be up-regulated in obese insulin-resistant patients (Pettinelli et al., 2009). The modulation of the activity of CAR and PXR by polyunsaturated fatty acids (PUFA) has also been reported (Finn et al., 2009).
In addition, changes in the architecture of the liver in hepatic cirrhosis have been reported to cause reduced liver blood flow, reduced functional hepatocytes and diminished functional capacity of the liver to synthesize serum proteins including albumin (Elbekai et al., 2004, Edginton and Willmann, 2008, Johnson et al., 2010). Collectively, the changes mediated by excess fatty acids, cytokines, oxidative stress, and other mechanisms in NAFLD and diabetes may affect the hepatic metabolism of certain drugs possibly through the alteration of the expression and activity of DMEs and transporters. This could result from host defence mechanisms at the transcriptional as well as pre- and post-translational levels (George et al., 1995, Renton, 2004, Aitken et al., 2006). These aberrant signals disrupt the normal hepatic signalling pathways and eventually dysregulate major drug-metabolism-associated nuclear factors leading to altered drug metabolism in NAFLD and diabetic patients (Naik et al., 2013).
Hepatic Drug Metabolism
Phase I reactions are mainly oxidative processes and are predominantly carried out by the cytochrome P450 (CYP) enzyme system (Guengerich and MacDonald, 1990, Guengerich, 2008, Guengerich and Munro, 2013). Of the 18 known families of CYP enzymes (Zanger and Schwab, 2013), only a few of the members belonging to families 1, 2 and 3 appear to be relevant to biotransformation of xenobiotics (Cholerton et al., 1992, Zanger and Schwab, 2013). These include CYP1A1, CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1, CYP2J2, CYP3A4, and CYP3A5. Non-CYP enzymes involved in phase I reactions include monoamine oxidase, flavin-containing monooxygenase (Rettie et al., 1995, Fisher et al., 2002) and aldehyde oxidase (Johns, 1967 ).
Phase II biotransformation on the other hand are primarily conjugation reactions and it includes glucuronidation (Meech and Mackenzie, 1997), sulfation (Negishi et al., 2001), and glutathione conjugation (Sofia et al., 1997). The enzymes responsible for these processes are Uridine diphosphate (UDP) - glucuronosyl transferases (UGTs), Sulfotransferases (SULT), and Glutathione -S-transferases (GSTs) respectively.
Drug transporters are crucial for metabolism of drugs and has been reviewed by several groups (Giacomini et al., 2010). Hepatic transporters are classified into uptake and efflux transporters (Mizuno and Sugiyama, 2002, Mizuno et al., 2003). The main uptake transporters belong to the solute carrier (SLC) superfamily and facilitate the movement of drugs into cells. These include OATPs (organic anion transporting polypeptides), OCTs (organic cation transporter), and OATs (organic anion transporter). The efflux transporters on the other hand belong to the ABC (ATP-binding cassette) superfamily and help move drugs out of cells (Mizuno et al., 2003, Sugiura et al., 2006). Examples include P-gp (P-glycoprotein), BCRP (Breast cancer resistance protein) and MRPs (Multidrug resistance-associated protein).
Several factors have been reported to affect DMEs and transporters. These include genetic polymorphisms, epigenetic factors, and non-genetic factors. Genetic polymorphisms result in alterations in DNA sequence of genes that regulate the expression of DMEs and transporters; and have led to loss-of-function or gain-of-function variants. The association between genetic polymorphisms and variation of plasma concentration levels of drugs as well as response has been extensively studied (Koren et al., 2006, Elens et al., 2011). Epigenetic influences on drug metabolism have also been reported. These are heritable changes in gene function that are not based on DNA sequence variation, but covalent modification of DNA, modification of histones or microRNA regulation (Pan et al., 2009, Mohri et al., 2010). In addition to the above, non-genetic factors like sex (Schmidt et al., 2001, Wolbold et al., 2003), age (Cotreau et al., 2005, Stevens et al., 2008) and disease state like diabetes (Dostalek et al., 2011, Dostalek et al., 2012a, Dostalek et al., 2012b) affect the expression and activity of DMEs and transporters.
Effect of NAFLD on Phase I Drug Metabolizing Enzymes (DMEs)
CYP3A
This gene is part of a cluster of cytochrome P450 genes on chromosome 7q21.1 and includes four genes - 3A4, 3A5, 3A7 and 3A43 (Zanger and Schwab, 2013). It is the most abundant human cytochrome P450 isoform in the liver and is involved in the metabolism of about half of clinically useful drugs (Guengerich, 1999). The CYP3A5 isoform is expressed mostly in Africans (Diczfalusy et al., 2011). It also exhibits wide inter-individual variability in its expression and activity through polymorphisms, epigenetic and non-genetic influences.
The influence of NAFLD on the expression and activity of CYP3A has been studied using animal and cell culture models, human hepatic tissues, and human subjects (Woolsey et al., 2015). Previous studies in rats and mice models are conflicting. However, a more consistent result have been emerging showing down-regulation of the mRNA and protein expressions, and the corresponding CYP3A activity in NAFLD (Table 2). This is perhaps due the use of models that are able to simulate better the metabolic and histological lesions of NAFLD. The activity of CYP3A decreased with severity of steatosis (Kolwankar et al., 2007) and with the progression of NAFLD (Woolsey et al., 2015). Dostalek et al. (2011) observed significantly lower protein levels, reduced enzymatic activity of CYP3A4 and unchanged mRNA levels in microsomal fractions of human diabetes mellitus livers (Dostalek et al., 2011). Again, the plasma levels of atorvastatin, a substrate of CYP3A4 (Lennernäs, 2003), has been reported to be elevated in patients with diabetes mellitus (Dostalek et al., 2012b). In view of the high prevalence of NAFLD in the diabetic population, it is likely that NAFLD could be involved in the down-regulation of CYP3A4 activity in the diabetic patients.
Table 2.
The effect of NAFLD on CYP3A4/CYP3A5. Overall, NAFLD progression seem to reduce the activity of CYP3A.
| Study | NAFLD Model | NAFLD category | mRNA | Protein | Activity | Activity Probe |
|---|---|---|---|---|---|---|
| (Kolwankar et al., 2007) | Human liver tissues (Ex vivo) | Steatosis | Decreased | Slight decrease | Decreased | Testosterone |
| (Fisher et al., 2009) | Human liver tissues (Ex vivo) | Steatosis | No change | Slight increase | Decreased | Testosterone |
| NASH (fatty) | No change | Decreased | Decreased | |||
| NASH (not fatty) | No change | Decreased | Decreased | |||
| (Woolsey et al., 2015) | Human Subjects (in vivo) | Steatosis | Not Reported | Not reported | Decreased (2.4 fold) | Midazolam |
| NASH | Decreased (2.5 fold) | |||||
| Human liver tissues (Ex vivo) | Steatosis | Decreased (60 %) | Not reported | Not reported | ||
| NASH | Decreased (69 %) | |||||
| Female Mice (in vivo) HFD | Steatosis | Not reported | Not reported | Not reported | CYP3A4 Luciferase Reporter plasmid | |
| Huh7 hepatoma cells (in vitro) | Steatosis | Decreased (80 %) | Not reported | Decreased (38 %) | Midazolam | |
| (Li et al., 2016) | ob/ob male Mouse (in vivo) (MCD) | Diabetic | Increase | Slight decrease | Slight decrease | Midazolam |
| Diabetic NASH | Increase | Decreased | Slight decrease |
CYP3A genes seem to be regulated by a multiplicity of signalling pathways via CCAAT-enhancer-binding proteins (C/EBP) (Martínez-Jiménez et al., 2005), HNF4 (Jover et al., 2009), PXR (Liu et al., 2008), and CAR (Timsit and Negishi, 2007). A reduced CYP3A4 luciferase reporter activity in steatotic mice suggested a reduced CYP3A4 transcription in NAFLD (Woolsey et al., 2015). The cytokine-mediated down-regulation of CYP3A4 (Werk and Cascorbi, 2014) in the course of the inflammatory response via the JAK/STAT (Janus kinase/Signal Transducer and Activator of Transcription) pathway (Jover et al., 2002 ) seem to be clinically relevant in NAFLD and diabetic patients due to circulating cytokines. Additionally, it has been suggested that the hepatic CYP3A4 expression is probably down-regulated by FGF21 (fibroblast growth factor 21) through the receptor-mitogen-activated protein kinase (MAPK) pathway which leads to reduced gene transcription (Woolsey et al., 2016).
CYP2
The CYP2 family contains several of the most important drug metabolizing CYPs including CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, and CYP2D6. Some of these members are highly polymorphic (Zanger and Schwab, 2013). The regulation of the subfamilies of CYP2 appears to involve nuclear factors like PXR, CAR, GR, and HNF4α. Conflicting results have been reported in NAFLD and diabetic models. This is perhaps due to differences in models used. Additionally, the polymorphic nature of some of the members of this family could be a source of discrepancy in findings especially where the genotypes involved are not considered. The effect of NAFLD on CYP2 enzymes has been studied by several groups. Reduced activity and mRNA expression of CYP2A6, CYP2B6, CYP2C9 and CYP2D6 have been reported in primary human cultured hepatocytes exposed to increasing concentrations (0.25 to 3 mM) of mixture (2:1) of oleic and palmitic acids (Donato et al., 2006). This study suggested probable alterations in some of the CYP2 enzymes in steatosis.
