Abstract
Although epidemiological studies reveal that cigarette smoking is inversely associated with Alzheimer’s disease (AD) and Parkinson’s disease (PD), the underlying mechanism remains largely unknown. Considering the facts that amyloid precursor protein-binding protein, family B, member 1 (APBB1) is mapped to a suggestive linkage region on chromosome 11 for nicotine dependence (ND), and has been implicated in the pathogenesis of AD and PD, it represents a plausible candidate for genetic study of ND. Five single nucleotide polymorphisms (SNPs) within APBB1 were genotyped in a sample consisting of 2,037 participants of either African-American (AA) or European-American (EA) origin, and examined their associations with ND assessed by three commonly used measures: Smoking Quantity (SQ), the Heaviness of Smoking Index (HSI), and the Fagerström Test for ND (FTND). Individual SNP-based association analysis showed that all five SNPs are associated with at least one ND measure in one of the three samples; however, only the association of SNP rs4758416 with SQ and HSI remained significant after correction for multiple testing in the pooled sample. Haplotype analysis demonstrated three major haplotypes significantly associated with ND after Bonferroni correction. Formed by rs4758416-rs10839562-rs1079199, haplotype C-C-T showed positive association with FTND in the AA and pooled samples, and conversely, haplotype G-C-T showed negative association with SQ and HSI in AA and EA samples. Another haplotype, C-T-G, formed by rs10839562-rs1079199-rs8164, was significantly associated with HSI in the EA sample. Based on these findings, we conclude that APBB1 represents an important candidate gene in the genetic study on ND and neurodegenerative diseases and warrants further investigation in future.
Introduction
Nicotine is commonly believed to be the primary addictive substance in tobacco smoking. Nicotine exerts its pharmacological effects on the central nervous system by binding to nicotinic acetylcholine receptors (Leonard and Bertrand 2001), and increases dopamine levels in the nucleus accumbens (Pontieri et al. 1996). Despite continued campaigns against cigarette smoking, it remains a leading preventable cause of death in the USA and globally. It has been estimated that one in every five deaths is attributable to cigarette smoking (Peto et al. 1992), resulting in 438,000 deaths and $157.7 billion in healthcare and related expenditures annually in the US (Leistikow 2000; Mokdad et al. 2004).
Considerable biochemical and pharmacological research indicates multiple genes and biochemical pathways are modulated by nicotine, leading to changes in downstream actions, neural circuit plasticity and the development of addiction. Further, twin and family studies reveal that nicotine dependence (ND) is highly heritable, with an average heritability of 0.56 for male and female smokers (Li et al. 2003a; Sullivan and Kendler 1999). A common approach for identifying vulnerability genes for ND is to conduct a linkage study followed by candidate gene-based association analysis. Susceptibility loci for ND have been reported in more than 20 linkage studies (for a recent review, see Li 2008). One of these chromosomal regions, 11p15, is where amyloid precursor protein-binding protein, family B, member 1 (APBB1) is located, which has been linked to ND in three independent samples: the Framingham Heart Study (FHS), Mid-South Tobacco Family (MSTF) samples, and Family Study for Panic Disorder (Gelernter et al. 2004; Li et al. 2003b; Wang et al. 2005).
APBB1 spans approximately 24 kb, consisting of 15 exons. The protein encoded by APBB1 is an adaptor protein localized in the nucleus, which belongs to Fe65 protein family. As a regulatory factor, APBB1 interacts with actions of amyloid precursor protein (APP). Through APBB1, the signaling pathway consisting of transient axonal glycoprotein-1 and APP (TAG1-APP) negatively modulates neurogenesis (Ma et al. 2008). Moreover, through enhanced production of the carboxyl-terminal fragment substrates of γ-secretase as well as direct stimulation of processing by γ-secretase, APBB1 stimulates γ-secrete-mediated libration of the APP intercellular domain. On the other hand, multiple splicing transcript variants of APBB1 lead to different stimulating capabilities of the proteins encoded (Wiley et al. 2007).
