Introduction
Most recent 2015 consensus guidelines on the use of magnetic resonance imaging (MRI) affect therapeutic decision making in smoldering multiple myeloma (SMM).1 Otherwise asymptomatic patients who have two or more definite focal lesions in the MRI are now considered to have multiple myeloma that needs treatment. Although clearly impactful, the new guidelines draw conclusions from retrospectively obtained data from small non-comparative descriptive studies.2, 3 Furthermore, authors stress that MRI is better suited to detect marrow involvement and not bone destruction,1 while the latter is the most common mode of presentation at the time of progression from SMM to multiple myeloma. Owing to the lack of comparative studies, several questions remain unanswered regarding the role of 18F-fluorodeoxyglucose (FDG)-PET/CT and newer PET tracers like 18F-sodium fluoride (NaF)-PET/CT on the incidence and significance of early bone marrow abnormalities and/or early cortical bone lysis in the myeloma precursor diseases. We conducted this clinical trial using functional and morphologic imaging along the spectrum from myeloma precursor diseases, including monoclonal gammopathy of undetermined significance (MGUS) and SMM to multiple myeloma to comprehensively evaluate both bone and bone marrow. A unique aspect of this study is that we compared in a head to head evaluation, three imaging modalities including FDG-PET/CT, NaF-PET/CT, and dynamic contrast enhanced MRI (DCE-MRI) of lumbosacral spine.
Patients and Methods
Patient Selection
Thirty patients (10 each with MGUS, SMM and multiple myeloma) enrolled on a prospective clinical imaging study (NCT01237054) were evaluated with FDG-PET/CT, NaF-PET/CT, and DCE-MRI of the lumbosacral spine, in addition to conventional skeletal surveys. The diagnosis of MGUS, SMM and multiple myeloma was made according to updated 2010 International Myeloma Working Group (IMWG) diagnostic criteria.4 Furthermore, for the purpose of expanding on the role of FDG-PET/CT in SMM, we also included 16 high risk SMM patients, those who were screened by FDG-PET/CT and skeletal surveys for enrollment on another treatment trial (NCT01572480). High risk SMM was defined according to the criteria by the Spanish study group (PETHEMA) or Mayo Clinic.5, 6 Thus, there were a total of 46 evaluable patients in this study. The trials were approved by the Institutional Review Board and ethics committee. Written informed consent was obtained from all patients. Imaging scans were reviewed by a radiologist and a nuclear medicine physician. The results of the morphologic component of the multi parametric DCE-MRI are reported for this study, while the purely functional (semi quantitative) measurements of DCE-MRI for assessing bone marrow angiogenesis have been published previously.7
Laboratory evaluation
All patients underwent myeloma specific work-up including serum and urine immunofixation electrophoresis, serum free light chain assay, serum immunoglobulin, serum beta-2-microglobulin and lactate dehydrogenase. Bone marrow aspirates and biopsies were obtained for cytological and histopathological evaluation of plasma cell infiltration. Clonality assessment and aberrant plasma cell percentage was determined in bone marrow aspirate by 8 color-flow cytometry using European Myeloma Network Gating criteria.8
NaF-PET/CT
Patients underwent three sequential 3D PET/CT scans of the torso in 60 minutes, followed by a whole body 3D PET/CT at 2 hours post intravenous injection of 3 to 5 mCi of 18F-NaF. The technical details of NaF-PET/CT and other imaging methods are available at request.
FDG-PET/CT
On a separate day, patients underwent FDG-PET/CT. Criteria for bone lesions on PET/CT were based on Zamagni et al.9; 1) presence of focal areas of detectable increased tracer uptake within bone on at least two consecutive slices excluding joints, without a lesion identified by CT, or 2) a SUVmax ≥ 2.5 within an osteolytic CT area exceeding 1 cm in diameter, or 3) a SUVmax > 1.5 within an osteolytic CT area 0.5 to 1 cm diameter. Bone marrow uptake on PET/CT was described as negative, diffuse, or focal according to the degree of FDG uptake. Bone marrow was considered diffusely involved if the tracer uptake was diffusely increased with a SUVmax equal to, or > the uptake in the spleen.