CYP2A6
CYP2A6 is clinically relevant for the hydroxylation of coumarin. The murine ortholog of CYP2A6, Cyp2a5, was found to be elevated in the presence of steatosis (Li et al., 2013, Cui et al., 2016) similar to the observations made in human hepatic tissues (Fisher et al., 2009). These observations however contradict the observations made by another group (Donato et al., 2006).
CYP2B6
CYP2B6 is an emerging enzyme with significant importance. It is involved in the biotransformation of several clinically relevant drugs like bupropion, efavirenz and cyclophosphamide. It also plays a role in the inactivation of environmental toxins. Recently, in vivo and in vitro studies using male Sprague Dawley rats and rat hepatic tissues respectively showed down-regulation of rat Cyp2b1(rat ortholog of human CYP2B6) activity, mRNA and protein expressions. This observation was made in both steatotic (HF diet) and NASH (MCD-diet) models with pronounce effect in NASH. It appears progression of NAFLD to hepatocellular carcinoma aggravates the decrease in CYP2B6 activity (Gao et al., 2016). Notwithstanding, Fisher and colleagues (Fisher et al., 2009) observed a slight increase in the mRNA levels, but did not observe any change in the protein level and activity of CYP2B6 in steatotic and NASH human liver tissues. Since CYP2B6 is less abundant and highly variable, evaluating the effect of heterogeneous NAFLD on its expression and activity poses a challenge.
CYP2C
The CYP2C family of CYPs are responsible for the metabolism of about 12 % (Wang and Tompkins, 2008) of clinically useful drugs. These include CYP2C8 (paclitaxel, amodiaquine), CYP2C9 (warfarin, tolbutamide) and CYP2C19 (phenytoin, omeperazole). There seems to be very little information about the CYP2Cs since the last reviews on NAFLD and DMEs (Merrell and Cherrington, 2011, Naik et al., 2013). The available reports suggest alterations of CYP2C in NAFLD. However, the direction of change is not clear as both increasing and decreasing trends have been observed (Fisher et al., 2009, Li et al., 2016). The AUC of rosiglitazone, an insulin sensitizer and a substrate of CYP2C8 and CYP2C9 (Baldwin et al., 1999), was found to be significantly increased in male mice after high fat and high fructose NAFLD induction (Kulkarni et al., 2016). Nevertheless, it is not clear whether this increase was mediated through down-regulation of the CYP2C8/9 or alteration in transport mechanisms.
CYP2D6
CYP2D6 constitutes about 4 % of total CYP content, yet it is involved in the biotransformation of more than 25 % (Wang and Tompkins, 2008) of clinically useful drugs including dextromethorphan and bufuralol. It is highly polymorphic (Ingelman-Sundberg, 2005) and the few reports are conflicting. In leptin-deficient (ob/ob) mice, the protein levels of Cyp2d22 (rat ortholog of human CYP2D6) (Li et al., 2016) were decreased. Similarly, in human liver tissues, CYP2D6 protein levels and activity showed a decreasing trend in NASH (Fisher et al., 2009).
CYP2E1
CYP2E1 is the most studied CYP enzyme in relation to NAFLD. CYP2E1 is involved in the biotransformation of acetaminophen, ethanol, acetone and fatty acid oxidation. It is known for the generation of ROS like hydrogen peroxide, and superoxide anion radicals (Aubert et al., 2011) due to uncoupling of oxygen consumption with NADPH (Nicotinamide adenine dinucleotide phosphate) oxidation and as a by-product of lipid peroxidation (Robertson et al., 2001). It is therefore considered to probably worsen the oxidative stress associated with diabetes and NAFLD, and may play a key role in the progression of NAFLD (Aubert et al., 2011). In fact, it is suspected to be a contributor to acetaminophen-induced liver injury in obesity and NAFLD (Michaut A1, 2014). There seem to be an increasing number of findings in the literature to support the enhancement of expression and activity of CYP2E1 in NAFLD in both humans and rodents (Chalasani et al., 2003, Abdelmegeed et al., 2012, Aljomah et al., 2015). Results in rat studies have shown a consistent trend of increase in Cyp2e1 expression and activity in MCD (Methionine choline deficient) diet fed rats (Weltman et al., 1996). Diabetes has also been reported to increase the mRNA and protein expressions of CYP2E1 (Lucas et al., 1998, Wang et al., 2003), and perhaps generating tissue-damaging hydroxyl radical in patients (Caro and Cederbaum, 2004).
CYP1A
The CYP1A subfamily has two functional members oriented head-to-head on chromosome 15q24.1. These are CYP1A1 and CYP1A2 (Zanger and Schwab, 2013). The two are highly inducible by ligands of CAR and AhR (aryl hydrocarbon receptor) (Zanger and Schwab, 2013). CYP1A2 constitutes approximately 15 % of total hepatic CYP enzymes (Wang and Tompkins, 2008). Its substrates include anticoagulants, antidepressants, antihistamines and anticancer agents (Zanger and Schwab, 2013). Reports from different groups about the down-regulation of CYP1A2 in NAFLD appears to be one of the most consistent despite some discrepancies (Merrell and Cherrington, 2011). The levels of expression of mRNA and protein are decreased in different rodent models of NAFLD (Zhang et al., 2007, Hanagama et al., 2008). In human related tissues, down-regulation of mRNA, protein and activity have been observed (Donato et al., 2006, Fisher et al., 2009).
Significant increases in the systemic clearance of antipyrine and protein levels of hepatic CYP1A2 were observed in diabetic rats possibly due to the enhancement of hepatic CYP1A2-mediated metabolism (Ueyama et al., 2007). Similarly, the metabolism of antipyrine was observed to be increased in patients with type 1 diabetes (Matzke et al., 2000). The hepatic metabolism of theophylline into 1, 3- dimethyluric acid (3-DMU) by CYP1A2 and CYP2E1 were studied using diabetes mellitus rat models (alloxan-induced and streptozotocin-induced). A significant increase in the exposure of 1, 3-DMU was observed in the diabetic rats compared to the controls. Based on in vitro rat hepatic microsomal studies, the increased clearance of theophylline was confirmed in the diabetic rats (Kim et al., 2005). Other studies in similar diabetic models have reported similar findings (Bae et al., 2006, DY et al., 2007).
Effect on Phase II Drug Metabolizing Enzymes (DMEs)
UDP-glucuronosyltransferases (UGTs)
Glucuronidation is the major route for phase II reactions catalyzed by the UDP-glucuronosyltransferases (UGTs). UGTs have been reported to be involved in the glucuronidation of more than 40 % of drugs in clinical use (Wells et al., 2004). They are anchored in the endoplasmic reticulum. Members of the UGT1A and 2B subfamilies appear relevant in humans due to their roles in the elimination of xenobiotics. In some reports, there was no change in Ugtb1 protein (rat) and UGT2B7 activity (humans) in NASH (Dzierlenga et al., 2015, Ferslew et al., 2015). An earlier work utilizing human liver and kidney microsomes, however, observed a decrease in the activity as well as reduction in the mRNA and protein expression of UGT2B7 in diabetes compared to control (Dostalek et al., 2011 ). Again, it is not clear whether the presence of NASH in the diabetic livers contributed to this observation. Limited literature on this subject matter does not allow a clear understanding of how the expression and activities of UGTs are modified by diabetes and NAFLD.
Sulfotransferases
Sulfotransferases (SULTs) are cytosolic enzymes that catalyze the sulfonation reaction of xenobiotics and endogenous compounds by adding a sulfonate moiety to a compound to increase its water solubility and decrease its biological activity. In humans, three SULT families, SULT1, SULT2, and SULT4 have been reported. PPARα mediates the induction of human SULTs, thus implicating a role for fatty acids as endogenous regulators of hepatic sulfonation in humans (Runge-Morris and Kocarek, 2005). In human patients, SULT1A2 was found to be down-regulated in NASH (Younossi et al., 2005); and resulted in decreased plasma levels of acetaminophen-sulfate (Canet et al., 2015). Yalcin and colleagues (Yalcin et al., 2013) also observed that sulfotransferase activity decreased significantly with severity of liver disease from steatosis to cirrhosis. Available reports therefore suggest that the activities of SULT1A1 and SULT1A3 were lower in disease states compared to non-steatotic tissues.
Glutatione-S-transferases
The Glutathione-S-transferases are present as different isoforms - α (A=alpha), μ (M=mu), π (P=pi), θ (T=theta), and ζ (Z=zeta) (Hayes et al., 2005). They are involved in the conjugation of glutathione (GSH) to reactive drug metabolites, though this reaction can be spontaneous without GST (Dragovic et al., 2010). A number of studies into GST activity in NAFLD and diabetes have found decreased enzymatic activity in ob/ob mice (Barnett et al., 1992, Roe et al., 1999) and human liver samples (Hardwick et al., 2010). GSTM2, M4 and M5 expressions were higher in African Americans with NASH than in Caucasians (Stepanova et al., 2010).
