Abstract
Background and Objectives
To support medication development with cannabinoids, smoked cannabis has been said to alleviate symptoms of opioid withdrawal. We evaluated that hypothesis.
Methods
We analyzed data from the methadone-taper phase of a clinical trial we had conducted. Participants were 116 outpatient heroin and cocaine users (of whom 46 were also cannabis users) who stayed for the 10-week taper. Main outcome measures were weekly urine screens for cannabinoids, plus every-two-week assessments of opioid-withdrawal symptoms.
Results
Opioid-withdrawal scores did not differ overall between users and nonusers of cannabis. In a lagged analysis in the 46 users, there was a slight (not statistically significant) indication that weeks of higher opiate-withdrawal symptoms preceded weeks of cannabis use (effect-size r =.20, 95% CI −.10 to .46, p =.52). Even if this finding is taken to suggest self-medication with cannabis, a lagged analysis in the other temporal direction showed no indication that cannabis use predicted lower opiate-withdrawal symptoms the next week (effect-size r =.01, 95% CI −.28 to .30, p =.69). These findings persisted in sensitivity analyses controlling for each of 17 potential confounds.
Discussion and Conclusion
With our findings, the clinical evidence for smoked cannabis as a reducer of opioid-withdrawal symptoms moves slightly further from “inconclusive” or “mixed” and closer to negative, at least in the context of a methadone dose taper like the one used here.
Scientific Significance
This finding may remove one rationale for medication development using cannabinoids to treat opioid withdrawal, but leaves other rationales intact.
INTRODUCTION
In a recent review, Scavone and colleagues1 gave multiple, persuasive rationales for the use of cannabinoid-based medications in treatment of opioid-use disorders. Discussing one of the rationales—observations that opioid-dependent individuals may use smoked cannabis to ease opioid withdrawal—Scavone and colleagues noted that the bulk of the evidence is equivocal. Nonetheless, they cited pilot data from which they concluded: “objective ratings of opiate withdrawal decrease in [methadone-maintained] patients using cannabis during stabilization.”2 The implication is that smoked cannabis—in some people, at some dosages—may alleviate opioid withdrawal.
The validity of that statement is not a make-or-break question for therapeutic use of synthetic cannabinoids in opioid withdrawal. But the question is worth addressing, if only for the sake of clarity about what cannabis can or cannot do. The idea that cannabis can treat opioid withdrawal has been in the medical literature since at least 1889, in the form of a case report in which cannabis extract seemed to produce immediate benefits for a laudanum addict whose withdrawal symptoms had otherwise been intractable.3 More recently and less anecdotally, Raby and colleages4 replicated their own unexpected finding: cannabis smoking was associated with better adherence to naltrexone maintenance immediately after inpatient opioid detoxification. Adherence to naltrexone maintenance may have been a proxy measure of absence of protracted opioid-withdrawal symptoms. One complicating detail is that the association was not linear (intermittent cannabis smoking was associated with better naltrexone adherence than either no smoking or frequent smoking), but this finding does not rule out an antiwithdrawal effect of cannabis, as Raby and colleagues discuss in their paper. Also, an antiwithdrawal effect was supported by “clinical observations that some opioid dependent patients on naltrexone reported benefit from cannabis use.”
Prior reports of those sorts of phenomenological data, as we are not the first to point out, have been mixed. For example, Gossop and colleagues5 reported that cannabis was among the drugs commonly used to self-medicate during self-detoxification attempts in heroin addicts, but in the 22 (out of 47) addicts who used cannabis to self-medicate, 12 spontaneously mentioned that it had actually made their withdrawal symptoms worse, whereas only six spontaneously mentioned that it had helped. The response profile was better in a study by Noble and colleagues,6 who found that cannabis had been used for self-medication in nine out of forty heroin addicts during attempts at self-detoxification. Of those nine, six reported that cannabis had helped, and two reported the opposite.