DCE-MRI
Patients underwent DCE-MRI of the lumbar spine. The pattern of marrow involvement on MRI was characterized as: (1) normal when there was no evidence of abnormal signal intensity; (2) focal, when there were localized areas of abnormal marrow; lesions are darker than normal yellow marrow and slightly darker than or isointense to red marrow on T1-weighted images, whereas on T2-weighted images, lesions are brighter than both red and yellow marrow, and on enhanced T1-weighted images they enhance to various degrees over the normal marrow; (3) diffuse, when the normal bone marrow signal intensity is completely absent; the intervertebral disks appear brighter than or isointense to the diseased marrow; diffuse decrease in the BM signal intensity on T1-weighted images, a variable increase in the signal intensity on T2-weighted images, and the abnormal marrow enhances diffusely and the intervertebral disks appear darker than the enhanced spine; and finally (4) heterogeneous, when there are innumerable small foci of abnormality on a background of intact marrow, with small dark lesions on T1-weighted images, small, bright lesions on T2-weighted images and multifocal areas of enhancement on DCE MRI.
Skeletal Surveys
The skeletal survey included a posteroanterior view of the chest, antero-posterior (AP) and lateral views of the cervical spine, thoracic spine, lumbar spine, humeri and femora, AP and lateral views of the skull and AP view of the pelvis. All images were obtained digitally and stored on a PACS system (Carestream Health, NY, USA)
Results
Clinical characteristics
Baseline characteristics of the study patients are listed in Table 1. The median age of all patients was 59 years (range 41-88 years), and there were equal numbers of men and women. The predominant monoclonal isotype was IgG. Extramedullary involvement was noted in 3 patients.
Table 1. Patients' clinical and laboratory characteristics.
| MGUS | SMM | Multiple Myeloma | ||
|---|---|---|---|---|
|
| ||||
| Imaging done on study | ||||
| FDG-PET/CT | + | + | + | + |
| NaF-PET/CT | + | + | - | + |
| DCE-MRI | + | + | - | + |
| Skeletal Survey | + | + | + | + |
|
| ||||
| Number, n | 10 | 10 | 16 | 10 |
|
| ||||
| Age, median(range) | 58(53-78) | 58(48-78) | 58(43-74) | 63(48-73) |
|
| ||||
| Sex, Male/Female | 1/9 | 6/4 | 7/9 | 5/5 |
|
| ||||
| Isotype | ||||
| IgG | 7 | 9 | 13 | 9 |
| IgA | 2 | 0 | 1 | 1 |
| IgM | 1 | 1 | 0 | |
| Light chain | 0 | 0 | 2 | |
|
| ||||
| Mayo Clinic risk | Not applicable | |||
| Low | 5 | 5 | 3 | |
| Intermediate | 4 | 4 | 11 | |
| High | 1 | 1 | 2 | |
|
| ||||
| PETHEMA risk | Not available | Not applicable | ||
| Low | 2 | 0 | ||
| Intermediate | 3 | 0 | ||
| High | ||||
| 5 | 16 | |||
|
| ||||
| Immunoparesis, yes/no | 2/8 | 7/3 | 16/0 | 6/2 |
|
| ||||
| Serum free light chain ratio, median(range) | 1.39 (0.26-9.26) | 2.4 (0.01-197) | 60.8 (0.01-8980) | 13.86 (0.06-58.4) |
|
| ||||
| Beta 2 microglobulin, median(range), mg/L | 1.85 (1.3-2.7) | 2.1 (0.7-3.6) | 2.15 (1.4-2.7) | 2 (1.2-3.5) |
|
| ||||
| Lactate dehydrogenase, median(range), U/L | 181.5 (131-277) | 148.5 (138-205) | 147.5 (106-205) | 176 (127-297) |
|
| ||||
| Creatinine, mg/dL | 0.77 (0.66-0.87) | 0.89 (0.95-1.15) | 0.8 (0.47-0.95) | 0.78 (0.55-1.1) |
|
| ||||
| Upstaged to multiple myeloma, n (%)* | 0 (0) | 1 (10) | 4 (25) | Not applicable |
Reclassified as multiple myeloma based on lytic lesions detectable by PET/CT (while skeletal survey was negative).
