Abstract
Angiogenesis is regulated by complex interactions between endothelial cells and support cells of the vascular microenvironment, such as tissue myeloid cells and vascular mural cells. Multicellular interactions during angiogenesis are difficult to study in animals, and challenging in a reductive setting. We incorporated stromal cells into an established bead-based capillary sprouting assay to develop assays that faithfully reproduce major steps of vessel sprouting and maturation. We observed that macrophages enhance angiogenesis, increasing the number and length of endothelial sprouts, a property we have dubbed “angiotrophism”. We found that polarizing macrophages towards a pro-inflammatory profile further increased their angiotrophic stimulation of vessel sprouting, and this increase was dependent on macrophage Notch signaling. To study endothelial/pericyte interactions, we added vascular pericytes directly to the bead-bound endothelial monolayer. These pericytes formed close associations with the endothelial sprouts, causing increased sprout number and vessel caliber. We found that Jagged1 expression and Notch signaling are essential for the growth of both endothelial cells and pericytes, and may function in their interaction. We observed that combining endothelial cells with both macrophages and pericytes in the same sprouting assay has additive effects on sprouting. These results significantly improve bead-capillary sprouting assays and provide an enhanced method for modeling interactions between the endothelium and the vascular microenvironment. Achieving this in a reductive in vitro setting represents a significant step toward a better understanding of the cellular elements that contribute to the formation of mature vasculature.
Keywords: Notch, in vitro, endothelial cell, pericyte, macrophage
Introduction
In both developmental and pathological phenomena, the formation of mature and functional blood vessel networks relies upon the interaction between endothelial cells and the stromal cells that surround them in the vascular microenvironment. These interactions are particularly relevant in anti-angiogenic disease therapy. The interaction of anti-angiogenic agents with vascular support cells has recently become a focus of interest, as macrophages and perivascular cells play indispensible roles in blood vessel growth and organization [1-4].
Macrophages are myeloid cells, derived from the bone marrow, which exist both in tissue resident states and as migratory cells [5]. Though macrophages were first described in their role as phagocytic mediators of passive immunity, it is now appreciated that macrophages can adopt a wide variety of context-specific phenotypes that allow them to function in diverse physiologic as well as pathologic processes [6]. Though the role of macrophages in the vasculature has been studied extensively in the context of tumorigenesis [6,7], they have also been implicated in physiological angiogenesis. In angiogenesis macrophages often take on an angiotrophic role: that is, they foster the growth of vessel networks directly and indirectly, through physical involvement in the process and by the secretion of angiogenic factors. Microglia, neuronal myeloid cells closely related to macrophages, can influence sprouting in a mouse aortic ring model of angiogenesis [2]. Microglia have also been implicated in vivo in the facilitation of anastomosis between nascent vessels [1], and have been found to limit angiogenesis by the expression of inhibitory VEGFR1 in a vascular bed-specific fashion [3].
Perivascular cells are stromal cells that interact closely with the abluminal surface of blood vessels to influence a wide range of vessel parameters. Different types of perivascular cells interact with different caliber vessels: vascular smooth muscle cells (VSMCs) interact with larger caliber vessels, while pericytes form close attachments with smaller caliber capillaries [4]. VSMCs and pericytes are distinct cell types, and the exact differences between them, and indeed the exact definition of a pericyte, have been the focus of considerable research [8,4]. Broadly, perivascular cells support vascular growth by forming close attachments with nascent vessels to promote vessel maturation, quiescence, and patency. VSMCs surrounding larger vessels and additionally regulate vessel tone [4].
The Notch receptors and their ligands are a family of well-conserved proteins that allow direct signaling between neighboring cells, and play roles in a wide array of physiological and pathological processes [9]. Notch signaling functions in several angiogenic mechanisms, most notably controlling the differentiation between endothelial tip- and stalk-cell identities [10]. More recently, Notch has been implicated in the interaction between endothelial cells and both macrophages and perivascular cells. In macrophages, Notch signaling has been found to be important for recruitment to sites of active angiogenesis in both developmental and pathological settings, and Notch signaling has been detected in macrophages at the sites of imminent or recent vessel anastomosis, suggesting a role in this process [11,12]. In perivascular cells, Notch signaling between the endothelium and smooth muscle cells has been shown to control VSMC differentiation and in influencing the intercellular adhesion between the endothelium and VSMC [13]. A study in zebrafish also pointed to a comparable role for Notch3 signaling in pericytes [14], but so far the field remains understudied.
Given the complexity of the interactions that lead to the formation of vascular networks, and the great potential utility of understanding these relationships, there is a pressing need for the development of reductive in vitro systems that faithfully recapitulate the angiogenic process. A wide variety of methods have been developed to model elements of angiogenesis, such as endothelial proliferation, migration, and network formation [15]. Few of these assays faithfully recreate angiogenesis in a multi-cellular setting [16]. Some of the more successful in vitro models for angiogenesis are bead-based capillary sprouting assays using three-dimensional matrices. In one form of this assay, endothelial cells are bound to micro-carrier beads and embedded in a fibrin gel, where they sprout to form lumenized vessels in response to cues from a fibroblast feeder layer [17,18]. This assay is typically focused on the endothelium, and while some recent studies have used the assay to describe interactions between endothelial cells and VSMCs [13], the potential of this assay as a tool for modeling the relationships between endothelial and vascular support cells remains largely untapped.
Methods
Cell culture
L929 fibroblasts were acquired from ATCC and maintained in DMEM (Gibco) 4.5g/dL glucose + 10% HI-FBS + 1× Penn/Strep. L929 fibroblasts were used solely in the creation of conditioned medium for use in derivation of bone marrow macrophages.
LADMAC were acquired from ATCC and maintained in EMEM (ATCC) + 10% HI-FBS + 1× Penn/Strep. Human umbilical vein endothelial cells (HUVEC) were isolated from human tissue according to established protocol [19]. Cells were maintained on collagen I (Corning) -coated plates in EGM2 (Lonza).
EOC2 microglia were purchased from ATCC and were maintained in DMEM (Gibco) 4.5g/dl glucose + 10% HI-FBS + 1× Penicillin/Streptomycin, supplemented with 20% LADMAC-conditioned medium as a source of M-CSF.
Bone marrow macrophages (BMM) were derived from bone marrow harvested from mouse femurs and tibias, according to established protocol [11]. Briefly, bone marrow was isolated and cultured on untreated petri dishes (Falcon) in RPMI (Gibco) + 10% HI-FBS supplemented with 20% L929-conditioned medium as a source of M-CSF with media changes every two days. Over the course of a week, the bone marrow differentiates to form a monoculture of f4/80+ macrophages, as other cells do not adhere to the plastic and are washed off.
Human Brain Vascular Pericytes were acquired from ScienCell. Cells were maintained on 1%-gelatin (Millipore) - coated plates in DMEM (Gibco) 1g/dL glucose + 10% HI-FBS + 1× Penn/Strep.
D551 human skin fibroblasts were purchased from ATCC and maintained in EMEM (Gibco) + 10% HI-FBS + 1× Penn/Strep. Medium was changed to EGM-2 24 hours prior to inclusion in capillary sprouting assay. 293T were acquired from (ATCC) and maintained in IMDM (Gibco) + 10% HI-FBS + 1× Penn/Strep.
