Introduction
To tackle some of the most fundamental questions in neurobiology at the molecular level, classical genetics and pharmacology have been effective tools to perturb the activity of neuronal receptors and ion channels. However, conventional pharmacology has several drawbacks that are especially problematic when studying neural circuits and synapses. First, standard diffusion and partitioning of the ligand mean poor spatial and temporal control of ligand activity. Second, even in cases of high specificity for ligand action, it is relatively common for the receptor or channel of interest to be expressed on multiple nearby cell types, or even on both sides of a synapse, making it difficult to isolate the effects of the target receptor within the circuit or synapse of interest. Light-based techniques that operate at the intersection of chemistry, biology, and neuroscience have been developed to overcome these challenges. We focus here on systems that contain a synthetic photoswitch, a small molecule that absorbs light to reversibly change its shape. The most commonly used photoswitch in biological applications is azobenzene due to its synthetic tractability, tunable photochemical properties, and biological compatibility (Fig. 1a). The lowest energy isomer, the straight trans-azobenzene, isomerizes to the bent cis-azobenzene configuration upon irradiation with near-UV light. Subsequent irradiation with longer wavelength visible light, or thermal relaxation, leads the metastable cis-azobenzene to revert to the trans-azobenzene isomer.
Figure 1.
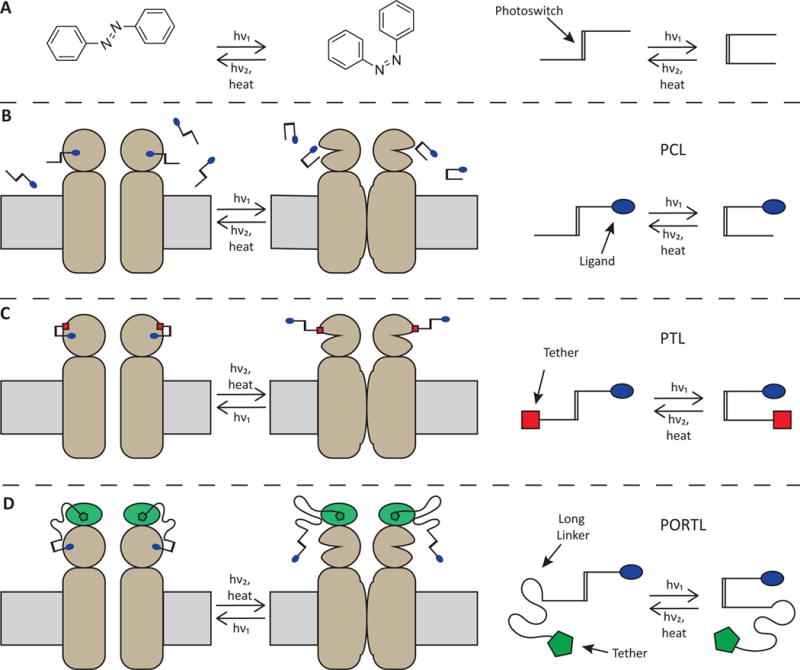
Strategies for incorporating synthetic photoswitches into neuroscience tools. A) The trans and cis isomers of azobenzene can be interconverted with different wavelengths of light. Cartoons show how the core azobenzene structure can be elaborated into photoswitchable tools. B) Photochromic ligands (PCLs) switch between active and inactive compounds that freely diffuse and function with endogenous channels and receptors. C) Photoswitchable Tethered Ligands (PTLs) covalently attach to an engineered protein to provide photocontrol. D) Photoswitchable orthogonal remotely tethered ligands (PORTLs) are conceptually a cross between PCLs and PTLs, they use a self-labeling protein fused to the target protein and a photoswitch ligand that has long linker to accommodate attachment farther from the ligand binding site.
To make a light-sensitive probe, the photoswitch is chemically attached to a biologically active ligand. Freely diffusible photoswitch-ligand constructs, called photochromic ligands (PCLs) (Fig. 1b), interact with the native target protein such that one photoisomer is active and the other is not. PCLs have the capability to operate as blockers (e.g. of a pore or active site), agonists, antagonists, or allosteric modulators. This one-component system, called photopharmacology, is an improvement upon conventional pharmacology in that PCLs can be rapidly photoswitched to provide exceptional spatial and temporal control. Despite this, selectivity for a particular receptor among related subtypes can vary, and diffusion makes it impossible to confine PCL action to a particular cell or group of cells. To overcome these drawbacks, photoswitchable tethered ligands (PTLs) incorporate a covalent linkage to a modified version of the target protein, thereby enabling subunit-specific control and cell-specific genetic targeting in a two-component approach called chemical optogenetics (Fig. 1c). Until recently, photoswitch linkage mainly used a maleimide on the PTL and an introduced cysteine on the target protein. While cysteines are not unique in proteins, on external domains they tend to be disulfide bonded (and not subject to interaction with maleimide) or absent from “sensitive areas” near the mouth of a pore or ligand binding site. Nevertheless, there would be advantages for a new bio-orthogonal linkage. Such a strategy was achieved recently. It merges the properties of PCLs and PTLs by placing a PCL on a leash that is covalently attached via highly selective chemistry near the ligand binding site on the target protein. The leash restricts binding to the targeted protein and assures a sufficiently high local concentration for high occupancy in the switched-on state. These compounds are called photoswitchable orthogonal remotely tethered ligands (PORTLs) (Fig. 1d), and they provide a gateway to multiplexed optical control of distinct photoswitches and their targets.