Bone and bone marrow imaging
SMM
A total of 26 newly diagnosed SMM patients were evaluated, and by IMWG definition had no lytic lesions on skeletal survey. Five (19%) patients were reclassified as having multiple myeloma requiring treatment, including 4 with lytic bone lesions on CT, and one with a biopsy-confirmed FDG-avid clavicular lesion that was negative on NaF-PET/CT (Figure 1). All these 5 patients had high risk disease by PETHEMA criteria; 2 were high risk, and 3 were intermediate risk by Mayo clinic criteria. Among the remaining 21 patients diagnosed with SMM, the distribution of risk categorization by both Mayo clinic and Spanish criteria is provided in Table 1. Among SMM, a group of six (23%) patients had bone marrow abnormalities on FDG-PET, either unifocal (n=3) or diffuse (n=3); the mean SUVmax of focal abnormalities were 1.6, 2.5 and 3.7. Risk categorization of these 6 patients were: high risk PETHEMA criteria (n=6); intermediate risk Mayo Clinic (n=5) and high risk Mayo Clinic (n=1). The SMM cohort with bone or marrow involvement on FDG-PET/CT did not differ in terms of other clinical features such as marrow plasmacytosis or M spike than those with normal imaging. DCE-MRI did not reveal bone marrow abnormalities in any of the evaluable patients, except one patient, who was upstaged to multiple myeloma based on biopsy confirmed FDG-avid focal abnormality, also had diffuse marrow involvement in MRI. Similarly, NaF-PET/CT was negative in patients analyzed. The mean normal bone SUVmax ranged from 1.6 to 2.4 (median 2) for FDG uptake and from 1.8 to 9.5 (median 7.25) for NaF uptake.
Figure 1.
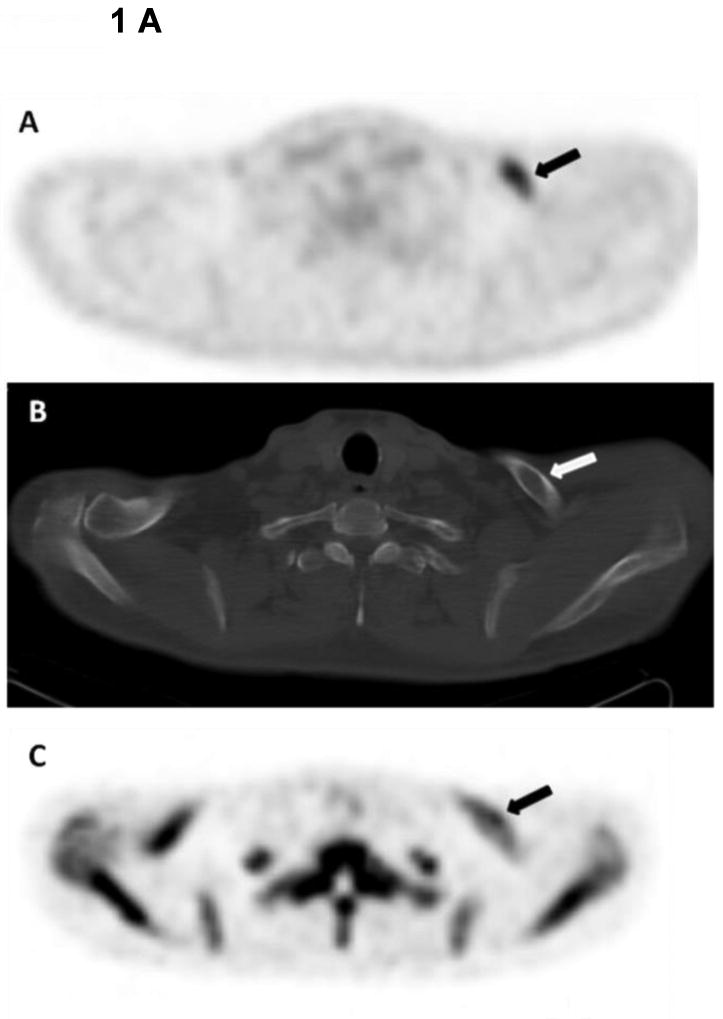
1 A. 48-year-old male with smoldering multiple myeloma. Axial 18F-FDG PET image shows increased tracer uptake within the left clavicle (arrow) (A). Corresponding axial CT image demonstrates a lytic bone lesion within the left clavicle (arrow) (B), This lytic bone lesion is likely within the trabecular bone as cortical bone seems intact, and was retrospectively identified with the help of 18F-FDG PET. The lytic bone lesion shows no significant uptake on the axial 18F-NaF PET (arrow) (C). This lytic bone lesion was biopsied under guidance of CT and the histopathology was consistent with myeloma involvement.