Previously, we reported that nicotine modulates expression of mRNA and protein levels of the APP and APLP2 genes in different mouse brain regions and in SH-SY5Y neuroblastoma cells (Gutala et al. 2006). Furthermore, there are the following evidences supporting the role of APBB1 in ND. First, APBB1 has been shown to influence learning and memory in Fe65 knockout mice (Wang et al. 2004), which is associated with the pharmacological effect of nicotine on the cognitive behavior. Second, it is known that APBB1 is involved in the modulation of β-amyloid secretion (Pietrzik et al. 2002, 2004; Sabo et al. 1999), suggesting that it regulates proteolytic events associated with Alzheimer’s disease (AD) pathogenesis. Third, epidemiological studies suggest that tobacco smoking is inversely correlated with neurodegenerative diseases such as AD and Parkinson’s disease (PD) (Birrenbach and Bocker 2004; Fratiglioni and Wang 2000; Sacco et al. 2004). These facts strongly indicate that APBB1 is a plausible candidate for a genetic study on ND.
An in-depth understanding of the role of APBB1 in tobacco smoking will not only provide insights into the etiology of ND, but also reveal its protective effect on cognitive function. In this study, we performed a family-based genetic association study of APBB1 with ND in a sample consisting of 2,037 participants of African-American (AA) and European-American (EA) origin. Both individual SNP-and haplotype-based association analyses indicated that APBB1 is significantly associated with ND in both ethnic populations.
Methods
All the participants involved in this study were recruited primarily from the mid-south states of Tennessee, Mississippi and Arkansas in the USA during 1999–2004, and are of either AA or EA origin. Proband smokers were required to smoke an average of 20 or more cigarettes per day for the last 12 months, to have smoked for at least the last 5 years, and to be at least 21 years of age. Once a smoker proband was identified, all his or her siblings and biological parents were recruited whenever possible, regardless of their smoking status. Participants included 1,366 individuals from 402 AA families and 671 individuals from 200 EA families, yielding a total of 2,037 individuals. The families varied in size from two to nine with an average size of 3.14 (±0.75; SD) for AAs and 3.17 (±0.69; SD) for EAs. Average age was 39.4 (±14.4; SD) years for the AA and 40.5 (±15.5; SD) years for EA participants. For more detailed demographic and clinical characteristics of this study, please refer to our previous reports (Li et al. 2005, 2006). All participants provided informed consent. The study protocol, forms and procedures were approved by all participating Institutional Review Boards.
In this study, the levels of ND for smokers were ascertained by three commonly-used measures in the literature: smoking quantity (SQ: defined as the number of cigarettes smoked per day), the Heaviness of Smoking Index (HSI: 0–6 scale), and the Fagerström Test for ND (FTND: 0–10 scale). Given the overlap in the content of these three ND measures, there exists fairly robust correlations among them in the AA (r = 0.88–0.97), EA (r = 0.91–0.97) and pooled (r = 0.89–0.97) samples.