In this work, we highlight recent developments, including the expansion of the available toolbox to include new receptors, channels, and binding sites, the incorporation of new bio-orthogonal attachment chemistry, the increased range of useful light wavelengths, and new in vivo applications. We will not address several related techniques and tools that have been well reviewed elsewhere, including optogenetics[1], chemogenetics[2], Photo-uncaging[3], and quasipharmacological interactions of cells with light[4].
Glutamate receptors (GluRs)
Glutamate is the primary excitatory neurotransmitter in the mammalian brain, with dozens of different receptor subunit types that mediate fast synaptic transmission and short- and long-term modulation of synaptic strength. Glutamate receptors (GluRs) comprise ionotropic receptors (iGluRs), which are tetrameric ion channels, and metabotropic receptors (mGluRs), which are class C dimeric G-protein coupled receptors (GPCRs). The iGluRs consist of three classes of receptors: NMDARs, AMPARs, and kainateRs.
NMDARs are cation channels with both ligand and voltage dependence. They function as synaptic regulators that play an important role in learning and memory. Recently both PCL and PTL approaches were developed for NMDARs. The PCL agonist ATG is unreactive in its relaxed trans-isomer, but when irradiated with UV light, the cis-isomer is an agonist for multiple NMDAR subunits (GluN2A,B,C,D)[5]. Experiments in slices of mouse cortex show that ATG can be used to induce currents and action potentials, even in place of synaptic stimulation for signal coincidence detection. A two-component system was also developed for three NMDAR subunits to create “LiGluNs”[6]. GluN1, GluN2A, and GluN2B were cysteine-substituted to enable PTLs called MAGs to generate photo-agonism (GluN2A, GluN2B) or photo-antagonism (GluN1a and GluN2A). In mouse hippocampal slices, photo-agonism can stimulate—and photo-antagonism inhibit—the dendritic spine growth associated with long-term potentiation (LTP) with single-spine resolution. Photo-antagonism also blocks the induction of LTP by synaptically-released glutamate. The relaxation of the active cis to inactive trans state of the photo-antagonist is slow, allowing for sustained light-block with brief pulses of light at long intervals. This was used over days of development to block NMDAR-dependent pruning of retinal ganglion cell axonal arbors innervating the brain of developing zebrafish[6].
AMPARs mediate fast excitatory synaptic transmission, and their modulation has strong effects on synaptic strength. Initial PCLs were agonists of GluA2, with the best compound being ATA[7]. The active trans-isomer of ATA allows photo-control of action potential firing in layer 2/3 neurons from mouse cortical slices. A complimentary PCL antagonist, ShuBQX-3, is quite selective over NMDARs, partially selective over kainateRs, and provides photo-antagonism of hippocampal CA1 neurons[8].
KainateRs also mediate fast excitatory synaptic transmission. In part due to limited selectivity of conventional pharmacological probes, the kainateRs were the first GluRs targeted for photocontrol with both one- and two-component systems[9]. Recent experiments showed that viruses containing LiGluR (GluK2 L439C) stereotactically injected into the V1 region of a mouse’s visual cortex led to targeted production of LiGluR. Subsequent labeling with a PTL made these LiGluRs photoactivatable in vivo: light pulses in the cortex led to neuronal firing with a frequency of up to 5 Hz[10]. This study exemplifies the type of experiments possible when using cell-type specific promoters to express the target protein with high specificity. A study on the effects of multiple ligands binding to multiple subunits of tetrameric kainateRs was only possible with LiGluR, and it suggested that multiple subunits must be occupied by ligand before the channel desensitizes[11].
mGluRs regulate signaling pathways within neurons to control synaptic strength and plasticity. An initial two-component system was developed for light-gated mGluRs, or “LimGluRs”[12]. They have been used to examine dimerization effects, receptor conformational dynamics, and receptor glutamate sensitivity[13], as well as to photoactivate the Gi GPCR pathway to show this pathway plays a role in astrocyte glutamate uptake[14]. A PCL negative allosteric modulator (NAM) targeting mGluR5, called alloswitch-1, was recently developed[15]. Alloswitch-1 photomodulates the activity of mGluR5 in vitro and tadpole swimming behavior in vivo. Medicinal chemistry refinements to find structure activity relationships for alloswitch-1 led to a series of phenylazopyridine-containing PCLs that also function as NAMs[16]. Impressively, some of these PCL NAMs produce photoswitchable analgesia in mice. Whereas mGluR5 has a potentiation effect on neural activity, mGluR4 works to inhibit glutamate release at the synapse. Accordingly, the PCL NAM for mGluR4, OptoGluNAM4.1, inhibits the activity of mGluR4 in vivo and has an opposite regulatory effect on the swimming behavior of zebrafish[17].
With these newest additions to the glutamate receptor family of tools, it is now possible to target at least one subunit from all three types of iGluR as well as to target or modulate at least one member of all three groups of mGluR.