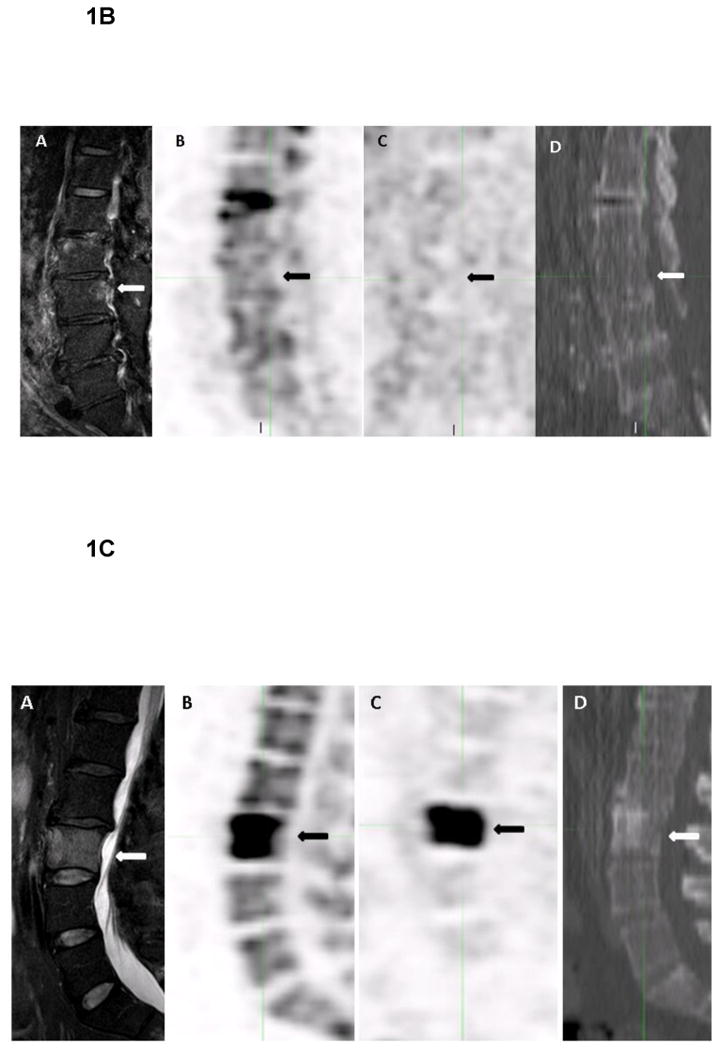
1B. 79-year-old male with monoclonocal gammopathy of undetermined significance (MGUS). Coronal T2W SPAIR MR image demonstrates a focal abnormal signal within the bone marrow in the posterior part of the L3 vertebral body (arrow) (A). The focal bone marrow abnormality shows no uptake on the coronal 18F-NaF PET (arrow) (B), and on 18F-FDG PET (arrow) (C). Corresponding CT from 18F-FDG PET demonstrates no lytic bone lesion (arrow) (D).
1C. 55-year-old male with multiple myeloma. Coronal T2W SPAIR MR image demonstrates a lytic bone lesion within the L3 vertebral body, which affects almost the whole vertebral body (arrow) (A). The lytic bone lesion shows uptake on the coronal 18F-NaF PET (arrow) (B), and on 18F-FDG PET (arrow) (C). Corresponding CT from 18F-FDG PET demonstrates a mixed blastic and lytic bone lesion within the L3 vertebral body (arrow) (D).
MGUS
No bone lesions were detected with either, FDG-PET/CT or NaF-PET/CT. On MRI, a single focal abnormality in a lumbar vertebral bone marrow was identified in one patient (Figure 2). The mean normal SUVmax ranged from 1.5 to 2.2 (median 1.75) for FDG uptake and from 5.3 to 8.6 (median 6.9) for NaF uptake.
Figure 2. Illustrative representation of overall imaging findings (lytic bone lesions and bone marrow abnormalities) for every patient with high risk smoldering multiple myeloma, monoclonal gammopathy of undetermined significance (MGUS) and multiple myeloma.