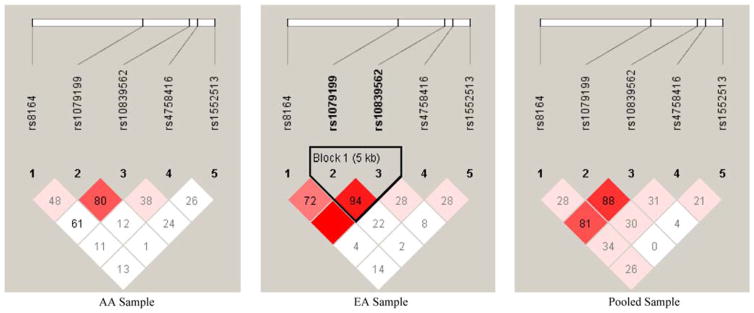
To uniformly cover the whole gene including 5′- and 3′-untranslated regions, five tagger SNPs were selected spanning the APBB1 gene, with an average inter-SNP distance of 5 kb per SNP, and were genotyped using the Illumina BeadChip system at the Center for Inherited Disease Research (CIDR) at Johns Hopkins University. The PedCheck program (O’Connell and Weeks 1998) was used to identify any possible errors, such as inconsistent Mendelian inheritance or other genotyping errors. Among over than 10,000 assays, 120 inconsistencies (83 in the AA sample, 1.24%; and 37 in the EA sample, 1.11%) were detected and excluded from the following association analyses. To further verify the quality of SNP-typing, we checked any significant departures from Hardy-Weinberg Equilibrium (HWE). Except for SNP rs1552513, which departed slightly from HWE in the AA sample, all other SNPs were in HWE (Table 1). Pair-wise Linkage Disequilibrium (LD) (Fig. 1) among all SNPs was determined using the Haploview program (Barrett et al. 2005), based on haplotype blocks as defined by Gabriel et al. (2002). One block formed by rs10839562-rs1079199 in the EA sample was detected; no significant LD blocks were detected for the AA or pooled samples.
Table 1.
Information for five SNPs within APBB1 and their minor allele frequencies (MAFs) from the NCBI dbSNP database and from this study
| dbSNfP ID | Alleles | Position (Chr.11) | SNP location | MAF
|
MAF
|
HWE
|
|||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| YRI | CEU | AA | EA | AA | EA | ||||
| rs1552513 | C/T | 6392604 | Intron 1 | 0.408 (T) | 0.408 (T) | 0.374 (T) | 0.397 (T) | 0.046 | 1.000 |
| rs4758416 | C/G | 6390725 | Intron 1 | 0.150 (G) | 0.375 (C) | 0.210 (G) | 0.428 (C) | 0.528 | 0.263 |
| rs10839562 | C/G | 6389841 | Intron 1 | 0.092 (G) | 0.300 (G) | 0.097 (G) | 0.275 (G) | 0.199 | 0.886 |
| rs1079199 | C/T | 6384682 | Intron 2 | 0.458 (T) | 0.333 (C) | 0.483 (C) | 0.289 (C) | 0.180 | 0.946 |
| rs8164 | A/G | 6372458 | 3′ UTR | 0.308 (A) | 0.133 (A) | 0.298 (A) | 0.162 (A) | 0.650 | 0.550 |
YRI is the sub-Saharan African sample; CEU is the European sample.
Both are from the international HapMap project Both MAF and HWE on the AA and EA samples are from this study
UTR untranslated region
Fig. 1.
LD structures for five SNPs within APBB1 in the three samples. The number in each box represents the D′ value for each SNP pair
The PBAT program (Lange et al. 2003) was employed to determine the association of individual SNPs with each ND measure. Haplotype association was analyzed by the FBAT program (Horvath et al. 2004). P values were evaluated under the null distribution of no linkage and no association. Additive, dominant, and recessive models were tested by incorporating age and gender as covariates for the AA and EA samples; for the pooled sample, age, gender and ethnicity covariates were employed. An association of an individual SNP with ND was considered significant if its P value was less than the corrected P value, based on the method of SNP spectral decomposition (SNPSpD) approach for multiple testing (Nyholt 2004). For haplotype analysis, Bonferroni correction was used to calculate the corrected P-value.
Results
Association analysis of individual SNPs
Heterogeneity is a major concern for any genetic association studies employing a sample comprised of mixed ethnicities. Thus, our data were analyzed separately for each ethnic-specific sample, while the results from the pooled sample were also computed to capitalize on improved power due to increased sample size (assuming a lack of heterogeneity across samples). As shown in Table 2, we found that all five SNPs examined in this study showed significant association with at least one ND measure in one of the three samples. However, only the association of SNP rs4758416 with SQ and HSI remained significant after correction for multiple testing in the pooled sample.
Table 2.