To address the question more systematically, we used data from a published clinical trial of ours7 that included repeated assessments of opioid-withdrawal symptoms during a methadone dose taper. We have not previously published the data presented here. We added to the extant literature by doing time-lagged analyses, so we could see whether weekly urine results for cannabinoids were associated with severity of opioid withdrawal either the week before (suggesting self-medication with cannabis) or the week after (suggesting either successful self-medication or exacerbation, depending on the direction of the effect). We did not have a formal a priori prediction about the outcome.
METHOD
Participants
Participants were outpatients who had just finished their participation in a clinical trial of a contingency-management (CM) intervention and a methadone-dose increase (the increase occurred 20 weeks before the taper started); details of the methods are in the publication in which we reported the main results.7
Eligibility criteria for initial enrollment into the main study were: age 18–65, cocaine and opiate use (by self-report and urine screen), and physical dependence on opiates. Exclusion criteria were: current psychotic, bipolar, or major depressive disorders; current physical dependence on alcohol or sedatives; unstable serious medical illness; estimated IQ below 80, per the Shipley Institute of Living Scale;8 and conditions precluding urine collection. Diagnostic and Statistical Manual (DSM-IV) diagnoses of heroin or cocaine dependence were not required. Eligibility for randomization to a treatment group in the main study was based on subsequent heroin and cocaine use during a five-week baseline (the criterion was that the participant had to test positive for heroin and cocaine at least four times each, not necessarily on the same days, in the 15 urine specimens collected during baseline). For ethical reasons, enrollees who tested positive less often than that were retained in our clinic as “nonstudy” patients for the duration of the study, and they completed the same outcome measures as the randomized participants. For the analyses reported here, we included everyone who stayed for a 10-week post-study methadone dose taper: 89 (out of 252) randomized participants plus 27 (out of 49) nonstudy patients. Thus, we had a total sample size of 116. Their demographics are shown in Table 1.
TABLE 1.
Participant characteristics
| Cannabis users (N =46) | Cannabis nonusers (N =70) | Total sample (N =116) | Group difference | |
|---|---|---|---|---|
| Women | 46% | 49% | 47% | Exact p = .85 |
| African American | 67% | 67% | 67% | Exact p = .58 |
| Age | 37 (SD =7.9, range 21–56) | 40 (SD =6.7, range 22–59) | 39 (SD =7.3, range 21–59) | t(114) =.54, p =.59 |
| Prior group assignment in main study (Epstein et al., 2009) | 30% cocaine-contingent CM; 28% split- contingent CM; 22% noncontingent; 20% nonrandomized | 29% cocaine-contingent CM; 30% split- contingent CM; 16% noncontingent; 26% nonrandomized | 29% cocaine-contingent CM; 29% split- contingent CM; 18% noncontingent; 23% nonrandomized | Exact p = .81 |
| Drug history, from Addiction Severity Index | ||||
| Cannabis: lifetime use (years) | 5.1 (SD =7.8, range 0–31) | 2.8 (SD =4.4, range 0–20) | 3.7 (SD =6.1, range 0–31) | t(114) =2.05, p =.0423 |
| Cannabis: Past 30 days | 3.4 (SD =6.1, range 0–30) | .6 (SD =1.5, range 0–8) | 1.7 (SD =4.2, range 0–30) | t(114) =3.71, p =.0003 |
| Cocaine: lifetime use (years) | 6.9 (SD =6.0, range 0–30) | 6.9 (SD = 5.4, range 0–20) | 6.9 (SD =5.6, range 0–30) | t(114) =.05, p =.96 |
| Cocaine: past 30 days | 18.5 (SD =9.9, range 4–30) | 17.2 (SD =9.6, range 4–30) | 17.7 (SD =5.6, range 0–30) | t(114) =.73, p =.47 |
| Heroin: lifetime use (years) | 10.3 (SD =7.9, range 1–32) | 10.5 (SD =7.7, range 1 32) | 10.4 (SD =7.8, range 1–32) | t(114) =.18, p =.86 |