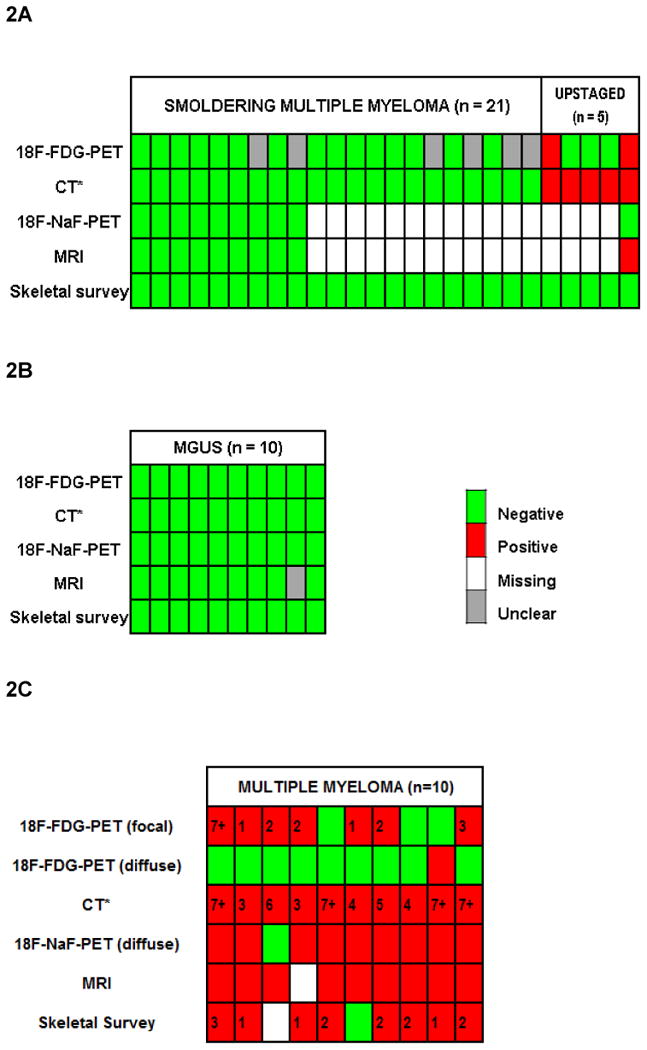
Each column represents a patient. Rows represent the image specific bone marrow and bone findings. 2A and 2B, from top: Row 1: FDG-avid bone marrow abnormalities and lytic bone lesions; coded as green (no activity), or gray (focal or diffuse bone marrow abnormalities, possibly representative of myelomatous infiltration, but not biopsy proven), or red (FDG-avid lytic bone lesion, either biopsy proven, one patient or unproven, one patient). Row 2: CT (*CT component of FDG-PET) detected lytic bone lesions coded as red (present) or green (absent). Row 3: NaF activity in lytic bone lesions; coded as green (absent) and white (if test was not done). Row 4: MRI detected focal bone marrow abnormalities; coded as green (absent) or gray (present, but of unclear significance in the absence of biopsy). Row 5: lytic bone lesions on skeletal survey coded as negative (green). Overall, among evaluable patients, bone marrow abnormalities were seen in 6 patients with smoldering multiple myeloma, and 1 patient with MGUS. Lytic bone lesions were detected on PET/CT in 5 patients with smoldering multiple myeloma; these patients were upstaged. 2C, from top: Row 1: FDG-avid focal bone marrow abnormalities with or without a CT correlate of lytic bone lesions; coded as green (no activity), or red (positive activity, indicated by number of lesions for that patient). Row 2: FDG-PET detected diffuse bone marrow abnormalities; coded as red (present) and green (absent). Row 3: CT (*CT component of FDG-PET) detected lytic bone lesions; coded as red (present, indicated by number of lesions for that patient). Row 4: NaF-avid lytic bone lesions; coded as green (absent) and white (test was not done). Row 5: MRI detected bone marrow abnormalities; coded as green (absent) or white (test was not done). Row 6: skeletal survey detected bone lytic lesions; coded as green (absent), red (present, indicated by number of lesions for that patient), and white (missing data). For the purpose of counting bone lytic lesions and bone marrow abnormalities we took into account 13 most important skeletal regions at risk for myeloma invasion: vertebral column (cervical, thoracic and lumbar), sternum and ribs, clavicles and scapulae, humeri, skull, pelvis and femora
Multiple Myeloma
On FDG-PET, focal or diffuse bone marrow abnormalities were detected in 8/10 (80%), and on MRI in 9/10 (90%) evaluable patients. CT identified lytic bone lesions in all patients, compared to 8/9 (89%) on skeletal survey. Overall CT detected more lesions [>7 sites in 4 (CT) vs. 0 patients (skeletal survey)]. As MRI was limited to the lumbosacral spine, FDG-PET/CT was more helpful in detecting EMD and appendicular lesions. NaF uptake was confined to rim of lytic lesions identified on CT (Figure 3), thus providing no additional clinical information. The mean background (normal vertebra) SUVmax ranged from 1 to 2.8 (median 1.75) for FDG uptake and from 3.5 to 11.7 (median 5.9) for NaF uptake.
The illustrative representation of overall imaging findings in every patient with SMM, MGUS and multiple myeloma is provided in Figure 2.
Discussion
Heightened interest in the use of newer imaging techniques in clinical practice has led IMWG to revise some of their recommendations on the use of imaging in diagnosis of SMM and multiple myeloma. The new guidelines recommend that one of PET-CT, low-dose whole-body CT, or MRI of the whole-body or spine be done in all patients with suspected SMM, with the exact imaging modality determined by availability and resources.9 Furthermore, according to the recent definition, a patient with SMM should undergo MRI, and if two or more focal lesions are found, the diagnosis is stepped up to multiple myeloma requiring treatment.1 Questions remain as to what is the best modality to choose, largely because comparative studies on the usefulness of combining functional and morphologic imaging for patients with MGUS and SMM have been lacking.