P -values, associative alleles and informative families (last two are in parentheses) for individual SNP-based associations with ND in the three samples
| dbSNP ID | AA | EA | Pooled | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| SQ | HSI | FTND | SQ | HSI | FTND | SQ | HSI | FTND | |
| rs1552513 | 0.210r | 0.218a | 0.190a | 0.330d | 0.191d | 0.038d | 0.524a | 0.361d | 0.353r |
| rs4758416 | 0.035a | 0.062a | 0.069a | 0.028a | 0.039a | 0.055a | 0.003a | 0.008a | 0.013a |
| rs10839562 | 0.593a | 0.651d | 0.794a | 0.092d | 0.035r | 0.076r | 0.149d | 0.219d | 0.388d |
| rs1079199 | 0.015d | 0.038r | 0.015d | 0.150d | 0.093r | 0.236r | 0.045r | 0.075d | 0.131d |
| rs8164 | 0.305a | 0.403a | 0.422a | 0.120d | 0.051d | 0.020d | 0.327d | 0.421d | 0.549d |
Adjusted P-value after correction for multiple testing at 0.05 significance level is 0.0104, 0.0123, and 0.0109 for the AA, EA, and pooled samples, respectively. Bold values indicate significant associations after correction for multiple testing
Only the lowest P-value given by any genetic model is shown for non-significant associations (bold values)
Superscript indicates the model used, a additive, d dominant, and r recessive models, respectively
Haplotype analysis
We employed the FBAT program to analyze the association between haplotypes and ND measures. We scanned all possible haplotypes consisting of three consecutive SNPs, and found three haplotypes that were significantly associated with ND after Bonferroni correction (Table 3). The first two haplotypes, formed by rs4758416-rs10839562-rs1079199, were C-C-T and G-C-T. The haplotype C-C-T showed positive associations with all three ND measures in the AA and pooled samples. However, after correction for multiple testing, only the association with FTND remained significant in the AA (Z = 2.50; P = 0.012; 91 informative families) and pooled (Z = 2.63; P = 0.008, 109 informative families) samples. On the other hand, the G-C-T haplotype showed significant inverse association with all three ND measures for the three samples. After correction for multiple testing, we found that this haplotype remained significant with at least one ND measure in each of the three samples. For example, this haplotype was significantly associated with SQ in the AA sample under both the additive (Z = −2.67; P = 0.008, 128 informative families) and dominant models (Z = −2.75; P = 0.006, 125 informative families), and with HSI under the recessive model in the EA sample (Z = −2.62; P = 0.009, 52 informative families) with a frequency of 47.5%. In the pooled sample, under the additive model, its Z score was −2.80 (P = 0.005, 235 informative families) for HSI and −2.95 (P = 0.003, 233 informative families) for SQ.
Table 3.
Z- and P-values and informative families (last two are in parentheses) for associations of major haplotypes with three ND measures in the three samples
| Haplotype | AA | EA | Pooled | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Freq (%) | SQ | HSI | FTND | Freq (%) | SQ | HSI | FTND | Freq (%) | SQ | HSI | FTND | |
| rs4758416-rs10839562-rs1079199 | ||||||||||||
| C-C-T | 39.1 | 1.98 (0.048, 91)r | 2.35 (0.019, 91)r | 2.50 (0.012, 91)r | 23 | 1.46 (0.143, 62)d | 1.32 (0.186,73)a | 1.29 (0.196,73)a | 34 | 2.02 (0.044,109)r | 2.53 (0.011, 108)r | 2.63 (0.008,109)r |
| G-C-T | 12.7 |
−2.67 (0.008, 128)a −2.75 (0.006, 125)d |
−2.23 (0.026, 126)d | −2.24 (0.025, 126)d | 47.5 | −2.47 (0.013, 52)r | −2.62 (0.009, 52)r | −2.18 (0.029, 52)r | 24.4 | −2.95 (0.003, 233)a | −2.80 (0.005, 235)a | −2.47 (0.014, 240)a |
| Global P-value | 0.080r | 0.052r | 0.039r | 0.045r | 0.027r | 0.078r | 0.017r | 0.006r | 0.014r | |||
| rs10839562-rs1079199-rs8164 | ||||||||||||
| C-T-G | 43.5 | −2.16 (0.031, 193)d | −1.69 (0.091, 193)d | −1.50 (0.133, 196)d | 57.7 | −2.25 (0.024, 70)r | −2.55 (0.011, 70)r | −2.20 (0.028, 70)r | 48.6 | −1.69 (0.091, 339)a | −1.21 (0.226, 338)a | −0.67 (0.503, 257)d |
| Global P-value | 0.227d | 0.175d | 0.100d | 0.069r | 0.039r | 0.038d | 0.377a | 0.446a | 0.448a | |||