| Heroin: past 30 days | 29.6 (SD =2.9, range 10 30) | 29.2 (SD =4.1, range 0 30) | 29.3 (SD =3.7, range 0–0) | t(114) =.54, p =.59 |
| Drug-use disorders, per DSM criteria (available for the 87 participants in the main study: 35 users, 52 nonusers) | ||||
| Cannabis dependence | 100% negative | 6% remitted; 94% negative | 3% remitted; 97% negative | Exact p = .27 |
| Cannabis abuse | 100% negative | 100% negative | 100% negative | Not applicable |
| Heroin dependence | 97% current; 3% negative | 92% current; 2% remitted; 6% negative | 94% current; 1% remitted; 5% negative | Exact p = .79 |
| Heroin abuse | 3% current; 97% negative | 2% current; 98% negative | 2% current; 98% negative | Exact p = .99 |
| Cocaine dependence | 74% current; 6% remitted; 20% negative | 71% current; 4% remitted; 25% negative | 72% current; 5% remitted; 23% negative | Exact p = .95 |
| Cocaine abuse | 6% current; 94% negative | 8% current; 92% negative | 7% current; 93% negative | Exact p = .99 |
The main study was approved by the Institutional Review Board of the NIDA Intramural Research Program (IRP); each participant gave written informed consent.
Methadone Dose and Taper Schedule
At the beginning of the taper, most participants were receiving a daily methadone dose of either 70 mg (n =66) or 100 mg (n =48); the other two had been stabilized at daily doses of 80 mg and 90 mg. Methadone doses had been stable for at least 20 weeks. During the taper, methadone dose was decreased by 10% per week over 10 weeks.
Measures
The main measure of opiate-withdrawal symptoms was a questionnaire on which participants rated the severity each of 24 items from 0 to 4 (0, not at all; 1, a little; 2, moderately; 3, quite a bit; 4, extremely), producing a maximum withdrawal score of 96. The original version of the questionnaire, based on symptoms listed by in 1938 by Kolb and Himmelsbach9 and in the Goodman & Gilman textbook,10 was developed at the NIDA IRP for a study of acutely precipitated withdrawal.11 We added items to capture symptoms likely to emerge during an outpatient taper, such as difficulty sleeping. The scale was completed every two weeks throughout the study, including the dose-taper phase. The instruction was to “rate each of the items below in terms of how you felt in the last 2 days” (emphasis in original). The 24 symptoms rated were: tense and jittery; gooseflesh; difficulty sleeping; anxious; restless; watery eyes; low energy; sick to stomach (nausea); runny nose; yawning; headache; vomiting; sweating; nervous; good mood [reverse-scored]; hot or cold feelings (chills); stomach cramps/muscle aches; heavy/sluggish feeling; backache; sleepy; irritable; skin clammy or damp; painful joints; relaxed [reverse-scored].
The main measure of cannabis use was weekly urine testing with the enzyme-multiplied immunoassay technique (EMIT). (The urine specimens were also tested for metabolites of opioids, cocaine, and other drugs of abuse.) Despite popular lore, carryover of cannabis excretion is not a major concern for occasional users,12,13 and in our participants, isolated cannabis-positive urines were common.
Data Analysis
First, in a between-subjects analysis, we classified participants as either cannabis users (n =46) or cannabis nonusers (n =70) on the basis of whether they ever tested positive for cannabinoids during the methadone dose taper. To test for an overall group difference in opiate-withdrawal symptoms during the dose taper, we ran a Proc Mixed model on opiate-withdrawal severity, with one between-subjects predictor (CannUser: yes vs. no) one within-subjects predictor (Week of dose taper: 2, 4, 6, and 8), and their interaction.
We then ran two lagged analyses, in which the outcome of interest was what happened week by week. For these analyses, we used the weekly urine data to classify each week, for each participant, as a cannabis-use week or a cannabis-nonuse week.