Effect of NAFLD on efflux and uptake transporters
The down-regulation of uptake and up-regulation of efflux transporters in obese and NAFLD have been observed in studies involving rodents and human samples (Canet et al., 2014, Canet et al., 2015). Though interspecies variation limits the use of rodents in modeling human NAFLD, concordance analysis has suggested that both mouse and rat MCD models, as well as mouse ob/ob and db/db NASH models show some similarity to human transporter mRNA and protein expression, and hence may be useful for predicting altered drug disposition (Canet et al., 2014). Canet et al. (2014) observed mainly up-regulation of mRNA and protein expressions of Mdr1 (multidrug resistance protein), Mrp1–4 (multidrug resistance-associated protein) and Bcrp (Breast cancer resistance protein) in rat and mouse NASH models. Conversely, the Oatps (organic anion transporting polypeptides) mainly showed a down-regulation (Canet et al., 2014). The plasma concentrations of metformin, an anti-hyperglycemic agent, were slightly increased in the WT/MCD and ob/Control groups. In ob/MCD mice compared to Wild Type, the plasma concentrations were 4.8-fold higher. These changes were attributed to decreases in the kidney mRNA expression of Oct2 and Mate1, the primary mediators of metformin elimination (Clarke et al., 2015).
In the literature, the influence of NAFLD on MRP2–3 appears more obvious compared to other transporters (Hardwick et al., 2012, Canet et al., 2015). Table 3 shows some of the published work on the effect of NAFLD on MRP3. In MCD diet-induced NASH male Sprague-Dawley rats, mis-localization of Mrp2, the canaliculi efflux transporter, was observed. Mrp2 appeared to pocket inward, resulting in a diminished function of effluxing substrates into bile. On the other hand, the sinusoidal Mrp3 efflux transporter increased with respect to protein expression leading to increased efflux of substrates into plasma (Dzierlenga et al., 2015). These findings were consistent with human clinical studies involving MRP3 and its morphine glucuronide (morphine 3 and 6 glucuronides) substrate in NASH subjects (Ferslew et al., 2015). The AUC of morphine glucuronide was 58 % higher in NASH subjects compared to healthy subjects. The Cmax also was also significantly higher in NASH subjects. In addition, fasting levels of total bile acids, glycocholate and taurocholate were also elevated in NASH subjects suggesting up-regulation of the basolateral efflux MRP-3 (Ferslew et al., 2015).
Table 3.
The effect of NAFLD on MRP3. Overall, NAFLD progression seem to increase the expression and activity of MRP3
| Study | Species | Ref/NAFLD | Endpoint | Change | Probe Substrate |
|---|---|---|---|---|---|
| (Hardwick et al., 2012) | Rats (male Sprague-Dawley) | Control/NASH | mRNA level | Significantly increased | |
| Protein | Significantly increased | ||||
| Plasma Concentration | Significantly increased | Ezetimibe glucuronide | |||
| (Dzierlenga et al., 2015) | Rats (male Sprague-Dawley) | Control/NASH | AUC | 150 % increase | Morphine glucuronide |
| Protein | Significantly increased | ||||
| Activity | Significantly increased | ||||
| (Ferslew et al., 2015) | Human | Healthy/NASH | Cmax | 52 % increase in NASH | Morphine glucuronide |
| AUC | 58 % increase in NASH | Morphine glucuronide | |||
| (Canet et al., 2015) | Human (Children) | Healthy/Steatosis/NASH | AUC | Increased | Acetaminophen glucuronide |
| Human Liver Tissues | Healthy/Steatosis/NASH | MRP3 Protein | Significantly increased |
Clinical impact of NAFLD/NASH on pharmacotherapy
Though very few clinical studies have reported the impact of NAFLD on pharmacotherapy, they strongly highlight the potential of NAFLD to cause variable drug response, adverse drug reaction and eventually toxicity through alteration of pharmacokinetic profile. Midazolam (Woolsey et al., 2015), morphine (Ferslew et al., 2015) and acetaminophen (Canet et al., 2015) have been evaluated in both healthy and NAFLD patients. NAFLD seem to increase the AUC of midazolam by reducing the activity of CYP3A4; and similarly increase the AUC of the glucuronide metabolites of morphine and acetaminophen via the up-regulation of the MRP3 efflux transporter. Perhaps, the available evidence in the literature is the main motivation behind the emerging interest in drug disposition in NAFLD patients. Hopefully, more clinical studies would be conducted to gain more insight into the nature and extent of impact of NAFLD on pharmacotherapy.
Challenges to studying the effect of NAFLD on DMEs and Transporters
Studying the effect of NAFLD is challenging. First, the pathogenesis of NAFLD is not clearly understood, and is usually asymptotic requiring biopsy for definitive diagnosis. Due to ethical reasons, researchers are unable to routinely obtain biopsies from patients for studies. In addition, the presence of co-morbidities particularly diabetes, which is highly prevalent in NAFLD patients, is not accounted for. For instance, it has been demonstrated that antipyrine elimination rate was dependent on the type of diabetes (type 1 versus type 2) and gender (Sotaniemi et al., 2002). It was observed that insulinopenia enhanced hepatic microsomal enzyme activity (probably through increased ketobodies), whereas relative insulin deficiency was associated with decreased metabolic activity (Sotaniemi et al., 2002). Since the presence of diabetes and other demographic characteristics could confound the effect of NAFLD on DMEs and transporters, it may be necessary to account for them. Finally, the absence of consensus on NASH models and NAFLD classification system to use for experiments has permitted the use of different NASH models and classification systems. For instance, a mice diabetic model of NASH only recapitulated human CYP alterations in NAFLD partially (Li et al., 2016); and hence may be inadequate for all CYPs. This has made comparison of results from some groups difficult. It is anticipated that as research advances in this area, these procedures would be harmonized to allow comparability of results.
CONCLUSION
NAFLD and diabetes are gradually becoming pandemic globally. Limited options are available for the treatment of NASH; hence, several pharmaceutical companies are trying to develop new molecules for this condition. However, lack of knowledge on the effect of NAFLD or NASH on the expression and activity of hepatic DMEs and transporters can impede drug development in this area. Current research findings, though limited and sometimes conflicting, suggest alterations in DMEs and transporters in NAFLD. Few of the results however are consistent across studies and species and includes the down-regulation of CYP3A; and up-regulation of CYP2E1 and MRP3. Results from other DMEs and transporters are either lacking or conflicting. Investigating the influence of NAFLD on DMEs and transporters is challenging because NAFLD is heterogeneous and involves a spectrum of hepatic lesions. The challenges introduce another layer of variability to NAFLD experimental studies. The presence of steatosis, oxidative stress and inflammatory mediators like TNF-α and IL-6 have been implicated in the alterations of nuclear factors in NAFLD. Consequently, the regulation of transcription factors like CAR, PXR, PPAR-α, etc. may change and eventually alter the expression of DMEs and transporters. These alterations could be potential sources of drug variability in patients and could have serious consequences on safety and efficacy. We recommend more studies in this area to augment our understanding on the effect of NAFLD on drug metabolism.
Acknowledgments
Support of grant # R15 GM101599 from the National Institutes of Health is gratefully acknowledged.
ABBREVIATIONS
- 3-DMU
1, 3- dimethyluric acid
- ABC
ATP-binding cassette
- AhR
Aryl hydrocarbon receptor
- ALT
Alanine transaminase
- ARE
Antioxidant response element
- AST
Aspartate transaminase
- AUC
Area under the curve
- BAAT
BMI, Age, ALT, Triglycerides
- BCRP
Breast cancer resistance protein
- BMI
Body Mass Index
- C/EBPs
CCAAT-enhancer-binding proteins (or C/EBPs)
- CAR
Constitutive androstane receptor
- chREBP
Carbohydrate-responsive element-binding protein
- CT
Computerized tomographic
- CYP
Cytochrome P450
- DAGs
Diacyl glycerols
- DME
Drug metabolizing enzyme
- ER
Endoplasmic Reticulum
- FAT/CD36
Fatty acid translocase
- FATPs
Fatty acid transport proteins
- FGF21
Fibroblast growth factor 21
- GR
Glucocorticoid receptor
- GSTs
Glutathione -S-transferases
- HAIR
Hypertension, ALT, Insulin resistance
- HDL
High-density lipoprotein
- HFD
High fat diet
- HNF-4
Hepatic nuclear factors 4
- IL-1β
Interleukin-1 β
- IL-6
Interleukin-6
- JAK/STAT
Janus kinase/Signal Transducer and Activator of Transcription
- keapl
Kelch-like ECH-associated protein 1
- LPS
Lipopolysaccharide
- LXRα
Liver X receptor alpha
- MAPK
Mitogen-activated protein kinase
- MCD
Methionine choline deficient
- MRI
Magnetic resonance imaging
- MRP
Multidrug resistance-associated protein
- NADPH
Nicotinamide adenine dinucleotide phosphate (reduced)
- NAFL
Non-alcoholic fatty liver
- NAFLD
Non-alcoholic fatty liver disease
- NAS
NAFLD activity score
- NASH
Non-alcoholic steatohepatitis
- NASH CRN
NASH Clinical Research Network
- NEFA
Nonesterified fatty acids
- NF-κβ
Nuclear factor – kappaβ
- NIDDK
National Institute of Diabetes & Digestive & Kidney Disease
- Nrf2
Nuclear factor erythroid 2–related factor 2
- OATPs
Organic anion transporting polypeptides
- OATs
Organic anion transporter
- OCTs
organic cation transporter
- P-gp
Permeability glycoprotein or P-glycoprotein
- PNPLA3
Patatin-like phospholipase domain containing 3
- PPARα
Peroxisome proliferator-activated receptor alpha
- PPAR-γ
Peroxisome proliferator-activated receptor gamma
- PUFA
Polyunsaturated fatty acids
- PXR
Pregnane X receptor
- QUICKI
Quantitative insulin sensitivity check index
- RXRα
Retinoid X receptor alpha
- SAF
Steatosis, activity and fibrosis
- SLC
Solute carrier superfamily
- SOCS3
Suppressor of cytokine signalling 3
- SREBP1c
Sterol regulatory binding protein-1c
- SULT
Sulfotransferases
- TAG
Triacylglycerol
- TM6SF2
Transmembrane 6 superfamily member 2
- TNF-α
Tumor necrosis factor - alpha
- UGTs
Uridine diphosphate (UDP) - glucuronosyl transferases
Footnotes
Declaration of interest statement: Author EC and Author FA declare that they have no conflict of interest.