GABA receptors (GABARs)
GABA is the primary inhibitory neurotransmitter in the mammalian brain. GABAARs are pentameric ion channels, while GABABRs are GPCRs. PCL versions of propofol[18,19] and gabazine[20] create photo-potentiation and photo-antagonism of GABAARs, respectively, both in vitro and in vivo. Two-component systems using PTLs that act as either an agonist (MAB-0), a potentiator (MPC100), or an antagonist (PAG-1C) have been developed to generate “LiGABARs” for specific GABAR subunits (α1-6, and γ). This has made it possible to map the subcellular location of different GABAAR subunits in living neurons[18,21,22]. An exciting series of experiments shows the great potential of knock-in animal models, which possibly provide the best assurance that no compensatory changes, from the molecular to the systems level, have taken place due to overexpression[22]. When observing γ oscillations in mice, the oscillations decreased when a nonselective GABAAR blockade was induced. However, in the α1-GABAAR photoswitch ready mouse (PhoRM, a knock-in mouse with all α1-LiGABAR), infusion or brain surface application of PTL allows subtype specific photo-antagonism of only GABAARs with α1, leading to enhanced γ oscillations. This opposite effect is evidence for inhibitory-inhibitory interactions for α1 subunit-containing GABAARs[22]. In a notable shift from ion channels and GPCRs, a PCL based on nipecotic acid photo-inhibits GABA transporter mGAT1 in vitro, which led to light-dependent tonic currents in dentate gyrus granule cells in mouse slices[23].
Acetylcholine receptors/acetylcholine esterase (AChRs/AChE)
Acetylcholine (ACh), the first neurotransmitter discovered, is found throughout the brain and peripheral nervous system. It plays a well-known role as the main neurotransmitter of the neuromuscular junction, but in the brain operates as a neuromodulator through both ionotropic and metabotropic receptors. Nicotinic acetylcholine receptors (nAChRs) are pentameric ion channels, and muscarinic acetylcholine receptors (mAChRs) are GPCRs. The many historic firsts of ACh include the first-ever examples of a PCL and a PTL[24]. Recent work has focused on nAChRs with a two-component system developed for heteropentameric forms (α3β4 and α4β2) that have PTLs functioning as a photoagonist (MAACh) and a photoantagonist (MAHoCh) in Xenopus oocytes[25]. A reevaluation of a classic PCL, BisQ, showed it to be a photoagonist of muscle-type nAChR, while a new PCL, AzoCholine, is a photoagonist of α7 nAChRs[26]. AzoCholine was further demonstrated to give photocontrol of bursting activity in mouse hippocampal brain slices. Additional regulation of ACh action at synapses is achieved by its degradation, an action performed by AChE. Photocontrol of AChE has recently been demonstrated with two different PCLs based on the AChE inhibitor tacrine[27,28].
Other Ion channels and promising targets
Several more of the channels and receptors involved in synaptic and neural function are targets for photocontrol. Recent work has targeted GIRK[29], TRPV1[30,31], TRPA1[32], P2X[33,34] and ASIC[33,35] channels. Of special note, two papers[33,34] on P2X channels employ a variant of the chemical optogenetic concept to create “molecular tweezers”: two subunits of the channel are tethered together with azobenzene via covalent cysteine-maleimide bonds at key positions in the channel, allowing the photoswitching azobenzene to open and close the pore.
The most light-accessible neurons in the central nervous system are those of the retina, and accordingly, much work has been done to make the non-photoreceptor cells of the retina light sensitive. Work using both one- and two-component systems on degenerated retinas lacking rods and cones (but possessing much of the rest of the retinal neural circuitry) shows great progress in restoring some degree of light sensitivity in both mouse and dog models of blindness[36–39].
Other developments outside of ion channels include a PCL derivative of the analgesic fentanyl that functions as a photo-agonist of the μ-opioid receptor, a family A GPCR[40]. Another paper describes a family of PCLs based on diacylglycerols, lipid signaling molecules, which provides photocontrol of a host of cellular targets (including protein kinase C) that, through phosphorylation of effector proteins, can modulate synaptic strength and the exocytosis of neurotransmitters[41]. Finally, two papers revealed PCL inhibitors of histone deacetylase, an enzyme that plays a role in gene expression and physiological processes like neuroplasticity[42,43].
Improving synthetic photoswitch utility: wavelength-shifting, two photon (2P) activation and orthogonal attachment chemistry
For synthetic photoswitches to reach their full potential, two goals must be met. First, in order to use these compounds simultaneously, their absorbance spectra shouldn’t overlap. To achieve this goal, a collection of compounds activated by various wavelengths of light is necessary. Particularly desirable is to red-shift the absorbance to red and infrared light, as these wavelengths better penetrate tissue. Much of the appeal of using azobenzene photoswitches is the ability to change the spectral properties of both isomers by changing chemical substitution patterns on the benzene rings[44,45]. Because chemical optogenetic methods are modular (individual tuning of both PTL and receptor), it is possible to use previously validated mutant receptors with a new PTL that has different photochemical properties. We have demonstrated this permissive labeling by attaching different red-shifted PTLs to LiGluR[46,47].
In addition to red-shifting photoswitches, it is also possible to activate these compounds with two photon (2P) excitation. When two photons hit a photoswitch nearly coincidentally, the photoswitch will isomerize as if it were hit by single photon of their combined energy. While this technique requires a high intensity laser, it allows activation of a photoswitch with significantly lower energy/longer wavelength light. The feasibility of 2P activation has been demonstrated for both PCLs and PTLs. Neurons[48,49] and astrocytes[48] expressing LiGluR and labeled with a PTL give a photocurrent with 2P excitation, and even neuron spiking. Further, 2P activation of LimGluRs[49,50], as well as the PCL ATG, which targets NMDARs[5] were reported.
The second goal is to implement PTLs with new bio-orthogonal attachment chemistry. To that end, targeted covalent photoswitches (TCPs) are a type of PTL that use a reactive group (e.g. NHS ester) to covalently bind to an endogenous protein and make it light sensitive. Light-dependent affinity labeling is possible, so patterns of light can shape where the TCP binds in the sample. When endogenous GluK1 channels are labeled with a TCP in DRG neurons and degenerate retina, the neurons become light sensitive[51].