This prospective clinical study is unique, as it compares three imaging modalities including FDG-PET/CT, NaF-PET/CT and lumbosacral DCE-MRI in patients with MGUS, SMM and multiple myeloma. Statistical power calculations for the imaging protocol ensured that we were able to test our hypothesis based on 30 patients in the comparative cohort, so that the data can be used to design larger confirmatory studies. This prospective clinical trial focusing on functional and morphologic imaging provides specific data on the percentage of SMM patients re-classified as multiple myeloma, an information that is hardly known from published series. Among 26 SMM patients evaluated, FDG PET/CT led to detection of bone lytic lesions in 19%, thereby reclassifying this subset of patients as having MM. In these cases, the low-dose CT component of the FDG-PET imparted sufficient resolution on bone settings and provided better detectability compared to skeletal survey. In the other subset of SMM patients (n=6), no lytic bone lesions were identified, but they exhibited either unifocal or diffuse increased FDG uptake in the bone marrow. This clinical study was not designed for longitudinal follow up to answer the specific question whether such lesions progress to full multiple myeloma. However, in a retrospective study, SMM patients with a positive PET-CT bone marrow findings and no underlying osteolysis had 66% rate (4 of 6 patients) of progression within 2 years.10 Similarly in another study, bone marrow abnormalities exclusive of bone lytic lesions were found in in 9/73 (12%) patients, with the probability of progression within 2 and 3 years for patients with positive PET/CT of 48% and 65%, respectively, in comparison to 32% and 42% for negative patients.11 In contrast to FDG-PET/CT, lack of MRI detected bone marrow abnormalities in our cohort of SMM could be attributed to the relatively small number of patients, and also to the fact that the imaging was limited to the lumbar spine and did not include the whole spine or body. Besides this prospective clinical trial focusing on functional and morphologic imaging, MRI has been focus of only a few other studies (reviewed in12) in SMM. In a sizeable study of 149 patients, whole body MRI revealed bone marrow focal abnormalities in 28% of patients, and the presence of two or more focal lesions was shown to have independent prognostic significance for progression to symptomatic disease.3 Based on the limited data deriving conclusions from retrospectively analyzed numbers with the inherent limitation of selection bias and follow-up bias, IMWG has proposed revised recommendations on the use of MRI in SMM that are practice changing. No clear guidance is provided as to why MRI and not FDG-PET/CT defined focal lesions would reclassify SMM patients as having multiple myeloma. We believe that more prospective studies are required to substantiate these guidelines, and in this regard our study focusing on functional and morphologic imaging provides important direction.
Regarding the role of functional imaging in MGUS, no bone lesions were detected by either FDG-PET/CT or NaF-PET/CT. Previous studies, supporting our findings, indicate that a negative FDG-PET and CT can be inferred to reliably predict stable MGUS, although these studies did not evaluate the role of NaF-PET/CT which is presumably more sensitive for bone disease.13, 14 On MRI, we identified a focal infiltration pattern in the lumbar vertebral bone marrow in only 1 of 10 MGUS patients. In the largest study to date that evaluated the role of whole body MRI in 137 consecutive patients with MGUS, a focal infiltration bone marrow pattern was identified in 23.4%, shown to have independent prognostic significance for progression to symptomatic disease.15 Whereas some of the MRI abnormalities might never progress, others might represent myelomatous deposits with high plasma cell burden and progress rapidly. Furthermore, MRI detected focal myelomatous infiltration may be a false positive finding related to benign bone lesions.16 Only about 0.5 to 1% of patients per year with MGUS will progress to myeloma, and less commonly to lymphoma, primary amyloidosis, or chronic lymphocytic leukemia.17-19 Collectively, this implies that only a very small subset of patients with abnormalities detected on MRI will eventually progress to multiple myeloma. Clearly, more data is needed before we justify clinical-decision making based solely on bone marrow MRI findings in MGUS.