In the first lagged analysis, we tested whether opiate-withdrawal severity was “predicted” by subsequent cannabis use—that is, whether periods of more severe opiate withdrawal were likely to be followed by cannabis use (suggesting attempts at self-medication). This was done in a Proc Mixed model with one time-varying predictor (CannWeekAfter: yes vs. no), one within-subjects predictor (Week of dose taper: 2, 4, 6, and 8), and their interaction. The reason we used CannWeekAfter (a dichotomous variable) as a predictor rather than as the dependent variable was to enable use of the same type of analysis for all of our models.
In the second lagged analysis, we tested whether opiate-withdrawal severity was predicted by prior cannabis use (suggesting either successful self-medication or exacerbation, depending on the direction of the effect). This was done in a Proc Mixed model with one time-varying predictor (Cann-WeekPrior: yes vs. no), one within-subjects predictor (Week of dose taper: 2, 4, 6, and 8), and their interaction.
After that, we ran 34 sensitivity analyses on our two lagged models, in which we controlled for each of 17 covariates in a separate model to avoid overspecification of any one model. (The covariates were: contingency group; maintenance dose; sex; race; age; Addiction Severity Index [ASI] heroin days; ASI heroin years; ASI cocaine days; ASI cocaine years; ASI cannabis days; ASI cannabis years; DSM-IV heroin dependence; DSM-IV heroin abuse; DSM-IV cocaine dependence; DSM-IV cocaine abuse; DSM-IV cannabis dependence; DSM-IV cannabis abuse.) Multiple tests of significance were not a concern because our goal was to have maximum power to detect confounding by the covariates—that is, to have maximum power to show that our initial conclusions were incorrect. Finally, we ran sensitivity analyses in which we omitted the Group x Time interaction term, or collapsed across week—again, trying to determine whether we had inadvertently buried a significant effect of cannabis.
We calculated effect sizes by converting F values into correlation coefficients (Pearson rs).14,15 This method is applicable only when the numerator df for the F-test is 1, but it has the advantage of generating 95% confidence intervals, which are preferred to post hoc power analyses as indicators of the reliability of null findings.16
For all analyses, we used autoregressive error structures, and the alpha level was p ≤.05, two-tailed.
RESULTS
Because some participants left before the end of the 10-week dose taper, or missed some urine collections, the mean ± standard error of the mean (SEM) number of weekly urine specimens tested during the taper was 8.0 ± .3 (range 2–10) for the 46 cannabis users and 6.8 ± .4 (range 1–10) for the 70 nonusers. The mean percentage of cannabinoid-positive urines was 66% ± 5 (range 10–100%) for the cannabis users. The mean percentage of opioid-positive urines was 54% ± 5 (range 0–100%) for the cannabis users and 52% ± 5 (range 0–100%) for the cannabis nonusers. The mean percentage of cocaine-positive urines was 53% ± 6 (range 0–100%) for the cannabis users and 56% ± 5 (range 0–100%) for the nonusers.
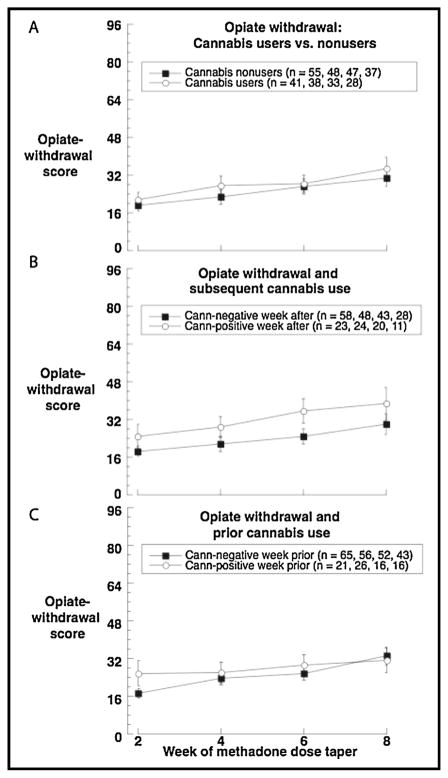
In the between-subjects analysis (Fig. 1a), as expected, opioid-withdrawal scores increased over time as methadone dose decreased [F(3,215) =12.64, p <.0001]. Cannabis users appeared to have very slightly higher opiate-withdrawal scores overall (least-squares means ± SEMs 28.29 ± 2.96 vs. 26.06 ± 2.55), but the main effect of group was not statistically significant [F(1,104) =.33, p =.57, effect-size r =.06, 95% CI −.13 to .25], nor was the interaction of group and week.