For the first SNP combination, P values correction at 0.05 significance level are 0.0167, 0.0125, and 0.0100 for the AA, EA and pooled samples, respectively; and 0.0100, 0.0167, and 0.0100, respectively, for the second SNP combination
Superscript indicates the genetic model used, a additive, d dominant, and r recessive models, respectively
The last significant haplotype, C-T-G, consisting of rs10839562-rs1079199-rs8164, showed negative associations with all three ND measures for all three samples. However, only the association in the EA sample with a frequency of 57.7% and a Z score of −2.55 (P = 0.011, 70 informative families) remained significant with HSI after Bonferroni correction.
Discussion
After genotyping five SNPs in APBB1 and observing several preliminary positive findings, we found only SNP rs4758416 remained significantly associated with SQ and HSI in the pooled sample after correction for multiple testing, providing further evidence of nominal association of rs4758416 with ND observed in each ethnic-specific sample. In addition, we found three major haplotypes significantly associated with ND after Bonferroni correction. Two halpotypes, C-C-T and G-C-T, consisting of rs4758416-rs10839562-rs1079199, showed significant associations with at least one ND measure in one of the three samples. C-C-T appears to be a risk haplotype for ND, while G-C-T seems to function as a protective haplotype. A comparison of the two haplotypes with opposite genetic effects on ND behaviors reveals they are primarily determined by rs4758416, suggesting this region may harbor causative SNP(s) affecting ND. Another haplotype, C-T-G, formed by rs10839562-rs1079199-rs8164, showed a significant protective effect in ND as measured by HSI in the EA sample.
This study has several unique strengths. First, this is a family-based association study, consisting of one of the largest cohorts recruited for studying the genetics of ND. Second, our samples include two major ethnicities in the USA, African-Americans and European-Americans, permitting an examination of potential genetic differences underlying the expression of ND. Finally, as this is a family-based association study, it is considered to be robust to population stratification and thus reduces the likelihood of false association caused by sample admixture. However, although the family-based association study can minimize the potential impact of population stratification or admixture on association results, it could not completely eliminate such an effect. Thus, more caution is needed when one uses samples comprised of participants with different ethnicity for an association study.
APBB1 is an important regulatory factor during cellular response to nicotinic stimulation and in the APP nuclear signaling process (Gutala et al. 2006). The existence of alternative splicing within APBB1 suggests the presence of various protein isoforms of this gene (Wiley et al. 2007). This is supported by a study reporting that a polymorphism in intron 13 of APBB1 is associated with AD that alters the splicing of the transcript (Hu et al. 2002). This suggests that the APBB1 SNPs associated with ND are likely important in the regulation of alternative splicing of APBB1, and thereby altering the expression of the isoforms, although they are all located in the introns of the gene.
Two of the three haplotypes showed significant inverse associations with ND, suggesting protective functions against the development of ND. Given (a) APBB1 is important in the etiology of AD and PD, (b) tobacco smoking is inversely associated with AD and PD, and (c) APBB1 variants are associated with ND, this study provides further evidence regarding the potential connection between smoking and both AD and PD at the gene level. In addition, a protective effect of a deletion in intron 13 of APBB1 for late-onset AD has been reported (Cousin et al. 2003; Hu et al. 2002; Lambert et al. 2000) although other studies failed to replicate such an association (Guenette et al. 2000; Prince et al. 2001). Despite these discrepancies observed among different studies, it is still suggested that APBB1 remains a potential candidate for a genetic study with AD (Cousin et al. 2003).