Mouse husbandry
BMMs were derived from mice with the following genotypes: LysMcre/cre; DNMAML-GFP+/+ (functionally wildtype), LysMcre/cre; DNMAML-GFPfl/+, and LysMcre/+; EYFPYFP/+. LysMcre mice were purchased from The Jackson Laboratory [20]. DNMAML-GFP mice were a kind gift from Warren Pear via Boris Reizis [21]. R26-EYFP mice were purchased from the Jackson Laboratory [22] Prior to sacrifice, mice were housed in the barrier facility at the Irving Cancer Research Center at Columbia University Medical Center according to institutional guidelines. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Labeling of macrophages for capillary sprouting assay
Bone marrow derived macrophages were labeled using Vybrant Dio Cell labeling solution (Molecular Probes) according to the manufacturer's instructions. Briefly, BMM were resuspended in serum-free RPMI, incubated with the labeling solution for 20 min at 37C, and washed twice with serum-free RPMI before being introduced to the fibrin for the capillary sprouting assay.
Macrophage polarization
Prior to inclusion in coculture, macrophages were treated for 24 hours with either LPS (100ng/mL)(Calbiotech) and recombinant murine Interferon Gamma (100U/mL) (Peprotech) or recombinant murine Interleukin 4 (5ng/mL)(R&D Systems). Factors were removed and cells were washed three times with sterile PBS prior to cell isolation and inclusion in the coculture. In order to examine whether the possibility of LPS carry-over into the capillary sprouting assay may affect endothelial sprouting in the absence of macrophages, a capillary sprouting assay was performed in which EGM2 containing LPS (0.1, 1.0, and 10ng/mL) was replaced each day of the assay.
Lentiviral infection
To allow expression of fluorescent markers, the DNMAML-GFP construct, and shRNA knockdowns, HUVEC and HBVP were infected via a third-generation lentiviral gene delivery system, as described previously [15]. Briefly, 293T cells were co-transfected via CaPO4 with the core lentiviral plasmids along with either one or two lentiviral expression vectors containing genes of interest. The transfected cells produced virus-containing media, which was filtered and placed onto target cells. Target gene plasmids used were pCCL-GFP, pCCL-RFP, PCCL-DNMAML-GFP, and pLKO-based knockdown constructs for Jagged1 and Dll4, as well as a scrambled pLKO control. Further plasmid information is available upon request.
Western blot
In order to confirm Jagged1 and Dll4 knockdowns, total cell lysates from HUVECs infected with lentiviral knockdown constructs were isolated in Tris-triton lysis buffer (10 mM Tris, 100 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1% Triton X-100, 10% glycerol, 0.1% SDS, 0.5% deoxycholate, pH 7.4) containing Halt™ protease and phosphate inhibitor cocktail (Thermo Scientific). Western blots were performed by 10% SDS-PAGE, transferred to nitrocellulose, blocked with 3% BSA, probed with antibodies to Dll4 (Santa Cruz Biotechnology, SC-18639) or alpha-tubulin (Sigma T6074) as a loading control. HRP-conjugated secondary antibodies were used with ECL (GE Lifesciences) to detect bands on X-ray film using a BioRad developer.
Proliferation assay
To measure the growth rate of endothelial cells, cells were seeded in triplicates at 5×10̂3 cells per well in 24-well plates coated with type I collgane. Cell number were assessed after cells settle down (4hr after seeding), and at day 4 with the Cell Couting Kit-8 assay (CK04-11; Dojindo Molecular Technologies, Inc., Gaithersburg, MD)
cDNA library creation and quantitative RT-PCR
mRNA was isolated from HUVEC, BMM, and HBVP via the RNEasy kit (Qiagen), and cDNA libraries were generated via the Verso cDNA synthesis kit (Fischer Scientific), all according to manufacturer specifications. Samples were then assayed for mRNA expression macrophage polarity markers iNOS and Arginase or for Jagged1 using SYBR Green (Applied Biosystems) in an Applied Biosystems 7300 Real Time PCR System. Target gene primer sequences available upon request.
Flow cytometry
BMM were washed in PCN buffer. Samples were assessed on a BD FACSCaliber. No antibodies were used, as the purpose was to assess the inherent fluorescence of the DNMAML-GFP construct.
Capillary sprouting assay
The capillary sprouting assay has been described previously [17], though the present study introduces substantive variations of the protocol. HUVEC were incubated with Cytodex 3 beads (Sigma-Aldritch) in EGM2 at a ratio of 400 cells per bead for 4 hours with periodic agitation. This ratio is a calculated excess - not all HUVEC will bind to a bead, but this ratio allows complete monolayer coverage of all beads within 4 hours. The beads were plated overnight on TC-treated plates in EGM2 to allow loose HUVEC to settle to the bottom. The following day, the beads were removed from the plate, washed in EGM2, and resuspended in a 1×PBS solution containing 3mg/mL fibrinogen (Sigma Aldritch) and 0.15TIU/mL aprotinin (Sigma Aldritch). This solution was mixed with 0.625U/mL thrombin (Sigma Aldritch) in a 24 well plate as a concentration of 150 beads in 500ul fibrinogen solution per well, and left for 25 minutes to polymerize. Wells were then covered with 1mL of EGM2 containing 1e5 D551 cells per mL, which settled to form a monolayer on top of the fibrin clot.
To include myeloid cells in the assay, the myeloid cells were added to the fibrinogen solution before the inclusion of the HUVEC-coated beads. The myeloid cells were added at a concentration of 1e4 cells per well.
To include pericytes in the assay, HUVEC were incubated as normal with Cytodex beads, and then washed thoroughly with EGM2. HBVP were then added to the mixture at a ratio of 400 HBVP per bead and incubated for a further 4 hours with periodic agitation. As before, this ratio is a calculated excess, and only a small proportion of HBVP bind to the HUVEC-coated beads. The beads were then plated overnight, and the rest of the assay completed as normal. Schematics of the expanded capillary sprouting assays are provided (Online Resource 1). The capillary sprouting assay matured between experimental days 4 and 6, with day 4 typically showing early vessel formation and day 6 exhibiting a matured plexus. There was some variation in growth tempo between separate experiments, which is internally controlled within individual experiments. Data presented highlighted the point of maximal difference between experimental groups, and the experimental day is noted in all figures.
Quantification
All capillary sprouting assays were performed in triplicate. Sprout number and length were tabulated from 5 low power (5×) images from each well, for a total of between 60 and 100 beads per group. To quantify sprout length, the number of sprouts greater than 100μm in length (approximately half the diameter of a bead) was normalized to the total number of sprouts. Sprout width was calculated from 5 mid-power (10×) images from each group. Only connecting, non-terminal sprouts were counted, and the value was expressed as the number of sprouts >40μm in width, normalized to the total number of sprouts counted. Each experiment was performed at least 3 times (except where noted), and the figures displayed here represent a single typical iteration of those experiments.
Results
The presence of myeloid cells accelerated sprouting in an in vitro model of angiogenesis
The complex process of sprouting angiogenesis is well modeled in vitro by a bead-capillary sprouting assay, in which human umbilical vein endothelial cells (HUVECs) are bound to collagen/dextran-coated beads. When embedded in a fibrin clot, these HUVECs sprout to form capillary-like structures in response to cues from a fibroblast feeder cell layer [23,17,24]. We have improved the visibility of the endothelial cells by lentivirally infecting HUVEC to express a red fluorescent protein (RFP) (Fig. 1a), which does not detectably alter the growth dynamics of the assay (data not shown).