We recently reported a two-component bio-orthogonal labeling technique taking advantage of genetically-encoded self-labeling proteins (e.g. SNAP-tag) fused to a target receptor[52]. The complimentary switches, called PORTLs (photoswitchable orthogonal remotely tethered ligands), covalently bind to the self-labeling protein via selective chemistry (e.g. a benzylguanine group in the case of SNAP). The technique was applied to a SNAP-mGluR2 fusion labeled with a glutamate-containing PORTL, making it possible to optically hyperpolarize and silence hippocampal neurons[52]. Perhaps the most exciting advantage with the addition of a new orthogonal chemical labeling strategy is the ability to individually express, label, and control more than one protein with light. When HEK293 cells expressing both SNAP-mGluR2 and LiGluR were labeled with different PTLs, each channel could be activated independently[52]. We recently expanded this technique to another orthogonal labeling system (the CLIP-tag and its benzylcytosine-selective PORTLs), as well as making the complete set of group II/III SNAP-mGluRs[50].
Conclusions/future directions
Chemical optogenetics and photopharmacology continue to mature. The major differences between these techniques and conventional optogenetics as tools to study neurons and synapses are significant and bear elaboration. Traditional optogenetics relies on the expression of an exogenous light sensitive channel or receptor, frequently an opsin, to generate or inhibit action potential firing. However, this top-down drive of function can obscure the behavior of endogenous receptors and channels. Chemical optogenetics shines in that by requiring minimal structural changes to endogenous proteins, minimal perturbations to the native modulation of that protein can be expected. This is especially important for GPCRs as they can affect downstream signaling in multiple pathways (e.g. G-Protein vs β-arrestin)[53]. Many of the known neurotransmitter small molecules and peptides have not yet been brought into the photopharmacology realm, and expansion to other neurotransmitters and neuropeptides is ongoing. Targets of great interest to our labs include the neuromodulatory class A GPCRs, especially the dopamine and serotonin receptors, due to their essential but unresolved role in many brain behaviors including mood and cognition.
Expanded bio-orthogonal labeling chemistries and photoswitch color palettes will give researchers the ability to construct elaborate experiments with multiplexed photoswitches and fluorophores with a high degree of spatial, temporal, and genetic targeting. Indeed, the proof of concept combining PTLs and PORTLs for both orthogonal labeling and light activation is described above, and we are excited to see this concept expanded to other attachment strategies in addition to SNAP- and CLIP-tags (e.g. HALO-tags). In fact, this and other recent chemical developments [54] encourages us to envision an ideal scenario where orthogonality of labeling a photochemistry allows precise targeting of a synapse (Fig. 2).
Figure 2.
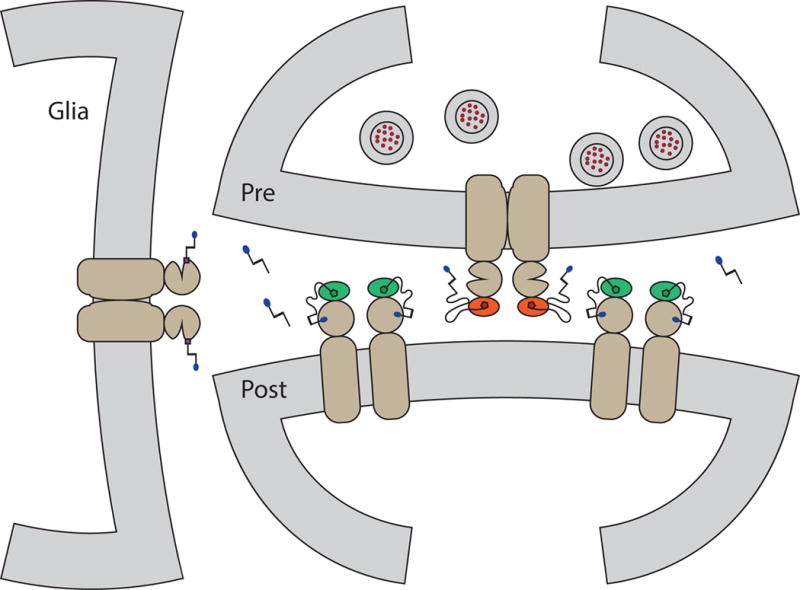
A cartoon of a hypothetical tripartite synapse. Here each piece, glia, presynaptic cell, and postsynaptic cell are all expressing a different form of receptor, which is labeled with an orthogonal photoswitchable ligand (PORTLs and PTLs). The synaptic cleft also has a freely diffusing PCL. Distinctly different absorption spectra for each photoswitchable ligand would allow exact spatiotemporal control at the subcellular level.
Incorporation of photoswitch unnatural amino acids is also an area of active research seeing rapid development; replacing photoswitchable ligands with photoswitch amino acids incorporated into proteins of interest at key positions near pores or fulcrums of movement is becoming feasible as our understanding of structure-function relationships grow[55]. We are also optimistic that methods to achieve both cell specificity and native expression levels will soon be practical, e.g. with cell-targeted CRISPR knock-ins[56]. Finally, some of the photopharmacology tools mentioned above show promise for applications in pain regulation via photoswitchable analgesia, a field in its infancy, and recently reviewed elsewhere[57].