Conclusion
We provide new evidence supporting our previous linkage results on chromosome 11p15 for ND by demonstrating that APBB1 is significantly associated with ND in both AA and EA samples. Two of the three haplotypes are inversely associated with ND, suggesting they are protective against the development of ND. This provides novel evidence for the reported inverse association of smoking with AD and PD from epidemiological studies. Thus, APBB1 plays an important role in the etiology of ND, implicating its importance as a candidate gene for future investigations of ND.
Acknowledgments
We acknowledge the invaluable contributions of personal information and blood samples by all participants in the study. We thank the Center for Inherited Disease Research (CIDR) for performing genotyping on our sample. This project was funded by National Institutes of Health Grant DA-12844 to MDL. We also thank the Rutgers University Cell and DNA Repository, the contractor for the NIDA Center for Genetic Studies, which is co-directed by Dr. Jay Tischfield and Dr. John Rice.
Contributor Information
Guo-Bo Chen, Institute of Bioinformatics, Zhejiang University, Hangzhou, Zhejiang, People’s Republic of China. Department of Psychiatry and Neurobehavioral Sciences, University of Virginia, Charlottesville, VA, USA.
Thomas J. Payne, ACT Center for Tobacco Treatment, Education and Research, Department of Otolaryngology, University of Mississippi Medical Center, Jackson, MS, USA
Xiang-Yang Lou, Department of Psychiatry and Neurobehavioral Sciences, University of Virginia, Charlottesville, VA, USA.
Jennie Z. Ma, Department of Public Health Sciences, University of Virginia, Charlottesville, VA, USA
Jun Zhu, Institute of Bioinformatics, Zhejiang University, Hangzhou, Zhejiang, People’s Republic of China.
Ming D. Li, Department of Psychiatry and Neurobehavioral Sciences, University of Virginia, Charlottesville, VA, USA. 1670 Discovery Drive, Suite 110, Charlottesville, VA 22911, USA
References
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Birrenbach T, Bocker U. Inflammatory bowel disease and smoking: a review of epidemiology, pathophysiology, and therapeutic implications. Inflamm Bowel Dis. 2004;10:848–859. doi: 10.1097/00054725-200411000-00019. [DOI] [PubMed] [Google Scholar]
- Cousin E, Hannequin D, Ricard S, Mace S, Genin E, Chansac C, Brice A, Dubois B, Frebourg T, Mercken L, Benavides J, Pradier L, Campion D, Deleuze JF. A risk for early-onset Alzheimer’s disease associated with the APBB1 gene (FE65) intron 13 polymorphism. Neurosci Lett. 2003;342:5–8. doi: 10.1016/s0304-3940(03)00225-8. [DOI] [PubMed] [Google Scholar]
- Fratiglioni L, Wang HX. Smoking and Parkinson’s and Alzheimer’s disease: review of the epidemiological studies. Behav Brain Res. 2000;113:117–120. doi: 10.1016/s0166-4328(00)00206-0. [DOI] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, Adeyemo A, Cooper R, Ward R, Lander ES, Daly MJ, Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Liu X, Hesselbrock V, Page GP, Goddard A, Zhang H. Results of a genomewide linkage scan: support for chromosomes 9 and 11 loci increasing risk for cigarette smoking. Am J Med Genet B Neuropsychiatr Genet. 2004;128:94–101. doi: 10.1002/ajmg.b.30019. [DOI] [PubMed] [Google Scholar]