Figure 1.

Macrophage presence increases vascular sprouting. a Visualization of endothelial sprouts (red), with and without the inclusion of bone marrow macrophages (labeled in green). b BMM inclusion increases number and length of endothelial sprouts at early timepoint day 3. c Sprout number and length remains increased through late timepoint day 5. d Quantification of sprout number. e Quantification of frequency of longer (>200um) sprouts. Scale bars represent 200um. Error bars represent standard error. * = p<0.05.
To assess the contribution of myeloid cells to angiogenesis, we incorporated either EOC2 immortalized microglia or primary bone marrow-derived macrophages (BMM) into the capillary sprouting model of angiogenesis. Myeloid cells were mixed into the fibrinogen, such that they were evenly spaced throughout the resulting polymerized matrix (Online Resource 1, Fig. 1a). Our visual assessments suggested that macrophages remained stationary within the gel, and were not observed to migrate relative to the endothelial beads.
BMM were able to influence the endothelial cells to augment the angiogenic growth occurring from beads, with increased number of endothelial sprouts, observable by day 3 of the assay (Fig. 1b) and persisting through day 5 (Fig. 1c, d). BMM inclusion caused an approximate 50% increase in overall number of sprouts at day 5. After 5 days of assay, the sprouts were longer in BMM-containing wells, with a significantly greater proportion of sprouts longer than 200μm when measured from the bead (Fig. 1c, e). There were no observed changes in branching, network formation, or lumen formation between experimental groups. EOC2 inclusion also increased sprout growth and length, though to a lesser degree than BMM (Online Resource 2a, b, c, d)
Inflammatory polarization of macrophages further increased their angiotrophic potential
Macrophages exhibit a wide range of context-specific phenotypes that allow them to participate in many physiological and pathological processes [5-7]. In vitro, macrophages can be activated “classically” or “alternatively” by treatment with specific factors and cytokines, and it is thought that this reflects one dimension of their differentiation capacity in vivo. We promoted macrophage classical polarization by pre-treatment of BMM with lipopolysaccharide (LPS) and interferon gamma (IFNγ). To achieve alternate activation, BMM were treated with IL-4 or IL-10. Classical and alternative activation was confirmed by qPCR for polarity markers iNOS and Arginase, respectively (Online Resource 3a, b).
LPS treatment of the capillary sprouting assay using endothelial cells alone did not affect angiogenesis (Online Resource 4a, b). LPS/IFNγ-treated macrophages promoted angiogenesis to a greater degree than either the non-stimulated or IL4-treated groups. By day 2 of the assay, the wells containing LPS/IFNγ-treated BMM produced nearly double the number of initial sprouts compared to non-stimulated BMM (Fig. 2a, b). This increased sprouting was less profound, but still significant, by day 4, with LPS/IFNγ-treated BMM inducing approximately 25% more sprouts than non-stimulated BMM (Fig. 2a, b). However, LPS/IFNγ stimulation did not affect sprout length; when normalized to the total number of sprouts, LPS/IFNγ-treated BMM induced the same proportion of longer sprouts as non-stimulated BMM (Fig. 2a, c).
Figure 2.

Pro-inflammatory macrophages show greater angiotrophism. a LPS/IFNγ-treated BMM cause increased sprouting in excess of that caused by unstimulated BMM, while IL4-treated BMM do not. b Quantification of sprout number. c Quantification of frequency of longer sprouts. The difference in longer sprout frequency between unstimulated and LPS/IFNγ-treated BMM is not significant. Scale bars represent 200μm. Error bars represent standard error. * = p<0.05.
IL4 treatment did not alter the number of sprouts compared to non-stimulated macrophages (Fig. 2a, b, c). However, it did appear that the sprouts induced by IL4-treated macrophages were less developed, with a smaller proportion of long sprouts (Fig. 2a, c).
Notch inhibition abrogated the angiotrophic advantage of inflammatory polarization
Notch signaling has been shown to function in macrophages, both as a regulator of macrophage polarization [25] and as a mediator of macrophage angiogenic functionality [12,11]. To examine the involvement of Notch signaling in macrophage angiotrophism using the co-culture model, we used transgenic macrophages that express a dominant negative mastermind-like (DNMAML) construct. This construct binds to the Notch/CSL complex and prevents activation of canonical Notch targets, functioning as a dominant negative repressor of Notch signaling [21]. We confirmed expression of the GFP-tagged transgene via FACS analysis (Online Resource 5).
Non-stimulated DNMAML-expressing BMM increased endothelial sprouting in a fashion comparable to wild-type BMM (Fig. 3a, c). However, BMM-DNMAML cells stimulated with LPS/IFNγ did not promote a further increase in sprouting in response to this treatment, in contrast to wild-type BMM (Fig. 3b, d). Transcript analysis showed that the relative transcript levels of iNOS, a marker of the pro-inflammatory state, was approximately halved in LPS/IFNγ-induced BMM-DNMAML compared to wildtype BMM (Fig. 3e). Thus, Notch inhibition tempered the ability of LPS/IFNγ-treated BMM to promote angiogenesis.
Figure 3.

Macrophage Notch signaling inhibition abrogates angiotrophic advantage of inflammatory polarization. a BMM expressing the Notch inhibitor DNMAML show similar angiotrophic properties to control BMM. b BMM-DNMAML do not show increased stimulation of angiogenesis when treated with LPS/IFNγ. c Quantification of sprout number in Fig. 3a. d Quantification of sprout number in Fig. 3b. e mRNA expression of inflammatory marker iNos is decreased in BMM expressing DNMAML. Scale bars represent 200μm. Error bars represent standard error. * = p<0.05. This capillary sprouting experiment was performed twice; results shown represent a typical iteration.
Pericyte and endothelial cell co-culture increased sprout stability and maturation in vitro
Endothelial cells rely on interactions with vascular smooth muscle cells (VSMCs) and pericytes to guide vessel growth and maturation. In small caliber capillaries, vascular pericytes are the physiologically relevant cell that participates in the angiogenic process. To study endothelial/pericyte interactions, we performed the capillary sprouting assay incorporating Human Brain Vascular Pericytes (HBVPs)[18]. In this variation of the assay, HUVEC-coated Cytodex beads were mixed with HBVPs and incubated together such that the HBVPs adhered to the outside of the endothelial monolayer already coating the beads. The beads were then embedded in a fibrin clot as normal.
HBVP co-culture with endothelial cells greatly altered the growth kinetics and dynamics of the capillarylike sprouting that occurred in the assay. In the presence of HBVP, sprouts grew faster and were clearly visible within 24 hours of the start of the assay (Fig. 4a), unlike assays using only endothelial cells. By day 5, the difference became even more apparent, as the HBVP-containing wells formed a much more complex vascular network, with more sprouts and an increased proportion of mature, large caliber vessels (Fig. 4b, c). The pericytes formed close associations with the endothelial sprouts, and can be seen in contact with the majority of sprouts (Fig. 4b). Some pericytes not associated with vessels were observed. As an alternative approach, pericytes were suspended in the fibrin gel, rather than bound directly to beads. In this approach, the association between endothelial cells and pericytes was much more limited than if they were both bound to the bead, and no change in the growth of the vessel networks were observed relative to endothelial cell-only assays (Online Resource 6).