We hope that we have enticed the reader into considering use of some of the many photoswitchable tools discussed in this review to address their research questions, as they now modulate many key synaptic proteins. At the same time, we hope that by showing so many different functioning systems, we can reinforce the general applicability of these approaches to molecular neuroscience. Should an interested reader find no tool currently in existence to deal with their research questions, joining forces with a local collaborator with complimentary skills is a realistic possibility. Indeed, while most of the effort in chemical optogenetic and photopharmacology has focused on ion channels and neurotransmitter receptors, the approaches are applicable to a host of proteins whose function can be controlled by an orthosteric or allosteric ligand or an active site blocker, a design process that was recently explained in detail[58,59].
Highlights.
Photoswitchable ligands allow high spatial and temporal control
Combining with genetic targeting gives cell-type specificity
Orthogonal attachment and photochemistry allows multiplexing
Acknowledgments
This work was supported by the National Institutes of Health Nanomedicine Center for the Optical Control of Biological Function (2PN2EY018241) and startup funding provided by the University of Maine and the Maine Economic Improvement Fund.
Glossary
- Optogenetics
A technique now common in neuroscience that uses genetically encodedlight-activated proteins to control neuronal activity.
- Chemogenetics
A technique that relies on an orthogonally active chemical (usually synthetic) to selectively activate a genetically encodedengineered receptor.
- Photoswitch A
structure that reversibly changes its shape and/or function on absorption of light.
- Photochromism
A reversible light dependent chemical change in a molecule that results in the formation of spectrally distinct compoundssometimes visible to the eye as a color change.
- Photoisomerization
A light dependent structural change in a moleculegenerally, but not exclusively, applied to reversible processes.
- PCL - Photochromic ligand
A freely diffusible photoswitchable ligand.
- Photopharmacology
A technique using a freely diffusiblebiologically active small molecule synthetically modified to include a photoswitchable element. Photoisomerization toggles between a more and less active form of the molecule.
- Optopharmacology
See photopharmacology.
- One-component system
Used here to mean a receptor or channel rendered photo-controllable with a just one syntheticnon-endogenous component. For example, photopharmacology.
- chemical optogenetics
A technique for photocontrol of protein activity that relies on the combination of two synthetic elements—a genetically encodedengineered protein and a covalently bound photoswitchable ligand.
- optochemical genetics
See chemical optogenetics.
- optogenetic pharmacology
See chemical optogenetics.
- Two-component system
Used here to mean a receptor or channel rendered photo-controllable using both a one synthetic photoswitch and an engineered receptor. For examplechemical optogenetics.
- PTL - photoswitchable tethered ligand
A photoswitchable ligand that is covalently attached to its target protein such that one photoisomer binds significantly better than the other.
- PORTL - photoswitchable orthogonal remotely tethered ligand
Conceptually a cross between a PCL and PTLit covalently binds to the target protein, but with a long tether, so photoisomerization affects binding efficacy and not access to the binding site.
- LiGluRs
A collection of engineered kainate receptors channels designed to become light-sensitive when a PTL covalently binds.
- LimGluRs
A collection of engineered group 2/3 glutamate receptor GPCRs designed to become light-sensitive when a PTL covalently binds.
- LiGABARs
A collection of engineered GABAAR channels designed to become light-sensitive when a PTL covalently binds.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: None.
References
- 1.Packer AM, Roska B, Häusser M. Targeting neurons and photons for optogenetics. Nat Neurosci. 2013;16:805–815. doi: 10.1038/nn.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sternson SM, Roth BL. Chemogenetic Tools to Interrogate Brain Functions. Annu Rev Neurosci. 2014;37:387–407. doi: 10.1146/annurev-neuro-071013-014048. [DOI] [PubMed] [Google Scholar]
- 3.Ellis-Davies GCR. Caged compounds: photorelease technology for control of cellular chemistry and physiology. Nat Methods. 2007;4:619–628. doi: 10.1038/nmeth1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go MA, Daria VR. Light-neuron interactions: key to understanding the brain. J Opt. 2017;19:023002. [Google Scholar]
- 5.Laprell L, Repak E, Franckevicius V, Hartrampf F, Terhag J, Hollmann M, Sumser M, Rebola N, DiGregorio DA, Trauner D. Optical control of NMDA receptors with a diffusible photoswitch. Nat Commun. 2015;6:8076. doi: 10.1038/ncomms9076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6*.Berlin S, Szobota S, Reiner A, Carroll EC, Kienzler MA, Guyon A, Xiao T, Trauner D, Isacoff EY. A family of photoswitchable NMDA receptors. eLife. 2016;5:e12040. doi: 10.7554/eLife.12040. This paper introduces subtype specific photo-agonism and -antagonism to NMDA receptors and demonstrates subtype specific contributions to synaptic plasticity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stawski P, Sumser M, Trauner D. A Photochromic Agonist of AMPA Receptors. Angew Chem Int Ed. 2012;51:5748–5751. doi: 10.1002/anie.201109265. [DOI] [PubMed] [Google Scholar]