References
- Abdelmegeed M, Banerjee A, Yoo S, Jang S, Gonzalez F, Song B. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. J Hepatol. 2012;57:860–6. doi: 10.1016/j.jhep.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abenavoli L, Milic N, Peta V, Alfieri F, De Lorenzo A, Bellentani S. Alimentary regimen in non-alcoholic fatty liver disease: Mediterranean diet. World J Gastroenterol. 2014;20:16831–40. doi: 10.3748/wjg.v20.i45.16831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken A, Morgan E. Gene-specific effects of inflammatory cytokines on cytochrome P450 2C, 2B6 and 3A4 mRNA levels in human hepatocytes. Drug Metab Dispos. 2007;35:1687–93. doi: 10.1124/dmd.107.015511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitken A, Richardson T, Morgan E. Regulation of drug-metabolizing enzymes and transporters in inflammation. Annu Rev Pharmacol Toxicol. 2006;46:123–49. doi: 10.1146/annurev.pharmtox.46.120604.141059. [DOI] [PubMed] [Google Scholar]
- Alberti K, Eckel R, Grundy S, Zimmet P, Cleeman J, Donato K, Fruchart J, James W, Loria C, Smith SJ. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- Aljomah G, Baker SS, Liu W, Kozielski R, Oluwole J, Lupu B, Baker RD, Zhu L. Induction of CYP2E1 in non-alcoholic fatty liver diseases. Exp Mol Pathol. 2015;99:677–81. doi: 10.1016/j.yexmp.2015.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson N, Borlak J. Molecular mechanisms and therapeutic targets in steatosis and steatohepatitis. Pharmacol Rev. 2008;60:311–57. doi: 10.1124/pr.108.00001. [DOI] [PubMed] [Google Scholar]
- Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–31. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- Angulo P, Keach J, Batts K, Lindor K. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–62. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- Argo C, Caldwell S. Epidemiology and natural history of non-alcoholic steatohepatitis. Clin Liver Dis. 2009;13:511–31. doi: 10.1016/j.cld.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Aubert J, Begriche K, Knockaert L, Robin M, Fromenty B. Increased expression of cytochrome P450 2E1 in nonalcoholic fatty liver disease: mechanisms and pathophysiological role. Clin Res Hepatol Gastroenterol. 2011;35:630–7. doi: 10.1016/j.clinre.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Bae S, Kim J, Yang S, Kim J, Kim T, Lee M. Pharmacokinetics of oltipraz in rat models of diabetes mellitus induced by alloxan or streptozotocin. Life Sci. 2006;78:2287–94. doi: 10.1016/j.lfs.2005.09.031. [DOI] [PubMed] [Google Scholar]
- Baldwin SJ, Clarke SE, Chenery RJ. Characterization of the cytochrome P450 enzymes involved in the in vitro metabolism of rosiglitazone. Br J Clin Pharmacol. 1999;48:424–32. doi: 10.1046/j.1365-2125.1999.00030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnett C, Abbott R, Bailey C, Flatt P, Ioannides C. Cytochrome P-450-dependent mixed-function oxidase and glutathione S-transferase activities in spontaneous obesity-diabetes. Biochem Pharmacol. 1992;43:1868–71. doi: 10.1016/0006-2952(92)90724-w. [DOI] [PubMed] [Google Scholar]
- Bazick J, Donithan M, Neuschwander-Tetri BA, Kleiner D, Brunt EM, Wilson L, Doo E, Lavine J, Tonascia J, Loomba R. Clinical Model for NASH and Advanced Fibrosis in Adult Patients With Diabetes and NAFLD: Guidelines for Referral in NAFLD. Diabetes Care. 2015;38:1347–55. doi: 10.2337/dc14-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedossa P, Poitou C, Veyrie N, Bouillot JL, Basdevant A, Paradis V, Tordjman J, Clement K. Histopathological algorithm and scoring system for evaluation of liver lesions in morbidly obese patients. Hepatology. 2012;56:1751–9. doi: 10.1002/hep.25889. [DOI] [PubMed] [Google Scholar]
- Bell M, Wang H, Chen H, Mclenithan J, Gong D, Yang R, Yu D, Fried S, Quon M, Londos C, Sztalryd C. Consequences of lipid droplet coat protein downregulation in liver cells: abnormal lipid droplet metabolism and induction of insulin resistance. Diabetes. 2008;57:2037–45. doi: 10.2337/db07-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellentani S. The epidemiology of non-alcoholic fatty liver disease. Liver Int. 2017;37(Suppl 1):81–84. doi: 10.1111/liv.13299. [DOI] [PubMed] [Google Scholar]
- Browning J, Horton J. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–52. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning J, Szczepaniak L, Dobbins R, Nuremberg P, Horton J, Cohen J, Grundy S, Hobbs H. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–95. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- Brunt E, Janney C, Di Bisceglie A, Neuschwander-Tetri B, Bacon B. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–74. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- Brunt E, Kleiner D, Wilson L, Belt P, Neuschwander-Tetri B. Nonalcoholic fatty liver disease (NAFLD) activity score and the histopathologic diagnosis in NAFLD: distinct clinicopathologic meanings. Hepatology. 2011;53:810–20. doi: 10.1002/hep.24127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunt E, Neuschwander-Tetri B, Oliver D, Wehmeier K, Bacon B. Nonalcoholic steatohepatitis: histologic features and clinical correlations with 30 blinded biopsy specimens. Hum Pathol. 2004;35:1070–82. doi: 10.1016/j.humpath.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Bugianesi E, Manzini P, D’antico S, Vanni E, Longo F, Leone N, Massarenti P, Piga A, Marchesini G, Rizzetto M. Relative contribution of iron burden, HFE mutations, and insulin resistance to fibrosis in nonalcoholic fatty liver. Hepatology. 2004;39:179–87. doi: 10.1002/hep.20023. [DOI] [PubMed] [Google Scholar]
- Canet MJ, Hardwick RN, Lake AD, Dzierlenga AL, Clarke JD, Cherrington NJ. Modeling human nonalcoholic steatohepatitis-associated changes in drug transporter expression using experimental rodent models. Drug Metab Dispos. 2014;42:586–95. doi: 10.1124/dmd.113.055996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canet MJ, Merrell MD, Hardwick RN, Bataille AM, Campion SN, Ferreira DW, Xanthakos SA, Manautou JE, Hh AK, Erickson RP, Cherrington NJ. Altered regulation of hepatic efflux transporters disrupts acetaminophen disposition in pediatric nonalcoholic steatohepatitis. Drug Metab Dispos. 2015;43:829–35. doi: 10.1124/dmd.114.062703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caro A, Cederbaum A. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol Toxicol. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- Chalasani N, Gorski J, Asghar M, Asghar A, Foresman B, Hall S, Crabb D. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology. 2003;37:544–50. doi: 10.1053/jhep.2003.50095. [DOI] [PubMed] [Google Scholar]
- Cholerton S, Daly A, Idle J. The role of individual human cytochromes P450 in drug metabolism and clinical response. Trends Pharmacol Sci. 1992;13:434–9. doi: 10.1016/0165-6147(92)90140-2. [DOI] [PubMed] [Google Scholar]
- Clark J, Diehl A. Nonalcoholic fatty liver disease: an underrecognized cause of cryptogenic cirrhosis. JAMA. 2003;289:3000–4. doi: 10.1001/jama.289.22.3000. [DOI] [PubMed] [Google Scholar]
- Clarke JD, Dzierlenga AL, Nelson NR, Li H, Werts S, Goedken MJ, Cherrington NJ. Mechanism of Altered Metformin Distribution in Nonalcoholic Steatohepatitis. Diabetes. 2015;64:3305–13. doi: 10.2337/db14-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey KE, Klebanoff MJ, Tramontano AC, Chung RT, Hur C. Screening for Nonalcoholic Steatohepatitis in Individuals with Type 2 Diabetes: A Cost-Effectiveness Analysis. Dig Dis Sci. 2016;61:2108–17. doi: 10.1007/s10620-016-4044-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotreau MM, Von Moltke LL, Greenblatt DJ. The influence of age and sex on the clearance of cytochrome P450 3A substrates. Clin Pharmacokinet. 2005;44:33–60. doi: 10.2165/00003088-200544010-00002. [DOI] [PubMed] [Google Scholar]
- Cui Y, Wang Q, Yi X, Zhang X. Effects of Fatty Acids on CYP2A5 and Nrf2 Expression in Mouse Primary Hepatocytes. Biochem Genet. 2016;54:29–40. doi: 10.1007/s10528-015-9697-6. [DOI] [PubMed] [Google Scholar]