Figure 4.
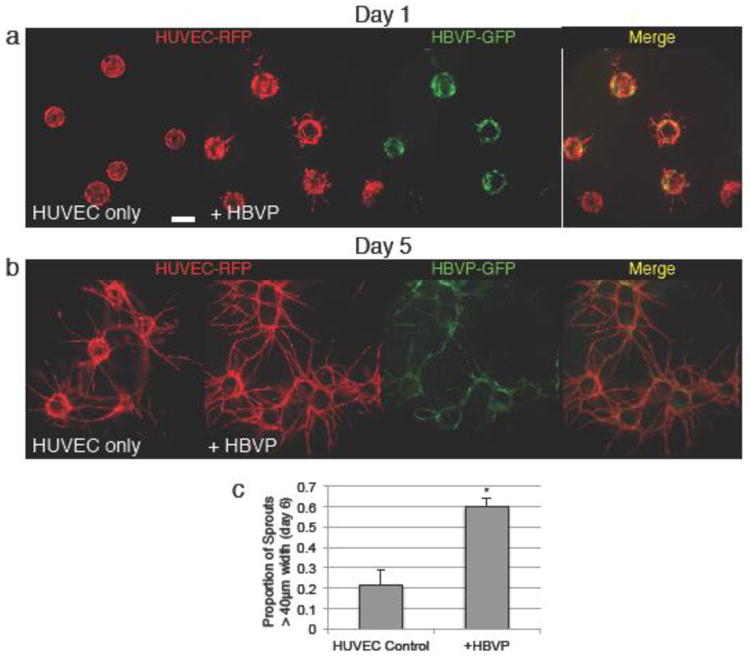
Pericyte coculture increases sprout number and vessel caliber. a Coculture with human brain vascular pericytes (HBVP), labeled green, causes increased initial sprouting at early timepoint day 1. b Coculture with HBVP leads to more complex vessel networks with wider vessel caliber by late timepoint day 5. c Quantification of sprout width. Scale bars represent 200μm. Error bars represent standard error. * = p<0.05.
Inhibition of endothelial Jagged1 function prevented endothelial sprouting
To assess the role of Notch signaling in the interaction between endothelial cells and pericytes, we established Jagged1 knockdown endothelial cells using an shRNA construct to reduce Jagged1 expression in HUVEC, and knockdown was confirmed by western blot (Online Resource 7). We found that Jagged1 knockdown almost completely abrogated sprouting in an endothelial monoculture bead-capillary sprouting assay (Fig. 5a). Co-culture of HUVEC-Jag1KD with pericytes was not able to significantly rescue this decreased sprouting, producing only scattered, scant vessels. (Fig. 5a, b, c). These few vessels were noted to be slightly wider, and showed limited association with the pericytes (Fig. 5a, d). Jagged1 knockdown also resulted in decreased HUVEC proliferation in monoculture (Online Resource 8). In contrast, we found that shRNA-mediated knockdown of Dll4 (Online Resource 9a) resulted in increased sprouting, both in endothelial-cell-only sprouting assay and in an endothelial-pericyte coculture assay (Online Resource 9b, c). Dll4 knockdown endothelial cells remained able to make extensive association with pericytes (Online Resource 9b).
Figure 5.
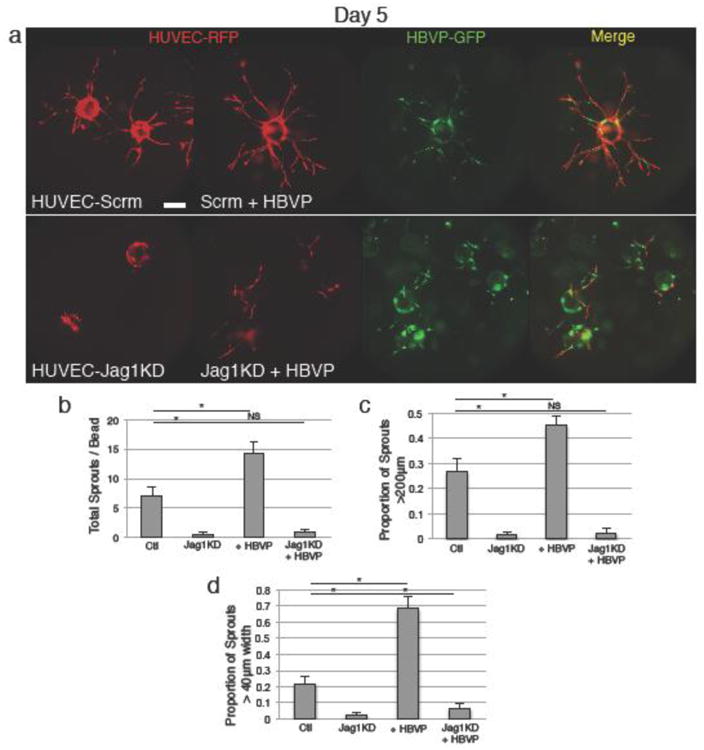
Endothelial Jagged1 knockdown abrogates endothelial sprouting. a Endothelial shRNA-mediated Jagged1 knockdown causes failure of sprouting by day 5. Co-culture with wildtype pericytes causes a minimal increase in presence of small, disorganized sprouts, with some endothelia-pericyte association. b Quantification of number of sprouts. c Quantification of frequency of longer (>200μm) sprouts. d Quantification of frequency of wide (>40μm) vessels. Scale bars represent 200μm. Error bars represent standard error. * = p<0.05.
Inhibition of endothelial Notch signaling decreased sprouting, and partially disrupted endothelial/pericyte interactions
To further examine endothelial Notch function in co-culture assays, we expressed the DNMAML Notch inhibitory construct in HUVEC, which decreased endothelial sprouting in monoculture (Fig. 6a, b, c). Expression of DNMAML appeared to disrupt growth of endothelial-pericyte containing sprouts, as co-culture of HUVEC-DNMAML with pericytes did not result in increased number or length of sprouts (Fig. 6a, b, c). However, the existing sprouts showed good association with pericytes and a roughly two-fold increase in the presence of wide, mature sprouts, compared to the monoculture assay, similar to the increase in vessel width seen in wild-type co-culture (Fig. 6a, d). This suggests that at least some elements of the interaction between endothelial cells and pericytes remain intact when using endothelial cells expressing DNMAML.
Figure 6.