- 8.Barber DM, Liu S-A, Gottschling K, Sumser M, Hollmann M, Trauner D. Optical control of AMPA receptors using a photoswitchable quinoxaline-2,3-dione antagonist. Chem Sci. 2017;8:611–615. doi: 10.1039/c6sc01621a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reiner A, Levitz J, Isacoff EY. Controlling ionotropic and metabotropic glutamate receptors with light: principles and potential. Curr Opin Pharmacol. 2015;20:135–143. doi: 10.1016/j.coph.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10**.Levitz J, Popescu AT, Reiner A, Isacoff EY. A Toolkit for Orthogonal and in vivo Optical Manipulation of Ionotropic Glutamate Receptors [Internet] Front Mol Neurosci. 2016;9 doi: 10.3389/fnmol.2016.00002. This paper shows that fine tuning of a chemical optogenetic system’s properties via modification of the receptor and/or photoswitch is feasible, even in awake behaving mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiner A, Isacoff EY. Tethered ligands reveal glutamate receptor desensitization depends on subunit occupancy. Nat Chem Biol. 2014;10:273–280. doi: 10.1038/nchembio.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levitz J, Pantoja C, Gaub B, Janovjak H, Reiner A, Hoagland A, Schoppik D, Kane B, Stawski P, Schier AF, et al. Optical control of metabotropic glutamate receptors. Nat Neurosci. 2013;16:507–516. doi: 10.1038/nn.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Levitz J, Habrian C, Bharill S, Fu Z, Vafabakhsh R, Isacoff EY. Mechanism of Assembly and Cooperativity of Homomeric and Heteromeric Metabotropic Glutamate Receptors. Neuron. 2016;92:143–159. doi: 10.1016/j.neuron.2016.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li D, Hérault K, Zylbersztejn K, Lauterbach MA, Guillon M, Oheim M, Ropert N. Astrocyte VAMP3 vesicles undergo Ca2+-independent cycling and modulate glutamate transporter trafficking. J Physiol. 2015;593:2807–2832. doi: 10.1113/JP270362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pittolo S, Gómez-Santacana X, Eckelt K, Rovira X, Dalton J, Goudet C, Pin J-P, Llobet A, Giraldo J, Llebaria A, et al. An allosteric modulator to control endogenous G protein-coupled receptors with light. Nat Chem Biol. 2014;10:813–815. doi: 10.1038/nchembio.1612. [DOI] [PubMed] [Google Scholar]
- 16.Gómez-Santacana X, Dalton JAR, Rovira X, Pin JP, Goudet C, Gorostiza P, Giraldo J, Llebaria A. Positional isomers of bispyridine benzene derivatives induce efficacy changes on mGlu5 negative allosteric modulation. Eur J Med Chem. 2017;127:567–576. doi: 10.1016/j.ejmech.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 17*.Rovira X, Trapero A, Pittolo S, Zussy C, Faucherre A, Jopling C, Giraldo J, Pin J-P, Gorostiza P, Goudet C, et al. OptoGluNAM4.1, a Photoswitchable Allosteric Antagonist for Real-Time Control of mGlu4 Receptor Activity. Cell Chem Biol. 2016;23:929–934. doi: 10.1016/j.chembiol.2016.06.013. Compound OptoGluNAM4.1 provides permanent photocontrol of endogenous mGluR4, allowing modulation l of larval zebrafish swimming behavior. [DOI] [PubMed] [Google Scholar]
- 18.Stein M, Middendorp SJ, Carta V, Pejo E, Raines DE, Forman SA, Sigel E, Trauner D. Azo-Propofols: Photochromic Potentiators of GABAA Receptors. Angew Chem Int Ed. 2012;51:10500–10504. doi: 10.1002/anie.201205475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yue L, Pawlowski M, Dellal SS, Xie A, Feng F, Otis TS, Bruzik KS, Qian H, Pepperberg DR. Robust photoregulation of GABAA receptors by allosteric modulation with a propofol analogue. Nat Commun. 2012;3:1095. doi: 10.1038/ncomms2094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huckvale R, Mortensen M, Pryde D, Smart TG, Baker JR. Azogabazine; a photochromic antagonist of the GABA A receptor. Org Biomol Chem. 2016;14:6676–6678. doi: 10.1039/c6ob01101b. [DOI] [PubMed] [Google Scholar]
- 21**.Lin W-C, Tsai M-C, Davenport CM, Smith CM, Veit J, Wilson NM, Adesnik H, Kramer RH. A Comprehensive Optogenetic Pharmacology Toolkit for In Vivo Control of GABAA Receptors and Synaptic Inhibition. Neuron. 2015;88:879–891. doi: 10.1016/j.neuron.2015.10.026. This paper demonstrates isoform specific photocontrol for all α isoforms of GABAA to control synaptic inhibition, most notably using photoswitch ready transgenic mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin W-C, Davenport CM, Mourot A, Vytla D, Smith CM, Medeiros KA, Chambers JJ, Kramer RH. Engineering a Light-Regulated GABAA Receptor for Optical Control of Neural Inhibition. ACS Chem Biol. 2014;9:1414–1419. doi: 10.1021/cb500167u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quandt G, Höfner G, Pabel J, Dine J, Eder M, Wanner KT. First Photoswitchable Neurotransmitter Transporter Inhibitor: Light-Induced Control of γ-Aminobutyric Acid Transporter 1 (GAT1) Activity in Mouse Brain. J Med Chem. 2014;57:6809–6821. doi: 10.1021/jm5008566. [DOI] [PubMed] [Google Scholar]