- Guenette SY, Bertram L, Crystal A, Bakondi B, Hyman BT, Rebeck GW, Tanzi RE, Blacker D. Evidence against association of the FE65 gene (APBB1) intron 13 polymorphism in Alzheimer’s patients. Neurosci Lett. 2000;296:17–20. doi: 10.1016/s0304-3940(00)01607-4. [DOI] [PubMed] [Google Scholar]
- Gutala R, Wang J, Hwang YY, Haq R, Li MD. Nicotine modulates expression of amyloid precursor protein and amyloid precursor-like protein 2 in mouse brain and in SH-SY5Y neuroblastoma cells. Brain Res. 2006;1093:12–19. doi: 10.1016/j.brainres.2006.03.100. [DOI] [PubMed] [Google Scholar]
- Horvath S, Xu X, Lake SL, Silverman EK, Weiss ST, Laird NM. Family-based tests for associating haplotypes with general phenotype data: application to asthma genetics. Genet Epidemiol. 2004;26:61–69. doi: 10.1002/gepi.10295. [DOI] [PubMed] [Google Scholar]
- Hu Q, Cool BH, Wang B, Hearn MG, Martin GM. A candidate molecular mechanism for the association of an intronic polymorphism of FE65 with resistance to very late onset dementia of the Alzheimer type. Hum Mol Genet. 2002;11:465–475. doi: 10.1093/hmg/11.4.465. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Mann D, Goumidi L, Harris J, Pasquier F, Frigard B, Cottel D, Lendon C, Iwatsubo T, Amouyel P, Chartier-Harlin MC. A FE65 polymorphism associated with risk of developing sporadic late-onset alzheimer’s disease but not with Abeta loading in brains. Neurosci Lett. 2000;293:29–32. doi: 10.1016/s0304-3940(00)01477-4. [DOI] [PubMed] [Google Scholar]
- Lange C, Silverman EK, Xu X, Weiss ST, Laird NM. A multivariate family-based association test using generalized estimating equations: FBAT-GEE. Biostatistics. 2003;4:195–206. doi: 10.1093/biostatistics/4.2.195. [DOI] [PubMed] [Google Scholar]
- Leistikow BN. The human and financial costs of smoking. Clin Chest Med. 2000;21:189–197. doi: 10.1016/s0272-5231(05)70017-4. [DOI] [PubMed] [Google Scholar]
- Leonard S, Bertrand D. Neuronal nicotinic receptors: from structure to function. Nicotine Tob Res. 2001;3:203–223. doi: 10.1080/14622200110050213. [DOI] [PubMed] [Google Scholar]
- Li MD. Identifying susceptibility loci for nicotine dependence: 2008 update based on recent genome-wide linkage analyses. Hum Genet. 2008;123:119–131. doi: 10.1007/s00439-008-0473-0. [DOI] [PubMed] [Google Scholar]
- Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003a;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- Li MD, Ma JZ, Cheng R, Dupont RT, Williams NJ, Crews KM, Payne TJ, Elston RC. A genome-wide scan to identify loci for smoking rate in the Framingham Heart Study population. BMC Genet. 2003b;4(Suppl 1):S103. doi: 10.1186/1471-2156-4-S1-S103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MD, Beuten J, Ma JZ, Payne TJ, Lou XY, Garcia V, Duenes AS, Crews KM, Elston RC. Ethnic- and gender-specific association of the nicotinic acetylcholine receptor alpha4 subunit gene (CHRNA4) with nicotine dependence. Hum Mol Genet. 2005;14:1211–1219. doi: 10.1093/hmg/ddi132. [DOI] [PubMed] [Google Scholar]
- Li MD, Payne TJ, Ma JZ, Lou XY, Zhang D, Dupont RT, Crews KM, Somes G, Williams NJ, Elston RC. A genomewide search finds major susceptibility loci for nicotine dependence on chromosome 10 in African Americans. Am J Hum Genet. 2006;79:745–751. doi: 10.1086/508208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma QH, Futagawa T, Yang WL, Jiang XD, Zeng L, Takeda Y, Xu RX, Bagnard D, Schachner M, Furley AJ, Karagogeos D, Watanabe K, Dawe GS, Xiao ZC. A TAG1-APP signalling pathway through Fe65 negatively modulates neurogenesis. Nat Cell Biol. 2008;10:283–294. doi: 10.1038/ncb1690. [DOI] [PubMed] [Google Scholar]