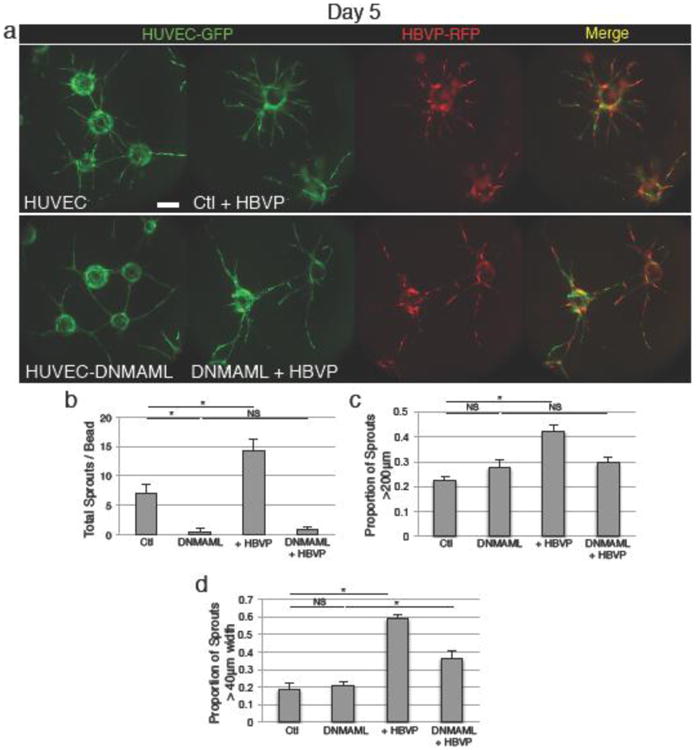
Endothelial Notch receptor signaling inhibition decreases sprouting and partially disrupts endothelial-pericyte interaction. a Endothelial expression of DNMAML causes reduced sprouting. Addition of wildtype pericytes results in only a slight increase in sprouting and sprout length, much less than that caused by pericyte addition to control endothelial cells. However, pericyte coculture does result in a >2-fold increase in wide, mature vessels, similar to control. b Quantification of number of sprouts. c Quantification of frequency of longer (>200μm) sprouts. d Quantification of frequency of wide (>40μm) vessels. Scale bars represent 200μm. Error bars represent standard error. * = p<0.05.
Pericyte Notch signaling was essential for cell survival and contribution to vessel maturation
We next employed the DNMAML construct to inhibit Notch signaling in pericytes and to determine whether pericyte Notch activity is necessary for growth of sprouts in the co-culture assay. DNMAML expressing pericytes grew very poorly, and when co-cultured with endothelial cells they initially bound to the endothelial-coated beads (data not shown), but were not detectable by day 5 (Fig. 7a). Unsurprisingly, co-culture with these pericytes had minimal effects on endothelial sprouting or vessel maturation (Fig. 7a, b, c, d). Interestingly, co-culture with DNMAML expressing pericytes actually decreased overall sprout length, resulting in only one-third the proportion of long sprouts compared to the endothelial monoculture assay (Fig. 7a, c).
Figure 7.

Pericyte Notch receptor signaling inhibition causes pericyte death and prevents their angiotrophic contribution. a Expression of DNMAML in pericytes causes degeneration of pericytes from the culture by endpoint day 5. Endothelial sprouting and maturation is unaffected by addition of Notch-inhibited pericytes, and show decreased sprout length. b Quantification of number of sprouts. c Quantification of frequency of longer (>200μm) sprouts. d Quantification of frequency of wide (>40μm) vessels. Scale bars represent 200μm. Error bars represent standard error. * = p<0.05.
Combination of macrophages and pericytes has multiplicative effects on angiogenesis
We tested whether the macrophage and pericyte co-cultures could be combined to create an even more accurate representation of the vascular microenvironment. We found that triple-cell cultures incorporating endothelial cells, macrophages, and pericytes grew well. These triple-cell assays exhibited a growth pattern suggesting additive angiotrophic contribution from both types of stromal cells (Fig. 8a). The vasculature in triple-cell cultures exhibited more sprouts than addition of either BMM or HBVP individually (Fig. 8a, b), and the vessels formed were of comparable caliber to the HBVP co-cultures (Fig. 8a, c). Additionally, the percentage change in sprouting with the addition of macrophages (approximately 20% in this iteration of the experiment) or pericytes (approximately 80% in this iteration) was preserved when the two were combined,. This suggests that the effects of macrophages and pericytes on the vasculature are multiplicative, and are neither synergistic nor completely redundant.
Figure 8.
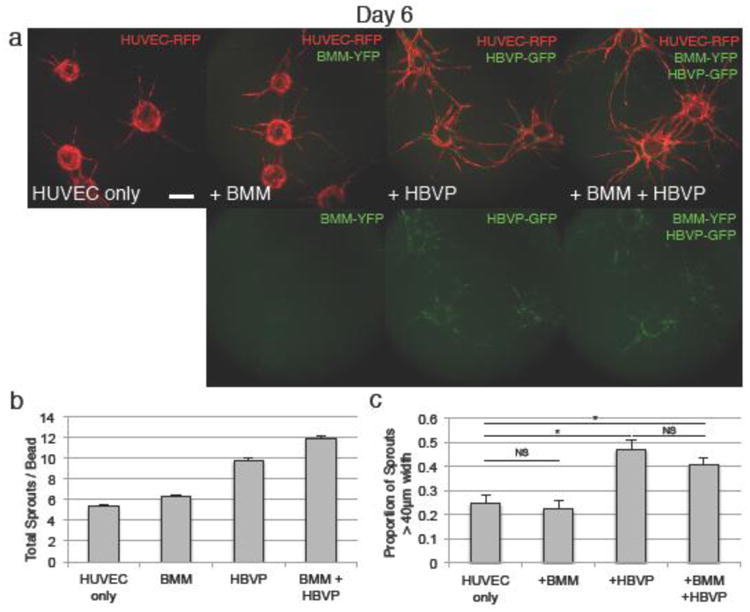
Endothelial, macrophage, and pericyte triple culture shows additive effects on angiogenesis. a Coculture of HUVEC with BMM and HBVP shows increased sprouting compared to BMM or HBVP alone, and maintains the increased vessel caliber seen in HBVP coculture. BMM-YFP and HBVP-GFP both appear in the green channel, and may be distinguished by morphology. b Quantification of sprout number at day 6, showing additive increase in sprouting in the triple culture. All experimental groups are statistically significantly different (p<0.05) from all other groups. c Quantification of vessel caliber. Scale bars represent 200μm. Error bars represent standard error. * = p<0.05.
Discussion
We demonstrated methodological advancements of a well-established capillary sprouting assay that uses beads coated with endothelial cells. This advancement allowed for examination of the important interactions between endothelial cells and the stromal cells of the vascular microenvironment. Using these methods, we documented the effect of macrophage and pericyte co-culture on endothelial sprouting, and describe a role for Notch signaling in both types of interaction.