- Cusi K. Treatment of patients with type 2 diabetes and non-alcoholic fatty liver disease: current approaches and future directions. Diabetologia. 2016;59:1112–20. doi: 10.1007/s00125-016-3952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diczfalusy U, Nylén H, Elander P, Bertilsson L. 4β-Hydroxycholesterol, an endogenous marker of CYP3A4/5 activity in humans. Br J Clin Pharmacol. 2011;71:183–9. doi: 10.1111/j.1365-2125.2010.03773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J, Bhathal P, O’brien P. Nonalcoholic fatty liver disease: predictors of nonalcoholic steatohepatitis and liver fibrosis in the severely obese. Gastroenterology. 2001;121:91–100. doi: 10.1053/gast.2001.25540. [DOI] [PubMed] [Google Scholar]
- Donato M, Lahoz A, Jiménez N, Pérez G, Serralta A, Mir J, Castell J, Gómez-Lechón M. Potential impact of steatosis on cytochrome P450 enzymes of human hepatocytes isolated from fatty liver grafts. Drug Metab Dispos. 2006;34:1556–62. doi: 10.1124/dmd.106.009670. [DOI] [PubMed] [Google Scholar]
- Dongiovanni P, Petta S, Maglio C, Fracanzani AL, Pipitone R, Mozzi E, Motta BM, Kaminska D, Rametta R, Grimaudo S, Pelusi S, Montalcini T, Alisi A, Maggioni M, Karja V, Boren J, Kakela P, Di Marco V, Xing C, Nobili V, Dallapiccola B, Craxi A, Pihlajamaki J, Fargion S, Sjostrom L, Carlsson LM, Romeo S, Valenti L. Transmembrane 6 superfamily member 2 gene variant disentangles nonalcoholic steatohepatitis from cardiovascular disease. Hepatology. 2015;61:506–14. doi: 10.1002/hep.27490. [DOI] [PubMed] [Google Scholar]
- Dostalek M, Akhlaghi F, Puzanovova M. Effect of diabetes mellitus on pharmacokinetic and pharmacodynamic properties of drugs. Clin Pharmacokinet. 2012a;51:481–99. doi: 10.2165/11631900-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Dostalek M, Court M, Hazarika S, Akhlaghi F. Diabetes mellitus reduces activity of human UDP-glucuronosyltransferase 2B7 in liver and kidney leading to decreased formation of mycophenolic acid acyl-glucuronide metabolite. Drug Metab Dispos. 2011;39:448–55. doi: 10.1124/dmd.110.036608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostalek M, Court M, Yan B, Akhlaghi F. Significantly reduced cytochrome P450 3A4 expression and activity in liver from humans with diabetes mellitus. Br J Pharmacol. 2011;163:937–47. doi: 10.1111/j.1476-5381.2011.01270.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostalek M, Sam W, Paryani K, Macwan J, Gohh R, Akhlaghi F. Diabetes mellitus reduces the clearance of atorvastatin lactone: results of a population pharmacokinetic analysis in renal transplant recipients and in vitro studies using human liver microsomes. Clin Pharmacokinet. 2012b;51:591–606. doi: 10.2165/11632690-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Dragovic S, Boerma J, Van Bergen L, Vermeulen N, Commandeur J. Role of human glutathione S-transferases in the inactivation of reactive metabolites of clozapine. Chem Res Toxicol. 2010;23:1467–76. doi: 10.1021/tx100131f. [DOI] [PubMed] [Google Scholar]
- Dy L, Mg L, Hs S, IL Changes in omeprazole pharmacokinetics in rats with diabetes induced by alloxan or streptozotocin: faster clearance of omeprazole due to induction of hepatic CYP1A2 and 3A1. J Pharm Pharm Sci. 2007;10:420–33. doi: 10.18433/j3wc7g. [DOI] [PubMed] [Google Scholar]
- Dzierlenga AL, Clarke JD, Hargraves TL, Ainslie GR, Vanderah TW, Paine MF, Cherrington NJ. Mechanistic basis of altered morphine disposition in nonalcoholic steatohepatitis. J Pharmacol Exp Ther. 2015;352:462–70. doi: 10.1124/jpet.114.220764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckel R, Alberti K, Grundy S, Zimmet P. The metabolic syndrome. Lancet. 2010;375:181–3. doi: 10.1016/S0140-6736(09)61794-3. [DOI] [PubMed] [Google Scholar]
- Edginton A, Willmann S. Physiology-based simulations of a pathological condition: prediction of pharmacokinetics in patients with liver cirrhosis. Clin Pharmacokinet. 2008;47:743–52. doi: 10.2165/00003088-200847110-00005. [DOI] [PubMed] [Google Scholar]
- Elbekai R, Korashy H, El-Kadi A. The effect of liver cirrhosis on the regulation and expression of drug metabolizing enzymes. Curr Drug Metab. 2004;5:157–67. doi: 10.2174/1389200043489054. [DOI] [PubMed] [Google Scholar]
- Elens L, Becker M, Haufroid V, Hofman A, Visser L, Uitterlinden A, Stricker B, Van Schaik R. Novel CYP3A4 intron 6 single nucleotide polymorphism is associated with simvastatin-mediated cholesterol reduction in the Rotterdam Study. Pharmacogenet Genomics. 2011;21:861–6. doi: 10.1097/FPC.0b013e32834c6edb. [DOI] [PubMed] [Google Scholar]
- European Association for the Study of the Liver (Easl) EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-Alcoholic Fatty Liver Disease. Obes Facts. 2016:65–90. doi: 10.1159/000443344. 2016/04/08 ed. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbrini E, Magkos F, Mohammed B, Pietka T, Abumrad N, Patterson B, Okunade A, Klein S. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. PNAS. 2009;106:15430–5. doi: 10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazel Y, Koenig AB, Sayiner M, Goodman ZD, Younossi ZM. Epidemiology and natural history of non-alcoholic fatty liver disease. Metabolism. 2016;65:1017–25. doi: 10.1016/j.metabol.2016.01.012. [DOI] [PubMed] [Google Scholar]
- Ferslew BC, Johnston CK, Tsakalozou E, Bridges AS, Paine MF, Jia W, Stewart PW, Barritt AST, Brouwer KL. Altered morphine glucuronide and bile acid disposition in patients with nonalcoholic steatohepatitis. Clin Pharmacol Ther. 2015;97:419–27. doi: 10.1002/cpt.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn RD, Henderson CJ, Scott CL, Wolf CR. Unsaturated fatty acid regulation of cytochrome P450 expression via a CAR-dependent pathway. Biochem J. 2009;417:43–54. doi: 10.1042/BJ20080740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher C, Jackson J, Lickteig A, Augustine L, Cherrington N. Drug metabolizing enzyme induction pathways in experimental non-alcoholic steatohepatitis. Arch Toxicol. 2008;82:959–64. doi: 10.1007/s00204-008-0312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher C, Lickteig A, Augustine L, Ranger-Moore J, Jackson J, Ferguson S, Cherrington N. Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab Dispos. 2009;37:2087–94. doi: 10.1124/dmd.109.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher M, Yoon K, Vaughn M, Strelevitz T, Foti R. Flavin-containing monooxygenase activity in hepatocytes and microsomes: in vitro characterization and in vivo scaling of benzydamine clearance. Drug Metab Dispos. 2002;30:1087–93. doi: 10.1124/dmd.30.10.1087. [DOI] [PubMed] [Google Scholar]
- Gao J, Zhou J, He XP, Zhang YF, Gao N, Tian X, Fang Y, Wen Q, Jia LJ, Jin H, Qiao HL. Changes in cytochrome P450s-mediated drug clearance in patients with hepatocellular carcinoma in vitro and in vivo: a bottom-up approach. Oncotarget. 2016;7:28612–23. doi: 10.18632/oncotarget.8704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George J, Liddle C, Murray M, Byth K, Farrell G. Pre-translational regulation of cytochrome P450 genes is responsible for disease-specific changes of individual P450 enzymes among patients with cirrhosis. Biochem Pharmacol. 1995;49:873–81. doi: 10.1016/0006-2952(94)00515-n. [DOI] [PubMed] [Google Scholar]
- Giacomini KM, Huang SM, Tweedie DJ, Benet LZ, Brouwer KL, Chu X, Dahlin A, Evers R, Fischer V, Hillgren KM. Membrane transporters in drug development. Nature reviews Drug discovery. 2010;9:215–236. doi: 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godos J, Federico A, Dallio M, Scazzina F. Mediterranean diet and nonalcoholic fatty liver disease: molecular mechanisms of protection. Int J Food Sci Nutr. 2017;68:18–27. doi: 10.1080/09637486.2016.1214239. [DOI] [PubMed] [Google Scholar]
- Greco D, Kotronen A, Westerbacka J, Puig O, Arkkila P, Kiviluoto T, Laitinen S, Kolak M, Fisher R, Hamsten A, Auvinen P, Yki-Järvinen H. Gene expression in human NAFLD. Am J Physiol Gastrointest Liver Physiol. 2008;294:G1281–7. doi: 10.1152/ajpgi.00074.2008. [DOI] [PubMed] [Google Scholar]