- Mokdad AH, Marks JS, Stroup DF, Gerberding JL. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245. doi: 10.1001/jama.291.10.1238. [DOI] [PubMed] [Google Scholar]
- Nyholt DR. A simple correction for multiple testing for single-nucleotide polymorphisms in linkage disequilibrium with each other. Am J Hum Genet. 2004;74:765–769. doi: 10.1086/383251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connell JR, Weeks DE. PedCheck: a program for identification of genotype incompatibilities in linkage analysis. Am J Hum Genet. 1998;63:259–266. doi: 10.1086/301904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peto R, Lopez AD, Boreham J, Thun M, Heath C., Jr Mortality from tobacco in developed countries: indirect estimation from national vital statistics. Lancet. 1992;339:1268–1278. doi: 10.1016/0140-6736(92)91600-d. [DOI] [PubMed] [Google Scholar]
- Pietrzik CU, Busse T, Merriam DE, Weggen S, Koo EH. The cytoplasmic domain of the LDL receptor-related protein regulates multiple steps in APP processing. EMBO J. 2002;21:5691–5700. doi: 10.1093/emboj/cdf568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrzik CU, Yoon IS, Jaeger S, Busse T, Weggen S, Koo EH. FE65 constitutes the functional link between the low-density lipoprotein receptor-related protein and the amyloid precursor protein. J Neurosci. 2004;24:4259–4265. doi: 10.1523/JNEUROSCI.5451-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature. 1996;382:255–257. doi: 10.1038/382255a0. [DOI] [PubMed] [Google Scholar]
- Prince JA, Feuk L, Sawyer SL, Gottfries J, Ricksten A, Nagga K, Bogdanovic N, Blennow K, Brookes AJ. Lack of replication of association findings in complex disease: an analysis of 15 polymorphisms in prior candidate genes for sporadic Alzheimer’s disease. Eur J Hum Genet. 2001;9:437–444. doi: 10.1038/sj.ejhg.5200651. [DOI] [PubMed] [Google Scholar]
- Sabo SL, Lanier LM, Ikin AF, Khorkova O, Sahasrabudhe S, Greengard P, Buxbaum JD. Regulation of beta-amyloid secretion by FE65, an amyloid protein precursor-binding protein. J Biol Chem. 1999;274:7952–7957. doi: 10.1074/jbc.274.12.7952. [DOI] [PubMed] [Google Scholar]
- Sacco KA, Bannon KL, George TP. Nicotinic receptor mechanisms and cognition in normal states and neuropsychiatric disorders. J Psychopharmacol. 2004;18:457–474. doi: 10.1177/0269881104047273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Kendler KS. The genetic epidemiology of smoking. Nicotine Tob Res. 1999;1(Suppl 2):S51–S57. doi: 10.1080/14622299050011811. discussion S69–S70. [DOI] [PubMed] [Google Scholar]
- Wang B, Hu Q, Hearn MG, Shimizu K, Ware CB, Liggitt DH, Jin LW, Cool BH, Storm DR, Martin GM. Isoform-specific knockout of FE65 leads to impaired learning and memory. J Neurosci Res. 2004;75:12–24. doi: 10.1002/jnr.10834. [DOI] [PubMed] [Google Scholar]
- Wang D, Ma JZ, Li MD. Mapping and verification of susceptibility loci for smoking quantity using permutation linkage analysis. Pharmacogenomics J. 2005;5:166–172. doi: 10.1038/sj.tpj.6500304. [DOI] [PubMed] [Google Scholar]
- Wiley JC, Smith EA, Hudson MP, Ladiges WC, Bothwell M. Fe65 stimulates proteolytic liberation of the beta-amyloid precursor protein intracellular domain. J Biol Chem. 2007;282:33313–33325. doi: 10.1074/jbc.M706024200. [DOI] [PubMed] [Google Scholar]