Inclusion of myeloid cells alongside endothelial-coated beads greatly increased the rate and extent of endothelial sprouting. The exact percentage increase in sprouting varies between approximately 20-50% in different iterations of the experiment, but is always a statistically significant increase. This variability may be due in part to variability in the macrophages, which are derived from mouse bone marrow for every separate experiment and thus may vary slightly in their angiotrophic potential. For this reason, every experiment is rigorously internally controlled and replicated, and sprout growth is always compared within a single experimental iteration. The pro-angiogenic character of myeloid cells has been documented previously in aortic ring explant studies [2], but the assay we utilized represents an even more reductive distillation of this interaction, as it incorporates only endothelial and myeloid cells. In addition, we show that polarizing the added macrophages impacted their ability to foster angiogenic sprouting. Notably, inflammatory polarization using LPS and interferon γ increased the angiotrophic character of the macrophages, meaning that they increased the ability of these macrophages to contribute to angiogenesis via the secretion of angiogenic factors. In contrast, treatment with alternative activator IL4 did not enhance macrophage angiotrophism. Alternatively activated macrophages are held to play a substantial role in the development of tumor angiogenesis [6,7]. Indeed, recent in vivo gel implantation studies have suggested that alternatively activated macrophages show additional angiotrophic character due to increased metalloproteinase activity, while inflammatory macrophages provided less pro-angiogenic stimulus [26]. It may be that chemical and structural differences between the collagen substrate of these in vivo experiments and the fibrin of the capillary sprouting assay alter the relative importance of metalloproteinases for the stimulation of angiogenesis. Additionally, the reductive nature of the bead-capillary sprouting assay may mean that direct pro-angiogenic effects of inflammatory macrophages are emphasized that may not be evident in the context of complex multicellular tissues. Though some studies have suggested that inflammatory polarized macrophages do not to contribute to angiogenesis [6,7,27], there is a growing body of evidence to indicate that inflammatory mediators can play a key pro-angiogenic role in certain contexts. A past study of in vitro angiogenesis employing mouse and rat aortic rings has demonstrated that treatment with TLR ligands such as LPS can recruit mural cells, cause macrophage transformation, and enhance angiogenesis in an NFκB-dependent fashion [28]. Additionally, it has been shown that secretion of pro-angiogenic factors such as VEGF-A by inflammatory macrophages represents an important part of the wound healing process [29]. It may be that similar functionality is being observed in this assay.
We observed that inhibition of macrophage Notch signaling was sufficient to abrogate the angiotrophic advantage of inflammatory polarization, such that Notch-inhibited, inflammatory-polarized macrophages increased angiogenic sprouting at a rate comparable to non-stimulated cells. This is consistent with the role of Notch as a mediator of macrophage inflammatory polarization [25]. However, we observed that the up-regulation of the inflammatory marker iNOS, while stunted compared to wildtype, is still substantially up-regulated in inflammatory polarized, Notch-inhibited macrophages. This suggests that Notch signaling, in addition to its role as a gatekeeper of inflammatory polarity, may also be involved mechanistically in the observed increase in angiotrophism.
We examined the interaction between pericytes and endothelial cells in the bead-capillary sprouting assay. This relationship has previously been examined during vasculogenesis in collagen gels, where randomly seeded endothelial cells and pericytes interact to form vascular networks [18]. The role of vascular smooth muscle cells (VSMCs) in angiogenesis has been explored using the bead-capillary sprouting assay [13]. In these experiments VSMCs were seeded evenly throughout the fibrin gel, such that they made limited but consistent contact with the endothelial sprouts [13]. Pericytes are perhaps better suited for the capillary sprouting assay than are VSMCs, as they are known to interact with small caliber capillaries, which resemble the sprouts in the bead-capillary sprouting assay, while VSMCs interact with larger caliber vessels. Rather than seeding pericytes throughout the gel, we allowed pericytes to adhere directly to the bead-bound endothelial monolayer, which caused pericytes to migrate out from an existing monolayer, presumably along with endothelial cells, to form uniform close interactions with the maturing sprouts in a fashion that closely resembled observed in vivo behavior. Association with these pericytes drastically altered the morphology of the growing sprouts, leading to an increase in the number of sprouts, possibly due to decreased sprout regression compared to endothelial-only controls. Most significantly, pericyte-containing beads had a greater proportion of mature, wide vessels than the endothelial-only wells, and more lumens are visible. Thus, the pericytes in the co-cultures are fulfilling many of their described in vivo functions within the reductive, in vitro context of the capillary sprouting assay.
We examined the role of Notch ligand-receptor signaling in the interactions between endothelial cells and pericytes using co-culture assays. This proved to be challenging, as both Notch signaling and ligand expression were important for the growth of endothelial cells in monoculture bead-capillary sprouting assays. As a result, both Jagged1- and Notch-inhibited endothelial cells had significantly decreased sprouting. Indeed, the endothelial cells knocked down for Jagged1 failed to sprout at all, and while addition of pericytes may have caused a small increase in sprouting, it is difficult to draw any concrete conclusions about the role of endothelial Jagged1 in this process. The role of endothelial Dll4 and Notch signaling is well studied [10], and the results of our experiments employing both Dll4 knockdown and Notch receptor inhibition are in line with these established relationships. By contrast, the demonstrated inhibitory role for Jagged1 in angiogenesis is controversial, as others have proposed that Jagged1 may act chiefly as an antagonist of Dll4-Notch interactions [30], in which case one would not expect Jagged inhibition to have such a profound anti-angiogenic effect. However, our group has previously demonstrated that Jagged inhibition via a Notch1 soluble decoy causes decreased sprout formation in the bead-capillary sprouting assay [31], consistent with the results of the present study. This context-dependent differential role for Jagged1 in angiogenesis may be fundamentally dependent upon the glycosylation state of the Notch receptors, which may bias the receptor affinity toward Delta-like or Jagged ligands. In contrast, endothelial Notch inhibition via DNMAML caused only a modest decrease in sprouting, such that pericyte-endothelial interactions could still be assessed. In this setting, addition of pericytes did not have a strong effect on endothelial sprout formation or sprout length, suggesting that some elements of the endothelial-pericyte interaction may rely on intact Notch signaling function in endothelial cells. Interestingly, these co-cultures did show an increase in vessel width and maturity comparable to wild-type co-cultures. This suggests that the two-fold contribution of pericytes to both initial sprouting and vessel maturation may occur via separate signaling modalities, and that endothelial Notch receptor signaling is only important in sprout initiation and lengthwise growth, and not in subsequent endothelial maturation.
We attempted to assay the effect of pericyte Notch signal inhibition on their contribution to endothelial sprouting and growth. Notch-inhibited pericytes grew very poorly, and when added to the co-cultures they failed to survive for the course of the experiment. Predictably, these degenerated pericytes failed to increase sprouting or vessel maturation in endothelial cells, and actually stunted the growth of the sprouts. This is likely due to secondary effects from local resource consumption and cell death rather than to a specific modulation of endothelial-pericyte interactions. Future studies employing Notch receptor-specific knockdown may help to separate out this primary effect on pericytes and allow specific examination of the role of pericyte Notch in the interplay between pericytes and the endothelium.
Lastly, we combined the two co-culture techniques to create an assay that contained endothelial cells alongside both macrophages and pericytes. We found that wells containing BMM and HBVP showed evidence of the angiotrophic effect of both cell types, with increased sprouting and wide vessel caliber. The increase in sprout number was approximately 20% over HBVP alone, which (in this iteration of the assay, at this time-point) corresponded to the difference in sprouting between HUVEC alone and BMM co-culture. This increased sprouting suggested that the angiotrophic effects of combining BMM and HBVP are additive rather than synergistic. The additive nature of the sprout increase may suggest non-redundant roles for BMM and HBVP in the stimulation of angiogenic sprouting. It is possible that macrophages provide pro-angiogenic factors necessary for sprouting to occur, while a key function of pericytes is to stabilize nascent sprouts, decreasing their rate of regression and leading to increased overall sprout number. With the validation of triple co-culture, this assay is well placed to tease apart the disparate contributions of these different cell types.