Among the multiple myeloma group, we specifically evaluated the role of NaF-PET. NaF-PET has been investigated in other malignancies for its desirable characteristics including high and rapid uptake in the bone with rapid blood clearance, producing a high bone-to-background ratio in a short time. However, in our group of patients, the NaF-PET provided no additional information besides that already present on the CT component. The uptake of NaF was confined to the rim of CT detected lytic bone lesions where metabolic bone activity is highest. Compared to FDG-PET, NaF-PET uptake was quite nonspecific as high background activity in the normal bone marrow tended to obscure subtle changes in true myelomatous lesions. Similar to our results, in another study of 60 patients with multiple myeloma FDG-PET/CT proved to be a more specific biomarker than NaF-PET/CT for skeletal assessment.20
In conclusion, using sensitive imaging tools we detected bone and marrow changes otherwise not identified on skeletal surveys in SMM patients, consistent with the concept of a disease continuum leading to multiple myeloma. This prospective study, based on sufficient statistical power allowed us to assess molecular imaging head-to-head in the spectrum from myeloma precursor disease to multiple myeloma. For the first time, we assessed NaF-PET/CT in a clinical trial targeting MGUS, SMM, and multiple myeloma. The information gained from this study, is of direct clinical relevance, and of value for the development of future prospective clinical studies designed to improve clinical outcomes based on molecular imaging. Future prospective imaging studies are needed to develop novel myeloma-specific PET tracers which can be used in the settings of PET/CT and PET/MRI. Key opportunities for molecular imaging in multiple myeloma include both quantification of disease burden (bone marrow and bone involvement) at diagnosis, and early assessment of treatment response (e.g., after 2 cycles) to better guide therapy.
Acknowledgments
This research was supported by the Intramural Research Program of the NCI, NIH.
Research Support: Supported by intramural funding from National Cancer Institute. Clinicaltrials.gov identifier: NCT01237054; NCT01572480
Footnotes
The abstract was accepted for general poster session at ASCO 2014 annual meeting, Chicago, IL
Authorship Contributions: OL was the principal investigator and takes primary responsibility for the paper; OL, NK, PLC, KK, SZU and MB contributed to the conception and design of the study. MB and OL wrote the manuscript and analyzed the data; OL, PLC, and KK directed implementation of the study, including quality assurance and control; MB, ET, NK, MR, EEM, NT, SM, ARM, and BMW recruited patients and acquired clinical data; MM, AC, and EL coordinated the research and acquired data; BT, PLC, EM, LL, and KK analyzed and reviewed imaging data; KRC and IM analyzed, and interpreted the diagnostic data. All the authors were involved in the interpretation of the results. All authors read, gave comments, and approved the final version of the manuscript. All the authors had full access to the data in the study and take responsibility for the accuracy of the data analysis.
Disclosure of Conflicts of Interest: The authors report no potential conflicts of interest.
References
- 1.Dimopoulos MA, Hillengass J, Usmani S, Zamagni E, Lentzsch S, Davies FE, et al. Role of magnetic resonance imaging in the management of patients with multiple myeloma: a consensus statement. J Clin Oncol. 2015 Feb 20;33(6):657–664. doi: 10.1200/JCO.2014.57.9961. [DOI] [PubMed] [Google Scholar]
- 2.Moulopoulos LA, Dimopoulos MA, Smith TL, Weber DM, Delasalle KB, Libshitz HI, et al. Prognostic significance of magnetic resonance imaging in patients with asymptomatic multiple myeloma. J Clin Oncol. 1995 Jan;13(1):251–256. doi: 10.1200/JCO.1995.13.1.251. [DOI] [PubMed] [Google Scholar]
- 3.Hillengass J, Fechtner K, Weber MA, Bauerle T, Ayyaz S, Heiss C, et al. Prognostic significance of focal lesions in whole-body magnetic resonance imaging in patients with asymptomatic multiple myeloma. J Clin Oncol. 2010 Mar 20;28(9):1606–1610. doi: 10.1200/JCO.2009.25.5356. [DOI] [PubMed] [Google Scholar]