FIGURE 1.
Cannabis use and opiate withdrawal scores during taper from methadone maintenance. (A) Between-person results: opioid-withdrawal scores assessed every two weeks in users vs. nonusers of cannabis. (B) Time-lagged analysis: opioid-withdrawal scores as a function of whether the participant was to test positve for cannabinoids in the subsequent week. (C) Time-lagged analysis: opioid-withdrawal scores as a function of whether the participant tested positive for cannabinoids in the prior week. All panels show raw means, not the least-squares means from the repeated-measures analyses. In panels B and C, some participants switch between the two lines in the graph depending on their cannabis use from week to week.
In the first of the two lagged analyses, using the following week’s urine cannabinoid results as a time-varying “predictor” of each week’s opiate-withdrawal symptoms, there was a slight indication that opiate-withdrawal symptoms preceded cannabis use (Fig. 1b). On weeks when participants tested positive for cannabinoids, their least-squares mean ± SEM opiate-withdrawal score from the preceding week was 27.44 ± 2.90, versus 25.44 ± 2.22 otherwise. However, this difference did not reach statistical significance [F(1,11) =.45, p =.52, effect-size r =.20, 95% CI −.10 to .46].
In the second of the two lagged analyses (Figure 1c), there was no consistent prediction of opiate-withdrawal score by prior cannabis use; the respective least-squares means were 26.23 ± 2.75 (cannabis-positive weeks) versus 26.29 ± 2.03 (cannabis-negative weeks) [F(1,10) =.001, p =.98, effect-size r =.01, 95% CI −.28 to .30]. Figure 1c suggests the possibility of an interaction with week, but this was not statistically significant either [F(3,185) =.49, p =.69].
We repeated the two lagged analyses to control for each of 17 potential covariates (listed in the Method section), using 34 (2 × 17) separate models so that no one model would be overspecified. None of them changed the magnitude or direction of the (always nonsignificant) association between cannabis use and opiate withdrawal. The only one of the 17 covariates with its own effect on withdrawal scores was maintenance dose: higher doses (among the actual final maintenance doses: 70, 80, 90, and 100) were associated with less severe withdrawal overall [F(3,74) =4.38, p =.007]. We ran two additional models to test for interactions between dose and cannabis use (Dose x CannWeekPrior, or Dose x CannWeekPost) as predictors of withdrawal scores; there were no interactions.
DISCUSSION
Given our findings, the clinical evidence for smoked cannabis as a reliever of opioid-withdrawal symptoms moves slightly further from “inconclusive” or “mixed” and slightly closer to negative. Even if our participants were trying to self-medicate with cannabis (Fig. 1b) during the methadone taper, we found no evidence that their efforts were effective (Fig. 1c).
This sort of finding—that smoked cannabis does not seem to have a specific clinical benefit as hypothesized—never exists in an ideological vacuum, even in a scientific journal. We think it is important to say that we approached the question with genuine clinical equipoise; we were prepared to report a positive outcome if we had found one. In hindsight, though, we do not find our null results surprising. Cannabis has been used as medicine since at least 2700 B.C.E.,17 there are currently an estimated 180 million past-year users worldwide,18 and, extrapolating from available data in 17 countries,19 we estimate that the current worldwide number of lifetime users may be greater than 500 million. With such widespread use over so many thousands of years, most of the reliable and robust therapeutic effects of cannabis for common conditions seem likely to have already been fortuitously discovered (as was the case with therapeutic effects of cannabis on intraocular pressure and nausea).