Taken together, our studies extend the functionality of the bead-capillary sprouting assay to not only faithfully recreate endothelial sprouting and maturation, but also to model endothelial interactions with macrophages and pericytes within the context of angiogenesis. This allows for the exploration of not just the role of Notch signaling, as demonstrated here, but myriad other signaling modalities in a simplified setting that nonetheless captures many of the important elements of sprouting angiogenesis that have been historically difficult to recreate in vitro. These advancements therefore represent an important step towards a greater understanding of intercellular dynamics within the vascular microenvironment, and potentially our ability to holistically understand and therapeutically manipulate the process of angiogenesis.
Supplementary Material
Online Resource 1. Schematic of the incorporation of macrophages and pericytes in the capillary sprouting assay. a Schematic of an endothelial cell-only capillary sprouting assay, involving coculture of HUVEC with an overlying D551 fibroblast feeder layer. b Schematics of capillary sprouting assays incorporating bone marrow macrophages (directly into the fibrin matrix) and pericytes (bound to the bead on top of the endothelial monolayer).
Online Resource 2. a EOC2 inclusion increases number and length of endothelial sprouts at early timepoint day 3. b Sprout number and length remains increased through late timepoint day 5. c Quantification of sprout number. d Quantification of frequency of longer (>200um) sprouts. Scale bars represent 200um. Error bars represent standard error. * = p<0.05.
Online Resource 3. Macrophage polarization was confirmed via qPCR. Myeloid cells treated with proinflammatory factors LPS and IFNγ show increased mRNA expression of pro-inflammatory marker iNOS/NOS2, while cells treated with alternative activator cytokine IL4 show increased mRNA expression of alternative activation marker Arginase. Error bars represent standard error. * = p<0.05.
Online Resource 4. LPS treatment does not alter angiogenesis in endothelial cell-only capillary sprouting assays. a Treatment with 0.1, 1.0, or 10 ng/mL LPS did not significantly alter sprout number or tip cell number. b Quantification of sprout number. No relationships are statistically significant for p<0.05. This experiment was performed once.
Online Resource 5. Macrophage expression of DNMAM-GFP was confirmed via FACS. Bone marrow macrophages derived from LysMcre/+; DNMAML-GFPfl/+ mice show widespread expression of GFP, indicating synthesis of the DNMAML-GFP gene product. LysMcre/+ control mice do not express GFP.
Online Resource 6. Pericyte dispersal in fibrin gel does not recapitulate effects of direct bead binding. HBVP resuspension in fibrin gel, rather than direct binding to the endothelial-coated beads, does not produce the profound phenotypic changes observed in the case of direct pericyte association with endothelial cells. Scale bar represents 200μm.
Online Resource 7 Pericyte/endothelial knockdown of Jagged1 qPCR was validated via western blot. Protein expression of Jagged1 in cells co-infected with RFP and the Jagged1KD shRNA construct are approximately half that of RFP/Scramble controls.
Online Resource 8 Knockdown of Jagged1 in pericytes reduces cell growth in monoculture. Growth assessed 4 days after plating. Error bar represents standard deviation. *=p<0.05
Online Resource 9. Dll4 inhibition increases sprouting in endothelial cell-only capillary sprouting assays and in endothelial-pericyte cocultures. a Protein expression of Dll4 in cells co-infected with RFP and the Dll4KD shRNA construct are greatly reduced compared to RFP/Scramble control. b Dll4KD HUVEC show increased sprouting in endothelial cell-only capillary sprouting assay. c Dll4KD HUVEC show increased sprouting in endothelial and pericyte coculture capillary sprouting assay. d Quantification of sprout number in both experiments. Error bars represent standard error. * = p<0.05. Experiments were performed once.
Acknowledgments
The authors would like to thank Carrie Shawber for her consultation and advice on experimental methodology and planning. In addition, they would like to thank the Columbia University Medical Scientist Training Program (and its MSTP training grant T32GM007367) for its support.
Funding: This study was funded by NIH grants: 1R01HL112626 (J.K.) and 1R01HL119043 (J.K.).
Footnotes
Conflict of interest: The authors declare that they have no conflicts of interest
References
- 1.Fantin A, Vieira JM, Gestri G, Denti L, Schwarz Q, Prykhozhij S, Peri F, Wilson SW, Ruhrberg C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood. 2010;116(5):829–840. doi: 10.1182/blood-2009-12-257832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rymo SF, Gerhardt H, Wolfhagen Sand F, Lang R, Uv A, Betsholtz C. A Two-Way Communication between Microglial Cells and Angiogenic Sprouts Regulates Angiogenesis in Aortic Ring Cultures. PloS one. 2011;6(1):e15846. doi: 10.1371/journal.pone.0015846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stefater IIIJA, Lewkowich I, Rao S, Mariggi G, Carpenter AC, Burr AR, Fan J, Ajima R, Molkentin JD, Williams BO, Wills-Karp M, Pollard JW, Yamaguchi T, Ferrara N, Gerhardt H, Lang RA. Regulation of angiogenesis by a non-canonical Wnt–Flt1 pathway in myeloid cells. Nature. 2011;474(7352):511–515. doi: 10.1038/nature10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Armulik A, Genové G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Developmental Cell. 2011;21(2):193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nature Reviews Immunology. 2011;11(11):723–737. doi: 10.1038/nri3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmid MC, Varner JA. Myeloid cells in tumor inflammation. Vasc Cell. 2012;4(1):14. doi: 10.1186/2045-824X-4-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wynn TA, Chawla A, Pollard JW. Macrophage biology in development, homeostasis and disease. Nature. 2013;496(7446):445–455. doi: 10.1038/nature12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circulation Research. 2005;97(6):512–523. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 9.Radtke F, Schweisguth F, Pear W. The Notch ‘gospel’. Paper presented at the EMBO reports. 2005 Dec; doi: 10.1038/sj.embor.7400585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tung JJ, Tattersall IW, Kitajewski J. Tips, stalks, tubes: notch-mediated cell fate determination and mechanisms of tubulogenesis during angiogenesis. Cold Spring Harbor perspectives in medicine. 2012;2(2):a006601. doi: 10.1101/cshperspect.a006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Outtz HH, Wu JK, Wang X, Kitajewski J. Notch1 deficiency results in decreased inflammation during wound healing and regulates vascular endothelial growth factor receptor-1 and inflammatory cytokine expression in macrophages. The Journal of Immunology. 2010;185(7):4363–4373. doi: 10.4049/jimmunol.1000720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Outtz HH, Tattersall IW, Kofler NM, Steinbach N, Kitajewski J. Notch1 controls macrophage recruitment and Notch signaling is activated at sites of endothelial cell anastomosis during retinal angiogenesis in mice. Blood. 2011;118(12):3436–3439. doi: 10.1182/blood-2010-12-327015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Scheppke LL, Murphy EAE, Zarpellon AA, Hofmann JJJ, Merkulova AA, Shields DJD, Weis SMS, Byzova TVT, Ruggeri ZMZ, Iruela-Arispe MLM, Cheresh DAD. Notch promotes vascular maturation by inducing integrin-mediated smooth muscle cell adhesion to the endothelial basement membrane. Blood. 2012;119(9):2149–2158. doi: 10.1182/blood-2011-04-348706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Pan L, Moens CB, Appel B. Notch3 establishes brain vascular integrity by regulating pericyte number. Development (Cambridge, England) 2014;141(2):307–317. doi: 10.1242/dev.096107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tung JJ, Hobert O, Berryman M, Kitajewski J. Chloride intracellular channel 4 is involved in endothelial proliferation and morphogenesis in vitro. Angiogenesis. 2009;12(3):209–220. doi: 10.1007/s10456-009-9139-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koh W, Stratman AN, Sacharidou A, Davis GE. In vitro three dimensional collagen matrix models of endothelial lumen formation during vasculogenesis and angiogenesis. Methods Enzymol. 2008;443:83–101. doi: 10.1016/S0076-6879(08)02005-3. [DOI] [PubMed] [Google Scholar]