- 24.Damijonaitis A, Barber DM, Trauner D. The photopharmacology of nicotinic acetylcholine receptors [Internet] Neurotransmitter. 2016;3 [Google Scholar]
- 25.Tochitsky I, Banghart MR, Mourot A, Yao JZ, Gaub B, Kramer RH, Trauner D. Optochemical control of genetically engineered neuronal nicotinic acetylcholine receptors. Nat Chem. 2012;4:105–111. doi: 10.1038/nchem.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Damijonaitis A, Broichhagen J, Urushima T, Hüll K, Nagpal J, Laprell L, Schönberger M, Woodmansee DH, Rafiq A, Sumser MP, et al. AzoCholine Enables Optical Control of Alpha 7 Nicotinic Acetylcholine Receptors in Neural Networks. ACS Chem Neurosci. 2015;6:701–707. doi: 10.1021/acschemneuro.5b00030. [DOI] [PubMed] [Google Scholar]
- 27.Broichhagen J, Jurastow I, Iwan K, Kummer W, Trauner D. Optical Control of Acetylcholinesterase with a Tacrine Switch. Angew Chem Int Ed. 2014;53:7657–7660. doi: 10.1002/anie.201403666. [DOI] [PubMed] [Google Scholar]
- 28.Chen X, Wehle S, Kuzmanovic N, Merget B, Holzgrabe U, König B, Sotriffer CA, Decker M. Acetylcholinesterase Inhibitors with Photoswitchable Inhibition of β-Amyloid Aggregation. ACS Chem Neurosci. 2014;5:377–389. doi: 10.1021/cn500016p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barber DM, Schönberger M, Burgstaller J, Levitz J, David Weaver C, Isacoff EY, Baier H, Trauner D. Optical control of neuronal activity using a light-operated GIRK channel opener (LOGO) Chem Sci. 2016;7:2347–2352. doi: 10.1039/c5sc04084a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frank JA, Moroni M, Moshourab R, Sumser M, Lewin GR, Trauner D. Photoswitchable fatty acids enable optical control of TRPV1. Nat Commun. 2015;6:7118. doi: 10.1038/ncomms8118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stein M, Breit A, Fehrentz T, Gudermann T, Trauner D. Optical Control of TRPV1 Channels. Angew Chem Int Ed. 2013;52:9845–9848. doi: 10.1002/anie.201302530. [DOI] [PubMed] [Google Scholar]
- 32.Kokel D, Cheung CYJ, Mills R, Coutinho-Budd J, Huang L, Setola V, Sprague J, Jin S, Jin YN, Huang X-P, et al. Photochemical activation of TRPA1 channels in neurons and animals. Nat Chem Biol. 2013;9:257–263. doi: 10.1038/nchembio.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33*.Browne LE, Nunes JPM, Sim JA, Chudasama V, Bragg L, Caddick S, North RA. Optical control of trimeric P2X receptors and acid-sensing ion channels. Proc Natl Acad Sci. 2014;111:521–526. doi: 10.1073/pnas.1318582111. See annotation Habermacher et al. (2016) [ref] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34*.Habermacher C, Martz A, Calimet N, Lemoine D, Peverini L, Specht A, Cecchini M, Grutter T. Photo-switchable tweezers illuminate pore-opening motions of an ATP-gated P2X ion channel. eLife. 2016;5:e11050. doi: 10.7554/eLife.11050. Together with Browne et al. (2014) [ref], these papers report the allosteric action of photoswitchable “molecular tweezers” to open and close a channel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shafaat OS, Winkler JR, Gray HB, Dougherty DA. Photoactivation of an Acid-Sensitive Ion Channel Associated with Vision and Pain. ChemBioChem. 2016;17:1323–1327. doi: 10.1002/cbic.201600230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaub BM, Berry MH, Holt AE, Reiner A, Kienzler MA, Dolgova N, Nikonov S, Aguirre GD, Beltran WA, Flannery JG, et al. Restoration of visual function by expression of a light-gated mammalian ion channel in retinal ganglion cells or ON-bipolar cells. Proc Natl Acad Sci. 2014;111:E5574–E5583. doi: 10.1073/pnas.1414162111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tochitsky I, Helft Z, Meseguer V, Fletcher RB, Vessey KA, Telias M, Denlinger B, Malis J, Fletcher EL, Kramer RH. How Azobenzene Photoswitches Restore Visual Responses to the Blind Retina. Neuron. 2016;92:100–113. doi: 10.1016/j.neuron.2016.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tochitsky I, Kramer RH. Optopharmacological tools for restoring visual function in degenerative retinal diseases. Curr Opin Neurobiol. 2015;34:74–78. doi: 10.1016/j.conb.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Laprell L, Hüll K, Stawski P, Schön C, Michalakis S, Biel M, Sumser MP, Trauner D. Restoring Light Sensitivity in Blind Retinae Using a Photochromic AMPA Receptor Agonist. ACS Chem Neurosci. 2016;7:15–20. doi: 10.1021/acschemneuro.5b00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schönberger M, Trauner D. A Photochromic Agonist for μ-Opioid Receptors. Angew Chem Int Ed. 2014;53:3264–3267. doi: 10.1002/anie.201309633. [DOI] [PubMed] [Google Scholar]