Our own prior paper on cannabis smoking during methadone maintenance, using data from 408 outpatients in a different clinical trial,20 has been cited as evidence for potentially beneficial effects of cannabis on treatment outcome. Our main finding in that paper was simply that there were no overt deleterious effects. We did report that heroin use during methadone maintenance was lower among participants who had met lifetime DSM-IV criteria for a cannabis-use disorder, but we also reported that actual use of cannabis and heroin during treatment was unrelated, despite a hint of an inverse association (not approaching statistical significance). For the question at hand—whether cannabis can alleviate opioid withdrawal—actual heroin use is less informative than opioid-withdrawal symptoms. Heroin use during methadone maintenance might vary inversely with cannabis use for reasons that have nothing to do with opioid-withdrawal symptoms—for example, limited money to spend on illicit drugs, or social influence by friends who prefer one drug to the other. In our prior paper, we did not examine opioid-withdrawal symptoms, and we did not use data from participants undergoing a methadone dose taper, as we do in the current analyses.
One limitation of our current analyses is that our sample size of 116 (of whom only 46 ever used cannabis) is at the low end of the range from which one might confidently assert the absence of an effect. However, the effect we found for cannabis-associated relief of opioid-withdrawal symptoms (r =.01) had 95% confidence limits of −.28 to .30. The upper limit is only slightly greater than a conventionally defined “small” r (.20) and less than a “medium” r (.50). Thus, it is unlikely that we missed an effect large enough to be clinically significant.
Other limitations are that our urine drug tests were qualitative, precluding biochemical assessment of the amounts of drugs used, and that our questionnaire on opioid-withdrawal symptoms was internally developed and not formally validated. A greater limitation is that we assessed opioid-withdrawal severity only once every two weeks (and only over a time frame of the past two days). We would have liked to have a much more sensitive measure of opioid-withdrawal severity as a function of cannabis use; it is possible that individual instances of cannabis use produced brief reductions in withdrawal severity that we could not detect in our design. Our reasoning, however, is that if cannabis use reliably eases opioid-withdrawal symptoms even briefly, and if our participants were using it for that purpose when they needed to, we should have seen some signal in our two-day “snapshots” of withdrawal severity.
What our findings leave intact is the possibility that opioid-withdrawal symptoms could be relieved by individual cannabinoids, such as cannabidiol21 or by inhibitors of endocannabinoid metabolism.22 Those approaches do not need to be justified by citing a clinical effect of smoked cannabis on opioid withdrawal.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH National Institute on Drug Abuse.
Footnotes
Declaration of Interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this paper.