- Groop L. Insulin resistance: the fundamental trigger of type 2 diabetes. Diabetes Obes Metab. 1999;1(Suppl 1):S1–7. doi: 10.1046/j.1463-1326.1999.0010s1001.x. [DOI] [PubMed] [Google Scholar]
- Guengerich F. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999;39:1–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- Guengerich F. Cytochrome p450 and chemical toxicology. Chem Res Toxicol. 2008;21:70–83. doi: 10.1021/tx700079z. [DOI] [PubMed] [Google Scholar]
- Guengerich F, Macdonald T. Mechanisms of cytochrome P-450 catalysis. FASEB J. 1990;4:2453–9. doi: 10.1096/fasebj.4.8.2185971. [DOI] [PubMed] [Google Scholar]
- Guengerich F, Munro A. Unusual cytochrome p450 enzymes and reactions. J Biol Chem. 2013;288:17065–73. doi: 10.1074/jbc.R113.462275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanagama M, Inoue H, Kamiya M, Shinone K, Nata M. Gene expression on liver toxicity induced by administration of haloperidol in rats with severe fatty liver. Leg Med (Tokyo) 2008;10:177–84. doi: 10.1016/j.legalmed.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Hardwick R, Fisher C, Canet M, Lake A, Cherrington N. Diversity in antioxidant response enzymes in progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos. 2010;38:2293–301. doi: 10.1124/dmd.110.035006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick RN, Fisher CD, Street SM, Canet MJ, Cherrington NJ. Molecular mechanism of altered ezetimibe disposition in nonalcoholic steatohepatitis. Drug Metab Dispos. 2012;40:450–60. doi: 10.1124/dmd.111.041095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S, Torgerson S, Hayashi P. The natural history of nonalcoholic fatty liver disease: a clinical histopathological study. Am J Gastroenterol. 2003;98:2042–7. doi: 10.1111/j.1572-0241.2003.07659.x. [DOI] [PubMed] [Google Scholar]
- Hayes J, Flanagan J, Jowsey I. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- Ingelman-Sundberg M. Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J. 2005;5:6–13. doi: 10.1038/sj.tpj.6500285. [DOI] [PubMed] [Google Scholar]
- Jaiswal A. Nrf2 signaling in coordinated activation of antioxidant gene expression. Free Radic Biol Med. 2004;36:1199–207. doi: 10.1016/j.freeradbiomed.2004.02.074. [DOI] [PubMed] [Google Scholar]
- Jiang W, Wu N, Wang X, Chi Y, Zhang Y, Qiu X, Hu Y, Li J, Liu Y. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep. 2015;5:8096. doi: 10.1038/srep08096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johns D. Human liver aldehyde oxidase: differential inhibition of oxidation of charged and uncharged substrates. J Clin Invest. 1967;46:1492–505. doi: 10.1172/JCI105641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T, Boussery K, Rowland-Yeo K, Tucker G, Rostami-Hodjegan A. A semi-mechanistic model to predict the effects of liver cirrhosis on drug clearance. Clin Pharmacokinet. 2010;49:189–206. doi: 10.2165/11318160-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Joshi-Barve S, Barve S, Amancherla K, Gobejishvili L, Hill D, Cave M, Hote P, Mcclain C. Palmitic acid induces production of proinflammatory cytokine interleukin-8 from hepatocytes. Hepatology. 2007;46:823–30. doi: 10.1002/hep.21752. [DOI] [PubMed] [Google Scholar]
- Jou J, Choi S, Diehl A. Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin Liver Dis. 2008;28:370–9. doi: 10.1055/s-0028-1091981. [DOI] [PubMed] [Google Scholar]
- Jover R, Bort R, Gómez-Lechón M, Castell J. Down-regulation of human CYP3A4 by the inflammatory signal interleukin-6: molecular mechanism and transcription factors involved. FASEB J. 2002;16:1799–801. doi: 10.1096/fj.02-0195fje. [DOI] [PubMed] [Google Scholar]
- Jover R, Moya M, Gómez-Lechón M. Transcriptional regulation of cytochrome p450 genes by the nuclear receptor hepatocyte nuclear factor 4-alpha. Curr Drug Metab. 2009;10:508–19. doi: 10.2174/138920009788898000. [DOI] [PubMed] [Google Scholar]
- Jump D. N-3 polyunsaturated fatty acid regulation of hepatic gene transcription. Curr Opin Lipidol. 2008;19:242–7. doi: 10.1097/MOL.0b013e3282ffaf6a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jump D, Botolin D, Wang Y, Xu J, Christian B, Demeure O. Fatty acid regulation of hepatic gene transcription. J Nutr. 2005;135:2503–6. doi: 10.1093/jn/135.11.2503. [DOI] [PubMed] [Google Scholar]
- Kim Y, Lee A, Lee J, Lee I, Lee D, Kim S, Kim S, Lee M. Pharmacokinetics of theophylline in diabetes mellitus rats: induction of CYP1A2 and CYP2E1 on 1,3-dimethyluric acid formation. Eur J Pharm Sci. 2005;26:114–23. doi: 10.1016/j.ejps.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Kirpich IA, Marsano LS, Mcclain CJ. Gut-liver axis, nutrition, and non-alcoholic fatty liver disease. Clin Biochem. 2015;48:923–30. doi: 10.1016/j.clinbiochem.2015.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, Mccullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- Kleiner DE, Makhlouf HR. Histology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in Adults and Children. Clin Liver Dis. 2016;20:293–312. doi: 10.1016/j.cld.2015.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolwankar D, Vuppalanchi R, Ethell B, Jones D, Wrighton S, Hall S, Chalasani N. Association between nonalcoholic hepatic steatosis and hepatic cytochrome P-450 3A activity. Clin Gastroenterol Hepatol. 2007;5:388–93. doi: 10.1016/j.cgh.2006.12.021. [DOI] [PubMed] [Google Scholar]
- Koren G, Cairns J, Chitayat D, Gaedigk A, Leeder S. Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine-prescribed mother. Lancet. 2006;368:704. doi: 10.1016/S0140-6736(06)69255-6. [DOI] [PubMed] [Google Scholar]
- Kulkarni NM, Malampati S, Mahat MY, Chandrasekaran S, Raghul J, Khan AA, Krishnan UM, Narayanan S. Altered pharmacokinetics of rosiglitazone in a mouse model of non-alcoholic fatty liver disease. Drug Metab Pers Ther. 2016;31:165–71. doi: 10.1515/dmpt-2016-0008. [DOI] [PubMed] [Google Scholar]
- Lennernäs H. Clinical pharmacokinetics of atorvastatin. Clin Pharmacokinet. 2003;42:1141–60. doi: 10.2165/00003088-200342130-00005. [DOI] [PubMed] [Google Scholar]
- Li H, Clarke JD, Dzierlenga AL, Bear J, Goedken MJ, Cherrington NJ. In vivo cytochrome P450 activity alterations in diabetic nonalcoholic steatohepatitis mice. J Biochem Mol Toxicol. 2016 doi: 10.1002/jbt.21840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Guo G, Cao Y, Wang Y, Liu F, Zhang X. Expression of cytochrome P450 2A5 in a C57BL/6J mouse model of nonalcoholic fatty liver disease. Pharmacology. 2013;92:26–31. doi: 10.1159/000348575. [DOI] [PubMed] [Google Scholar]
- Liu F, Song X, Yang D, Deng R, Yan B. The far and distal enhancers in the CYP3A4 gene co-ordinate the proximal promoter in responding similarly to the pregnane X receptor but differentially to hepatocyte nuclear factor-4alpha. Biochem J. 2008;409:243–50. doi: 10.1042/BJ20070613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas D, Farez C, Bardou L, Vaisse J, Attali J, Valensi P. Cytochrome P450 2E1 activity in diabetic and obese patients as assessed by chlorzoxazone hydroxylation. Fundam Clin Pharmacol. 1998;12:553–8. doi: 10.1111/j.1472-8206.1998.tb00985.x. [DOI] [PubMed] [Google Scholar]
- Marchesini G, Brizi M, Morselli-Labate A, Bianchi G, Bugianesi E, Mccullough A, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–5. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- Martínez-Jiménez C, Gómez-Lechón M, Castell J, Jover R. Transcriptional regulation of the human hepatic CYP3A4: identification of a new distal enhancer region responsive to CCAAT/enhancer-binding protein beta isoforms (liver activating protein and liver inhibitory protein) Mol Pharmacol. 2005;67:2088–101. doi: 10.1124/mol.104.008169. [DOI] [PubMed] [Google Scholar]
- Matteoni C, Younossi Z, Gramlich T, Boparai N, Liu Y, Mccullough A. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116:1413–9. doi: 10.1016/s0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- Matzke G, Frye R, Early J, Straka R, Carson S. Evaluation of the influence of diabetes mellitus on antipyrine metabolism and CYP1A2 and CYP2D6 activity. Pharmacotherapy. 2000;20:182–90. doi: 10.1592/phco.20.3.182.34775. [DOI] [PubMed] [Google Scholar]