- 41*.Frank JA, Yushchenko DA, Hodson DJ, Lipstein N, Nagpal J, Rutter GA, Rhee J-S, Gottschalk A, Brose N, Schultz C, et al. Photoswitchable diacylglycerols enable optical control of protein kinase C. Nat Chem Biol. 2016;12:755–762. doi: 10.1038/nchembio.2141. A set of PCLs are used to control several diacylglycerol dependent cell processes including protein kinase C activity and vesicle release in hippocampal neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reis SA, Ghosh B, Hendricks JA, Szantai-Kis DM, Törk L, Ross KN, Lamb J, Read-Button W, Zheng B, Wang H, et al. Light-controlled modulation of gene expression by chemical optoepigenetic probes. Nat Chem Biol. 2016;12:317–323. doi: 10.1038/nchembio.2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Szymanski W, Ourailidou ME, Velema WA, Dekker FJ, Feringa BL. Light-Controlled Histone Deacetylase (HDAC) Inhibitors: Towards Photopharmacological Chemotherapy. Chem – Eur J. 2015;21:16517–16524. doi: 10.1002/chem.201502809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dong M, Babalhavaeji A, Samanta S, Beharry AA, Woolley GA. Red-Shifting Azobenzene Photoswitches for in Vivo Use. Acc Chem Res. 2015;48:2662–2670. doi: 10.1021/acs.accounts.5b00270. [DOI] [PubMed] [Google Scholar]
- 45.Bléger D, Hecht S. Visible-Light-Activated Molecular Switches. Angew Chem Int Ed. 2015;54:11338–11349. doi: 10.1002/anie.201500628. [DOI] [PubMed] [Google Scholar]
- 46.Rullo A, Reiner A, Reiter A, Trauner D, Isacoff EY, Woolley GA. Long wavelength optical control of glutamate receptor ion channels using a tetra- ortho -substituted azobenzene derivative. Chem Commun. 2014;50:14613–14615. doi: 10.1039/c4cc06612j. [DOI] [PubMed] [Google Scholar]
- 47.Kienzler MA, Reiner A, Trautman E, Yoo S, Trauner D, Isacoff EY. A Red-Shifted, Fast-Relaxing Azobenzene Photoswitch for Visible Light Control of an Ionotropic Glutamate Receptor. J Am Chem Soc. 2013;135:17683–17686. doi: 10.1021/ja408104w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48*.Izquierdo-Serra M, Gascón-Moya M, Hirtz JJ, Pittolo S, Poskanzer KE, Ferrer È, Alibés R, Busqué F, Yuste R, Hernando J, et al. Two-Photon Neuronal and Astrocytic Stimulation with Azobenzene-Based Photoswitches. J Am Chem Soc. 2014;136:8693–8701. doi: 10.1021/ja5026326. See annotation Carroll et al. (2015) [ref] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49*.Carroll EC, Berlin S, Levitz J, Kienzler MA, Yuan Z, Madsen D, Larsen DS, Isacoff EY. Two-photon brightness of azobenzene photoswitches designed for glutamate receptor optogenetics. Proc Natl Acad Sci. 2015;112:E776–E785. doi: 10.1073/pnas.1416942112. Together with Izquierdo-Serra et al (2014) [ref], these papers demonstrate 2P photoswitching of ionotropic and metabotropic glutamate receptors, improving both the 3D spatial precision and tissue penetration of required light. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50**.Levitz J, Broichhagen J, Leippe P, Konrad D, Trauner D, Isacoff EY. Dual optical control and mechanistic insights into photoswitchable group II and III metabotropic glutamate receptors. Proc Natl Acad Sci. 2017;114:E3546–E3554. doi: 10.1073/pnas.1619652114. This paper uses multiple PORTLs to install orthogonal photocontrol in genetically targeted receptors simultaneously. Several new engineered group 2/3 metabotropic glutamate receptors are presented. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51*.Izquierdo-Serra M, Bautista-Barrufet A, Trapero A, Garrido-Charles A, Díaz-Tahoces A, Camarero N, Pittolo S, Valbuena S, Pérez-Jiménez A, Gay M, et al. Optical control of endogenous receptors and cellular excitability using targeted covalent photoswitches. Nat Commun. 2016;7:12221. doi: 10.1038/ncomms12221. The authors disclose PTLs that allow photoaffinity labeling of endogenous kainate receptors, photosensitizing neurons in blind mouse retinas. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Broichhagen J, Damijonaitis A, Levitz J, Sokol KR, Leippe P, Konrad D, Isacoff EY, Trauner D. Orthogonal Optical Control of a G Protein-Coupled Receptor with a SNAP-Tethered Photochromic Ligand. ACS Cent Sci. 2015;1:383–393. doi: 10.1021/acscentsci.5b00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spangler SM, Bruchas MR. Optogenetic approaches for dissecting neuromodulation and GPCR signaling in neural circuits. Curr Opin Pharmacol. 2017;32:56–70. doi: 10.1016/j.coph.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lerch MM, Hansen MJ, Velema WA, Szymanski W, Feringa BL. Orthogonal photoswitching in a multifunctional molecular system. Nat Commun. 2016;7:12054. doi: 10.1038/ncomms12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoppmann C, Lacey VK, Louie GV, Wei J, Noel JP, Wang L. Genetically Encoding Photoswitchable Click Amino Acids in Escherichia coli and Mammalian Cells. Angew Chem Int Ed. 2014;53:3932–3936. doi: 10.1002/anie.201400001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Platt RJ, Chen S, Zhou Y, Yim MJ, Swiech L, Kempton HR, Dahlman JE, Parnas O, Eisenhaure TM, Jovanovic M, et al. CRISPR-Cas9 Knockin Mice for Genome Editing and Cancer Modeling. Cell. 2014;159:440–455. doi: 10.1016/j.cell.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gazerani P. Shedding light on photo-switchable analgesics for pain. Pain Manag. 2016;7:71–74. doi: 10.2217/pmt-2016-0039. [DOI] [PubMed] [Google Scholar]
- 58.Lerch MM, Hansen MJ, van Dam GM, Szymanski W, Feringa BL. Emerging Targets in Photopharmacology. Angew Chem Int Ed. 2016;55:10978–10999. doi: 10.1002/anie.201601931. [DOI] [PubMed] [Google Scholar]
- 59.Broichhagen J, Frank JA, Trauner D. A Roadmap to Success in Photopharmacology. Acc Chem Res. 2015;48:1947–1960. doi: 10.1021/acs.accounts.5b00129. [DOI] [PubMed] [Google Scholar]