References
- 1.Scavone JL, Sterling RC, Van Bockstaele EJ. Cannabinoid and opioid interactions: implications for opiate dependence and withdrawal. Neuroscience. 2013;248:637–654. doi: 10.1016/j.neuroscience.2013.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scavone JL, Sterling RC, Weinstein SP, et al. Impact of cannabis use during stabilization on methadone maintenance treatment. Am J Addict. 2013;22:344–351. doi: 10.1111/j.1521-0391.2013.12044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birch EA. The use of Indian hemp in the treatment of chronic chloral and chronic opium poisoning. The Lancet. 1889;133:625. [Google Scholar]
- 4.Raby WN, Carpenter KM, Rothenberg J, et al. Intermittent marijuana use is associated with improved retention in naltrexone treatment for opiate-dependence. Am J Addict. 2009;18:301–308. doi: 10.1080/10550490902927785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gossop M, Battersby M, Strang J. Self-detoxification by opiate addicts: A preliminary investigation. Br J Psychiatry. 1991;159:208–212. doi: 10.1192/bjp.159.2.208. [DOI] [PubMed] [Google Scholar]
- 6.Noble A, Best D, Man LH, et al. Self-detoxification attempts among methadone maintenance patients: what methods and what success? Addict Behav. 2002;27:575–584. doi: 10.1016/s0306-4603(01)00194-0. [DOI] [PubMed] [Google Scholar]
- 7.Epstein DH, Schmittner J, Umbricht A, et al. Promoting abstinence from cocaine and heroin with a methadone dose increase and a novel contingency. Drug Alcohol Depend. 2009;101:92–100. doi: 10.1016/j.drugalcdep.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zachary R. Shipley Institute of Living Scale. Revised Manual. Los Angeles: Western Psychological Services; 1986. [Google Scholar]
- 9.Kolb L, Himmelsbach CK. Clinical studies of drug adduction, III: A critical review of the withdrawal treatments with method of evaluating abstinence syndromes. Am J Psychiatry. 1938;94:759–799. [Google Scholar]
- 10.Gilman AG, Goodman LS, Roll TW, Murad F. The Pharmacological Basis of Therapeutics. New York: McMillan; 1985. [Google Scholar]
- 11.Preston KL, Bigelow GE, Liebson IA. Butorphanol-precipitated withdrawal in opioid-dependent human volunteers. J Pharmacol Exp Ther. 1988;246:441–448. [PubMed] [Google Scholar]
- 12.Huestis MA, Mitchell JM, Cone EJ. Detection times of marijuana metabolites in urine by immunoassay and GC-MS. J Anal Toxicol. 1995;19:443–449. doi: 10.1093/jat/19.6.443. [DOI] [PubMed] [Google Scholar]
- 13.Smith-Kielland A, Skuterud B, Morland J. Urinary excretion of 11-nor-9-carboxy-delta9-tetrahydrocannabinol and cannabinoids in frequent and infrequent drug users. J Anal Toxicol. 1999;23:323–332. doi: 10.1093/jat/23.5.323. [DOI] [PubMed] [Google Scholar]
- 14.Rosnow RL, Rosenthal R. Computing contrasts, effect sizes, and counternulls on other people’s published data: General procedures for research consumers. Psychol Methods. 1996;1:331–340. [Google Scholar]
- 15.Rosnow RL, Rosenthal R, Rubin DB. Contrasts and correlations in effect-size estimation. Psychol Sci. 2000;11:446–453. doi: 10.1111/1467-9280.00287. [DOI] [PubMed] [Google Scholar]
- 16.Colegrave N, Ruxton GD. Confidence intervals are a more useful complement to nonsignificant tests than are power calculations. Behav Ecol. 2003;14:446–450. [Google Scholar]
- 17.Zuardi AW. History of cannabis as a medicine: A review. Rev Bras Psiquiatr. 2006;28:153–157. doi: 10.1590/s1516-44462006000200015. [DOI] [PubMed] [Google Scholar]
- 18.United Nations Office on Drugs and Crime. World Drug Report. Vienna: United Nations: 2013. [Google Scholar]
- 19.Degenhardt L, Chiu WT, Sampson N, et al. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: Findings from the WHO World Mental Health Surveys. PLoS Med. 2008;5:e141. doi: 10.1371/journal.pmed.0050141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Epstein DH, Preston KL. Does cannabis use predict poor outcome for heroin-dependent patients on maintenance treatment? Past findings and more evidence against. Addiction. 2003;98:269–279. doi: 10.1046/j.1360-0443.2003.00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ren Y, Whittard J, Higuera-Matas A, et al. Cannabidiol, a non-psychotropic component of cannabis, inhibits cue-induced heroin seeking and normalizes discrete mesolimbic neuronal disturbances. J Neurosci. 2009;29:14764–14769. doi: 10.1523/JNEUROSCI.4291-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Negus SS, Banks ML. Medications development for opioid abuse. Cold Spring Harb Perspect Med. 2014;3:a012104. doi: 10.1101/cshperspect.a012104. [DOI] [PMC free article] [PubMed] [Google Scholar]