- 17.Nakatsu MN, Hughes CCW. An optimized three-dimensional in vitro model for the analysis of angiogenesis. Methods in enzymology. 2008;443:65–82. doi: 10.1016/S0076-6879(08)02004-1. [DOI] [PubMed] [Google Scholar]
- 18.Stratman AN, Malotte KM, Mahan RD, Davis MJ, Davis GE. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood. 2009;114(24):5091–5101. doi: 10.1182/blood-2009-05-222364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. The Journal of clinical investigation. 1973;52(11):2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clausen BE, Burkhardt C, Reith W, Renkawitz R, Förster I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic research. 1999;8(4):265–277. doi: 10.1023/a:1008942828960. [DOI] [PubMed] [Google Scholar]
- 21.Tu L, Fang TC, Artis D, Shestova O, Pross SE, Maillard I, Pear WS. Notch signaling is an important regulator of type 2 immunity. The Journal of experimental medicine. 2005;202(8):1037–1042. doi: 10.1084/jem.20050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, Costantini F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC developmental biology. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakatsu MN, Davis J, Hughes CCW. Optimized fibrin gel bead assay for the study of angiogenesis. Journal of visualized experiments : JoVE. 2007;(3):186. doi: 10.3791/186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nakatsu MN, Sainson RCA, Aoto JN, Taylor KL, Aitkenhead M, Pérez-del-Pulgar S, Carpenter PM, Hughes CCW. Angiogenic sprouting and capillary lumen formation modeled by human umbilical vein endothelial cells (HUVEC) in fibrin gels: the role of fibroblasts and Angiopoietin-1. Microvascular Research. 2003;66(2):102–112. doi: 10.1016/s0026-2862(03)00045-1. [DOI] [PubMed] [Google Scholar]
- 25.Wang YC, He F, Feng F, Liu XW, Dong GY, Qin HY, Hu XB, Zheng MH, Liang L, Feng L, Liang YM, Han H. Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Research. 2010;70(12):4840–4849. doi: 10.1158/0008-5472.CAN-10-0269. [DOI] [PubMed] [Google Scholar]
- 26.Zajac E, Schweighofer B, Kupriyanova TA, Juncker-Jensen A, Minder P, Quigley JP, Deryugina EI. Angiogenic capacity of M1- and M2-polarized macrophages is determined by the levels of TIMP-1 complexed with their secreted proMMP-9. Blood. 2013;122(25):4054–4067. doi: 10.1182/blood-2013-05-501494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jetten N, Verbruggen S, Gijbels MJ, Post MJ, De Winther MP, Donners MM. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis. 2014;17(1):109–118. doi: 10.1007/s10456-013-9381-6. [DOI] [PubMed] [Google Scholar]
- 28.Aplin AC, Ligresti G, Fogel E, Zorzi P, Smith K, Nicosia RF. Regulation of angiogenesis, mural cell recruitment and adventitial macrophage behavior by Toll-like receptors. Angiogenesis. 2014;17(1):147–161. doi: 10.1007/s10456-013-9384-3. [DOI] [PubMed] [Google Scholar]
- 29.Brancato SK, Albina JE. Wound macrophages as key regulators of repair: origin, phenotype, and function. The American journal of pathology. 2011;178(1):19–25. doi: 10.1016/j.ajpath.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Benedito R, Roca C, Sorensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137(6):1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 31.Kangsamaksin T, Murtomaki A, Kofler NM, Cuervo H, Chaudhri RA, Tattersall IW, Rosenstiel PE, Shawber CJ, Kitajewski J. Notch Decoys that Selectively Block Dll/Notch or Jagged/Notch Disrupt Angiogenesis by Unique Mechanisms to Inhibit Tumor Growth. Cancer discovery. 2014 doi: 10.1158/2159-8290.CD-14-0650. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Resource 1. Schematic of the incorporation of macrophages and pericytes in the capillary sprouting assay. a Schematic of an endothelial cell-only capillary sprouting assay, involving coculture of HUVEC with an overlying D551 fibroblast feeder layer. b Schematics of capillary sprouting assays incorporating bone marrow macrophages (directly into the fibrin matrix) and pericytes (bound to the bead on top of the endothelial monolayer).
Online Resource 2. a EOC2 inclusion increases number and length of endothelial sprouts at early timepoint day 3. b Sprout number and length remains increased through late timepoint day 5. c Quantification of sprout number. d Quantification of frequency of longer (>200um) sprouts. Scale bars represent 200um. Error bars represent standard error. * = p<0.05.
Online Resource 3. Macrophage polarization was confirmed via qPCR. Myeloid cells treated with proinflammatory factors LPS and IFNγ show increased mRNA expression of pro-inflammatory marker iNOS/NOS2, while cells treated with alternative activator cytokine IL4 show increased mRNA expression of alternative activation marker Arginase. Error bars represent standard error. * = p<0.05.
Online Resource 4. LPS treatment does not alter angiogenesis in endothelial cell-only capillary sprouting assays. a Treatment with 0.1, 1.0, or 10 ng/mL LPS did not significantly alter sprout number or tip cell number. b Quantification of sprout number. No relationships are statistically significant for p<0.05. This experiment was performed once.
Online Resource 5. Macrophage expression of DNMAM-GFP was confirmed via FACS. Bone marrow macrophages derived from LysMcre/+; DNMAML-GFPfl/+ mice show widespread expression of GFP, indicating synthesis of the DNMAML-GFP gene product. LysMcre/+ control mice do not express GFP.
Online Resource 6. Pericyte dispersal in fibrin gel does not recapitulate effects of direct bead binding. HBVP resuspension in fibrin gel, rather than direct binding to the endothelial-coated beads, does not produce the profound phenotypic changes observed in the case of direct pericyte association with endothelial cells. Scale bar represents 200μm.
Online Resource 7 Pericyte/endothelial knockdown of Jagged1 qPCR was validated via western blot. Protein expression of Jagged1 in cells co-infected with RFP and the Jagged1KD shRNA construct are approximately half that of RFP/Scramble controls.
Online Resource 8 Knockdown of Jagged1 in pericytes reduces cell growth in monoculture. Growth assessed 4 days after plating. Error bar represents standard deviation. *=p<0.05
Online Resource 9. Dll4 inhibition increases sprouting in endothelial cell-only capillary sprouting assays and in endothelial-pericyte cocultures. a Protein expression of Dll4 in cells co-infected with RFP and the Dll4KD shRNA construct are greatly reduced compared to RFP/Scramble control. b Dll4KD HUVEC show increased sprouting in endothelial cell-only capillary sprouting assay. c Dll4KD HUVEC show increased sprouting in endothelial and pericyte coculture capillary sprouting assay. d Quantification of sprout number in both experiments. Error bars represent standard error. * = p<0.05. Experiments were performed once.