- Meech R, Mackenzie PI. Structure and function of uridine diphosphate glucuronosyltransferases. Clinical and experimental pharmacology and physiology. 1997;24:907–915. doi: 10.1111/j.1440-1681.1997.tb02718.x. [DOI] [PubMed] [Google Scholar]
- Merrell M, Cherrington N. Drug metabolism alterations in nonalcoholic fatty liver disease. Drug Metab Rev. 2011;43:317–34. doi: 10.3109/03602532.2011.577781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaut A1 MC, Robin Ma, Fromenty B. Acetaminophen-induced liver injury in obesity and nonalcoholic fatty liver disease. Liver Int. 2014 doi: 10.1111/liv.12514. [DOI] [PubMed] [Google Scholar]
- Mizuno N, Niwa T, Yotsumoto Y, Sugiyama Y. Impact of drug transporter studies on drug discovery and development. Pharmacol Rev. 2003;55:425–61. doi: 10.1124/pr.55.3.1. [DOI] [PubMed] [Google Scholar]
- Mizuno N, Sugiyama Y. Drug transporters: their role and importance in the selection and development of new drugs. Drug Metab Pharmacokinet. 2002;17:93–108. doi: 10.2133/dmpk.17.93. [DOI] [PubMed] [Google Scholar]
- Mofrad P, Contos M, Haque M, Sargeant C, Fisher R, Luketic V, Sterling R, Shiffman M, Stravitz R, Sanyal A. Clinical and histologic spectrum of nonalcoholic fatty liver disease associated with normal ALT values. Hepatology. 2003;37:1286–92. doi: 10.1053/jhep.2003.50229. [DOI] [PubMed] [Google Scholar]
- Mohri T, Nakajima M, Fukami T, Takamiya M, Aoki Y, Yokoi T. Human CYP2E1 is regulated by miR-378. Biochem Pharmacol. 2010;79:1045–52. doi: 10.1016/j.bcp.2009.11.015. [DOI] [PubMed] [Google Scholar]
- Morgan E. Impact of infectious and inflammatory disease on cytochrome P450-mediated drug metabolism and pharmacokinetics. Clin Pharmacol Ther. 2009;85:434–8. doi: 10.1038/clpt.2008.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik A, Belič A, Zanger U, Rozman D. Molecular Interactions between NAFLD and Xenobiotic Metabolism. Front Genet. 2013;4:1–14. doi: 10.3389/fgene.2013.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata K, Tanaka Y, Nakano T, Adachi T, Tanaka H, Kaminuma T, Ishikawa T. Nuclear receptor-mediated transcriptional regulation in Phase I, II, and III xenobiotic metabolizing systems. Drug Metab Pharmacokinet. 2006;21:437–57. doi: 10.2133/dmpk.21.437. [DOI] [PubMed] [Google Scholar]
- Negishi M, Pedersen LG, Petrotchenko E, Shevtsov S, Gorokhov A, Kakuta Y, Pedersen LC. Structure and function of sulfotransferases. Archives of Biochemistry and Biophysics. 2001;390:149–157. doi: 10.1006/abbi.2001.2368. [DOI] [PubMed] [Google Scholar]
- Pan Y, Gao W, Yu A. MicroRNAs regulate CYP3A4 expression via direct and indirect targeting. Drug Metab Dispos. 2009;37:2112–7. doi: 10.1124/dmd.109.027680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persico M, Capasso M, Persico E, Svelto M, Russo R, Spano D, Croce L, La Mura V, Moschella F, Masutti F, Torella R, Tiribelli C, Iolascon A. Suppressor of cytokine signaling 3 (SOCS3) expression and hepatitis C virus-related chronic hepatitis: Insulin resistance and response to antiviral therapy. Hepatology. 2007;46:1009–15. doi: 10.1002/hep.21782. [DOI] [PubMed] [Google Scholar]
- Pettinelli P, Del Pozo T, Araya J, Rodrigo R, Araya A, Smok G, Csendes A, Gutierrez L, Rojas J, Korn O, Maluenda F, Diaz J, Rencoret G, Braghetto I, Castillo J, Poniachik J, Videla L. Enhancement in liver SREBP-1c/PPAR-alpha ratio and steatosis in obese patients: correlations with insulin resistance and n−3 long-chain polyunsaturated fatty acid depletion. Biochim Biophys Acta. 2009;1792:1080–6. doi: 10.1016/j.bbadis.2009.08.015. [DOI] [PubMed] [Google Scholar]
- Ramadori G, Armbrust T. Cytokines in the liver. Eur J Gastroenterol Hepatol. 2001;13:777–84. doi: 10.1097/00042737-200107000-00004. [DOI] [PubMed] [Google Scholar]
- Ratziu V, Charlotte F, Heurtier A, Gombert S, Giral P, Bruckert E, Grimaldi A, Capron F, Poynard T, Group LS. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology. 2005;128:1898–1906. doi: 10.1053/j.gastro.2005.03.084. [DOI] [PubMed] [Google Scholar]
- Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, Khalil L, Turpin G, Opolon P, Poynard T. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117–23. doi: 10.1016/s0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- Renton K. Cytochrome P450 regulation and drug biotransformation during inflammation and infection. Curr Drug Metab. 2004;5:235–43. doi: 10.2174/1389200043335559. [DOI] [PubMed] [Google Scholar]
- Rettie A, Meier G, Sadeque A. Prochiral sulfides as in vitro probes for multiple forms of the flavin-containing monooxygenase. Chem Biol Interact. 1995;96:3–15. doi: 10.1016/0009-2797(94)03579-w. [DOI] [PubMed] [Google Scholar]
- Rinella ME, Sanyal AJ. Management of NAFLD: a stage-based approach. Nat Rev Gastroenterol Hepatol. 2016;13:196–205. doi: 10.1038/nrgastro.2016.3. [DOI] [PubMed] [Google Scholar]
- Robertson G, Leclercq I, Farrell G. Nonalcoholic steatosis and steatohepatitis. II. Cytochrome P-450 enzymes and oxidative stress. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1135–9. doi: 10.1152/ajpgi.2001.281.5.G1135. [DOI] [PubMed] [Google Scholar]
- Roe A, Howard G, Blouin R, Snawder J. Characterization of cytochrome P450 and glutathione S-transferase activity and expression in male and female ob/ob mice. Int J Obes Relat Metab Disord. 1999;23:48–53. doi: 10.1038/sj.ijo.0800756. [DOI] [PubMed] [Google Scholar]
- Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–5. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth A, Looser R, Kaufmann M, Meyer U. Sterol regulatory element binding protein 1 interacts with pregnane X receptor and constitutive androstane receptor and represses their target genes. Pharmacogenet Genomics. 2008;18:325–37. doi: 10.1097/FPC.0b013e3282f706e0. [DOI] [PubMed] [Google Scholar]
- Runge-Morris M, Kocarek T. Regulation of sulfotransferases by xenobiotic receptors. Curr Drug Metab. 2005;6:299–307. doi: 10.2174/1389200054633871. [DOI] [PubMed] [Google Scholar]
- Sanyal A, Campbell-Sargent C, Mirshahi F, Rizzo W, Contos M, Sterling R, Luketic V, Shiffman M, Clore J. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–92. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- Sanyal AJ, Brunt EM, Kleiner DE, Kowdley KV, Chalasani N, Lavine JE, Ratziu V, Mccullough A. Endpoints and clinical trial design for nonalcoholic steatohepatitis. Hepatology. 2011;54:344–53. doi: 10.1002/hep.24376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanyal AJ, Friedman SL, Mccullough AJ, Dimick-Santos L. Challenges and opportunities in drug and biomarker development for nonalcoholic steatohepatitis: Findings and recommendations from an American Association for the Study of Liver Diseases–U.S. Food and Drug Administration Joint Workshop. Hepatology. 2015;61:1392–1405. doi: 10.1002/hep.27678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayiner M, Koenig A, Henry L, Younossi ZM. Epidemiology of Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis in the United States and the Rest of the World. Clin Liver Dis. 2016;20:205–14. doi: 10.1016/j.cld.2015.10.001. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Baumann F, Hanschmann H, Geissler F, Preiss R. Gender difference in ifosfamide metabolism by human liver microsomes. Eur J Drug Metab Pharmacokinet. 2001;26:193–200. doi: 10.1007/BF03190396. [DOI] [PubMed] [Google Scholar]
- Shulman G. Cellular mechanisms of insulin resistance. J Clin Invest. 2000;106:171–6. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofia B, Segura-Aguilar J, Widersten M, Johansson AS, Mannervik B. Glutathione transferases catalyse the detoxication of oxidized metabolites (o-quinones) of catecholamines and may serve as an antioxidant system preventing degenerative cellular processes. Biochemical Journal. 1997;324:25–28. doi: 10.1042/bj3240025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sookoian S, Pirola CJ. Meta-analysis of the influence of I148M variant of patatin-like phospholipase domain containing 3 gene (PNPLA3) on the susceptibility and histological severity of nonalcoholic fatty liver disease. Hepatology. 2011;53:1883–94. doi: 10.1002/hep.24283. [DOI] [PubMed] [Google Scholar]