- 4.Kyle RA, Durie BG, Rajkumar SV, Landgren O, Blade J, Merlini G, et al. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering (asymptomatic) multiple myeloma: IMWG consensus perspectives risk factors for progression and guidelines for monitoring and management. Leukemia. 2010 Jun;24(6):1121–1127. doi: 10.1038/leu.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez-Persona E, Vidriales MB, Mateo G, Garcia-Sanz R, Mateos MV, de Coca AG, et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood. 2007 Oct 1;110(7):2586–2592. doi: 10.1182/blood-2007-05-088443. [DOI] [PubMed] [Google Scholar]
- 6.Dispenzieri A, Kyle RA, Katzmann JA, Therneau TM, Larson D, Benson J, et al. Immunoglobulin free light chain ratio is an independent risk factor for progression of smoldering (asymptomatic) multiple myeloma. Blood. 2008 Jan 15;111(2):785–789. doi: 10.1182/blood-2007-08-108357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhutani M, Turkbey B, Tan E, Kemp TJ, Pinto LA, Berg AR, et al. Bone marrow angiogenesis in myeloma and its precursor disease: a prospective clinical trial. Leukemia. 2014 Feb;28(2):413–416. doi: 10.1038/leu.2013.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rawstron AC, Orfao A, Beksac M, Bezdickova L, Brooimans RA, Bumbea H, et al. Report of the European Myeloma Network on multiparametric flow cytometry in multiple myeloma and related disorders. Haematologica. 2008 Mar;93(3):431–438. doi: 10.3324/haematol.11080. [DOI] [PubMed] [Google Scholar]
- 9.Rajkumar SV, Dimopoulos MA, Palumbo A, Blade J, Merlini G, Mateos MV, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014 Nov;15(12):e538–548. doi: 10.1016/S1470-2045(14)70442-5. [DOI] [PubMed] [Google Scholar]
- 10.Dykstra BKS, Dispenzieri A, Lacy M, Buadi F, Dingli D, et al. PET-CT has major diagnostic value in the evaluation of smoldering multiple myeloma. Blood. 2014;124(21):3382. [Google Scholar]
- 11.Zamagni E, N C, Gay F, Pezzi A, Bello M, Rambaldi I, et al. The presence of FDG PET/CT focal, not osteolytic, lesion(s) identifies a sub-group of patients with smoldering multiple myeloma with high-risk of progression into symptomatic disease. Blood. 2014;124(21):3371. [Google Scholar]
- 12.Bhutani M, Landgren O. [Imaging in smoldering (asymptomatic) multiple myeloma. Past, present and future] Radiologe. 2014 Jun;54(6):572, 574–581. doi: 10.1007/s00117-014-2694-7. [DOI] [PubMed] [Google Scholar]
- 13.Spira D, Weisel K, Brodoefel H, Schulze M, Kaufmann S, Horger M. Can whole-body low-dose multidetector CT exclude the presence of myeloma bone disease in patients with monoclonal gammopathy of undetermined significance (MGUS)? Acad Radiol. 2012 Jan;19(1):89–94. doi: 10.1016/j.acra.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Durie BG, Waxman AD, D'Agnolo A, Williams CM. Whole-body (18)F-FDG PET identifies high-risk myeloma. J Nucl Med. 2002 Nov;43(11):1457–1463. [PubMed] [Google Scholar]
- 15.Hillengass J, Weber MA, Kilk K, Listl K, Wagner-Gund B, Hillengass M, et al. Prognostic significance of whole-body MRI in patients with monoclonal gammopathy of undetermined significance. Leukemia. 2014 Jan;28(1):174–178. doi: 10.1038/leu.2013.244. [DOI] [PubMed] [Google Scholar]
- 16.Hanrahan CJ, Christensen CR, Crim JR. Current concepts in the evaluation of multiple myeloma with MR imaging and FDG PET/CT. Radiographics. 2010 Jan;30(1):127–142. doi: 10.1148/rg.301095066. [DOI] [PubMed] [Google Scholar]
- 17.Turesson I, Kovalchik SA, Pfeiffer RM, Kristinsson SY, Goldin LR, Drayson MT, et al. Monoclonal gammopathy of undetermined significance and risk of lymphoid and myeloid malignancies: 728 cases followed up to 30 years in Sweden. Blood. 2014 Jan 16;123(3):338–345. doi: 10.1182/blood-2013-05-505487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kyle RA, Therneau TM, Rajkumar SV, Offord JR, Larson DR, Plevak MF, et al. A long-term study of prognosis in monoclonal gammopathy of undetermined significance. N Engl J Med. 2002 Feb 21;346(8):564–569. doi: 10.1056/NEJMoa01133202. [DOI] [PubMed] [Google Scholar]
- 19.Kyle RA, Therneau TM, Rajkumar SV, Larson DR, Plevak MF, Melton LJ., 3rd Long-term follow-up of 241 patients with monoclonal gammopathy of undetermined significance: the original Mayo Clinic series 25 years later. Mayo Clin Proc. 2004 Jul;79(7):859–866. doi: 10.4065/79.7.859. [DOI] [PubMed] [Google Scholar]
- 20.Sachpekidis C, Goldschmidt H, Hose D, Pan L, Cheng C, Kopka K, et al. PET/CT studies of multiple myeloma using (18) F-FDG and (18) F-NaF: comparison of distribution patterns and tracers' pharmacokinetics. Eur J Nucl Med Mol Imaging. 2014 Jul;41(7):1343–1353. doi: 10.1007/s00259-014-2721-y. [DOI] [PubMed] [Google Scholar]