- Sorensen HT, Mellemkjaer L, Jepsen P, Thulstrup AM, Baron J, Olsen JH, Vilstrup H. Risk of cancer in patients hospitalized with fatty liver: a Danish cohort study. J Clin Gastroenterol. 2003;36:356–9. doi: 10.1097/00004836-200304000-00015. [DOI] [PubMed] [Google Scholar]
- Sotaniemi EA, Pelkonen O, Arranto AJ, Tapanainen P, Rautio A, Pasanen M. Diabetes and elimination of antipyrine in man: an analysis of 298 patients classified by type of diabetes, age, sex, duration of disease and liver involvement. Pharmacol Toxicol. 2002;90:155–60. doi: 10.1034/j.1600-0773.2002.900308.x. [DOI] [PubMed] [Google Scholar]
- Starley B, Calcagno C, Harrison S. Nonalcoholic fatty liver disease and hepatocellular carcinoma: a weighty connection. Hepatology. 2010;51:1820–32. doi: 10.1002/hep.23594. [DOI] [PubMed] [Google Scholar]
- Stepanova M, Hossain N, Afendy A, Perry K, Goodman Z, Baranova A, Younossi Z. Hepatic gene expression of Caucasian and African-American patients with obesity-related non-alcoholic fatty liver disease. Obes Surg. 2010;20:640–50. doi: 10.1007/s11695-010-0078-2. [DOI] [PubMed] [Google Scholar]
- Stevens J, Marsh S, Zaya M, Regina K, Divakaran K, Le M, Hines R. Developmental changes in human liver CYP2D6 expression. Drug Metab Dispos. 2008;36:1587–93. doi: 10.1124/dmd.108.021873. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Kato Y, Tsuji A. Role of SLC xenobiotic transporters and their regulatory mechanisms PDZ proteins in drug delivery and disposition. J Control Release. 2006;116:238–46. doi: 10.1016/j.jconrel.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Tarantino G, Finelli C. What about non-alcoholic fatty liver disease as a new criterion to define metabolic syndrome? World J Gastroenterol. 2013;19:3375–84. doi: 10.3748/wjg.v19.i22.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timsit Y, Negishi M. CAR and PXR: the xenobiotic-sensing receptors. Steroids. 2007;72:231–46. doi: 10.1016/j.steroids.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiniakos D, Vos M, Brunt E. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010;5:145–71. doi: 10.1146/annurev-pathol-121808-102132. [DOI] [PubMed] [Google Scholar]
- Torisu T, Sato N, Yoshiga D, Kobayashi T, Yoshioka T, Mori H, Iida M, Yoshimura A. The dual function of hepatic SOCS3 in insulin resistance in vivo. Genes Cells. 2007;12:143–54. doi: 10.1111/j.1365-2443.2007.01044.x. [DOI] [PubMed] [Google Scholar]
- Ueyama J, Wang D, Kondo T, Saito I, Takagi K, Takagi K, Kamijima M, Nakajima T, Miyamoto K, Wakusawa S, Hasegawa T. Toxicity of diazinon and its metabolites increases in diabetic rats. Toxicol Lett. 2007;170:229–37. doi: 10.1016/j.toxlet.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Vos B, Moreno C, Nagy N, Fery F, Cnop M, Vereerstraeten P, Deviere J, Adler M. Lean non-alcoholic fatty liver disease (Lean-NAFLD): a major cause of cryptogenic liver disease. Acta Gastroenterol Belg. 2011;74:389–94. [PubMed] [Google Scholar]
- Wang H, Tompkins LM. CYP2B6: New Insights into a Historically Overlooked Cytochrome P450 Isozyme. Curr Drug Metab. 2008;9:598–610. doi: 10.2174/138920008785821710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Hall S, Maya J, Li L, Asghar A, Gorski J. Diabetes mellitus increases the in vivo activity of cytochrome P450 2E1 in humans. Br J Clin Pharmacol. 2003;55:77–85. doi: 10.1046/j.1365-2125.2003.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells P, Mackenzie P, Chowdhury J, Guillemette C, Gregory P, Ishii Y, Hansen A, Fk K, Kim P, Chowdhury N, Ritter J. Glucuronidation and the UDP-glucuronosyltransferases in health and disease. Drug Metab Dispos. 2004;32:281–90. doi: 10.1124/dmd.32.3.281. [DOI] [PubMed] [Google Scholar]
- Weltman M, Farrell G, Liddle C. Increased hepatocyte CYP2E1 expression in a rat nutritional model of hepatic steatosis with inflammation. Gastroenterology. 1996;111:1645–53. doi: 10.1016/s0016-5085(96)70028-8. [DOI] [PubMed] [Google Scholar]
- Werk A, Cascorbi I. Functional Gene Variants of CYP3A4. Clin Pharmacol Ther. 2014 doi: 10.1038/clpt.2014.129. [DOI] [PubMed] [Google Scholar]
- Wieckowska A, Feldstein AE. Seminars in liver disease ©. Thieme Medical Publishers; Diagnosis of nonalcoholic fatty liver disease: invasive versus noninvasiveed; pp. 386–395. [DOI] [PubMed] [Google Scholar]
- Williams J, Hyland R, Jones B, Smith D, Hurst S, Goosen T, Peterkin V, Koup J, Ball S. Drug-drug interactions for UDP-glucuronosyltransferase substrates: a pharmacokinetic explanation for typically observed low exposure (AUCi/AUC) ratios. Drug Metab Dispos. 2004;32:1201–8. doi: 10.1124/dmd.104.000794. [DOI] [PubMed] [Google Scholar]
- Wolbold R, Klein K, Burk O, Nüssler A, Neuhaus P, Eichelbaum M, Schwab M, Zanger U. Sex is a major determinant of CYP3A4 expression in human liver. Hepatology. 2003;38:978–88. doi: 10.1053/jhep.2003.50393. [DOI] [PubMed] [Google Scholar]
- Woolsey SJ, Beaton MD, Mansell SE, Leon-Ponte M, Yu J, Pin CL, Adams PC, Kim RB, Tirona RG. A Fibroblast Growth Factor 21-Pregnane X Receptor Pathway Downregulates Hepatic CYP3A4 in Nonalcoholic Fatty Liver Disease. Mol Pharmacol. 2016;90:437–46. doi: 10.1124/mol.116.104687. [DOI] [PubMed] [Google Scholar]
- Woolsey SJ, Mansell SE, Kim RB, Tirona RG, Beaton MD. CYP3A Activity and Expression in Nonalcoholic Fatty Liver Disease. Drug Metab Dispos. 2015;43:1484–90. doi: 10.1124/dmd.115.065979. [DOI] [PubMed] [Google Scholar]
- Yalcin E, More V, Neira K, Lu Z, Cherrington N, Slitt A, King R. Downregulation of sulfotransferase expression and activity in diseased human livers. Drug Metab Dispos. 2013;41:1642–50. doi: 10.1124/dmd.113.050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasui K, Hashimoto E, Komorizono Y, Koike K, Arii S, Imai Y, Shima T, Kanbara Y, Saibara T, Mori T, Kawata S, Uto H, Takami S, Sumida Y, Takamura T, Kawanaka M, Okanoue T. Characteristics of patients with nonalcoholic steatohepatitis who develop hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2011;9:428–33. doi: 10.1016/j.cgh.2011.01.023. quiz e50. [DOI] [PubMed] [Google Scholar]
- Yoshioka Y, Hashimoto E, Yatsuji S, Kaneda H, Taniai M, Tokushige K, Shiratori K. Nonalcoholic steatohepatitis: cirrhosis, hepatocellular carcinoma, and burnt-out NASH. J Gastroenterol. 2004;39:1215–8. doi: 10.1007/s00535-004-1475-x. [DOI] [PubMed] [Google Scholar]
- Younossi Z, Baranova A, Ziegler K, Del Giacco L, Schlauch K, Born T, Elariny H, Gorreta F, Vanmeter A, Younoszai A, Ong J, Goodman Z, Chandhoke V. A genomic and proteomic study of the spectrum of nonalcoholic fatty liver disease. Hepatology. 2005;42:665–74. doi: 10.1002/hep.20838. [DOI] [PubMed] [Google Scholar]
- Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, Racila A, Hunt S, Beckerman R. The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 2016;64:1577–1586. doi: 10.1002/hep.28785. [DOI] [PubMed] [Google Scholar]
- Younossi ZM, Stepanova M, Negro F, Hallaji S, Younossi Y, Lam B, Srishord M. Nonalcoholic fatty liver disease in lean individuals in the United States. Medicine (Baltimore) 2012;91:319–27. doi: 10.1097/MD.0b013e3182779d49. [DOI] [PubMed] [Google Scholar]
- Zanger U, Schwab M. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther. 2013;138:103–41. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- Zein CO, Yerian LM, Gogate P, Lopez R, Kirwan JP, Feldstein AE, Mccullough AJ. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610–9. doi: 10.1002/hep.24544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev. 2006;38:769–89. doi: 10.1080/03602530600971974. [DOI] [PubMed] [Google Scholar]
- Zhang W, Ramzan I, Murray M. Impaired microsomal oxidation of the atypical antipsychotic agent clozapine in hepatic steatosis. J Pharmacol Exp Ther. 2007;322:770–7. doi: 10.1124/jpet.107.124024. [DOI] [PubMed] [Google Scholar]