Abstract
The understanding that recovery of brain function after stroke is imperfect has prompted decades of effort to engender speedier and better recovery through environmental manipulation. Clinical evidence has shown that the performance plateau exhibited by patients with chronic stroke, usually signaling an end of standard rehabilitation, might represent a period of consolidation rather than a performance optimum. These results highlight the difficulty of translating pertinent neurological data into pragmatic changes in clinical programs. This opinion piece focuses on upper limb impairment reduction after robotic training. We propose that robotic devices be considered as novel tools that might be used alone or in combination with novel pharmacology and other bioengineered devices. Additionally, robotic devices can measure motor performance objectively and will contribute to a detailed phenotype of stroke recovery.
Attempts to improve recovery after stroke have quickened because the prevalence and incidence of those with stroke disability has increased, driven by an aging population and improved survival after the initial injury. The phenotype of the post-stroke condition is based on the neurological deficit that comprises a complex interaction of cognitive and sensorimotor impairments and that depends on the size and location of the brain injury. Standard treatments for the sensorimotor impairment focus, in part, on teaching patients to use the so-called unaffected limbs to adapt, compensate, and, especially, improve motor abilities with respect to feeding, grooming, and toileting. Lower extremity function, in particular walking a few steps, even with a prosthesis or assistance, shows more reliable improvement than upper extremity function. Treatment of the affected upper limb is a tedious and difficult process that occurs in short episodes and concentrates on passive movement, especially for patients with moderate to severe stroke. As it turns out, passive movement is best suited for maintaining joint integrity.1 Although there is compelling evidence that specialized stroke recovery units provide care that leads to decreased mortality and morbidity, there is less agreement on which particular treatment program is superior, or even that significant recovery beyond standard treatment is possible. Most change from the acute state occurs within weeks, but smaller incremental improvement may occur later and has been shown to result from additional intervention with a variety of protocols and devices.
The extent of the functional recovery occurring after training with robotic devices needs to stand the test of multicenter randomized control trials.2 Nevertheless, the available literature on robotic studies demonstrates clear incremental reductions of motor impairment that offer the opportunity to build a better outcome.3,4 Furthermore, when looking at the ensemble of robotic studies, several issues come to the fore. Intensive treatment protocols for sensorimotor impairment have demonstrated benefit compared with standard care.5–7 Motor learning principles suggest that to optimize the outcome of motor training, distributed training (timing and session scheduling given over longer intervals) will be more effective than massed training (scheduled within narrow time windows). Incidentally, distributed training also guarantees that an adequate challenge occurs throughout treatment, thus maintaining task interest.8,9 Robots are tireless agents that produce reliable, highly reproducible control of movement sequences and, thus, act as tools to lighten the workload of intensive training protocols. Another issue is whether clinical scales adequately capture the variability that occurs among patients with similar brain lesions. Robotic devices can measure the kinematics and dynamics of movement performance objectively and, coupled with neuroimaging methods that capture brain blood flow or metabolic activity,10–15 will provide a richer description of stroke phenotype. Amore objective phenotype will become more important as we understand the genotypic differences that influence recovery.16 A final issue is whether standard rehabilitation techniques should include robotic training in combination with electromyographic triggering, transcranial direct current stimulation (tDCS), transcranial magnetic stimulation, and pharmacological sensitization.17–22
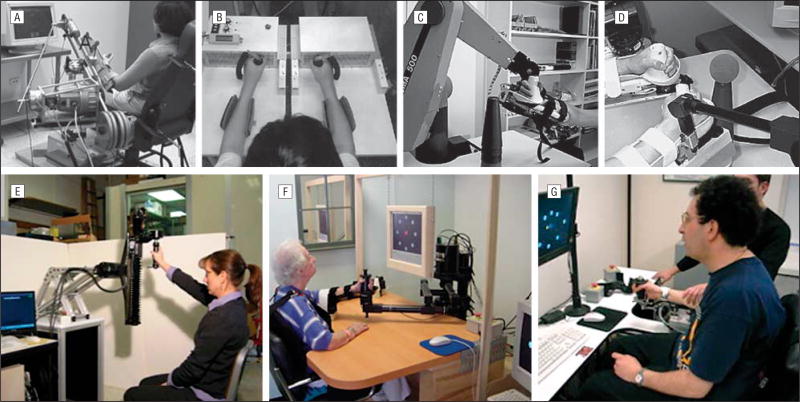
Some time ago, a group of engineers and clinicians met to discuss the range of robotic devices that could potentially be used in stroke recovery (Figure 1). This led to the first robotic treatments of patients; some occurred within weeks, others occurred within months of stroke, in a rehabilitation setting, to measure whether robotic devices would improve recovery.24,26,28–32 The trials were encouraging and, although the numbers of patients were relatively small, a recent meta-analysis demonstrated significant improvement for those trained with devices that targeted movement of the shoulders and elbows.3 Training with devices that targeted the wrists and hands, and the translation into “functional abilities,” was less convincing.3
Figure 1.
Different robotic devices. A, The assisted rehabilitation measurement and guide device23 requires that the patient move the end of the robotic arm. The position and speed of movement of the robotic arm are represented by a cursor on a video screen, and the reaching movements are executed in one direction at a time. If the patient cannot move the robotic arm, the device will assist. B, The robot-assisted bilateral arm trainer24 requires the patient to attempt to move the affected and unaffected limbs, including wrist pronation and supination, at the same time in response to visual cues. This device moves the patients’ limbs. C and D, The Mirror Image Movement Enabler (MIME) robotic device25 can be used for the affected limb or paired with a second robotic arm to execute mirror movements with the affected and unaffected limbs simultaneously. If the patient cannot execute the movement, the device will assist. E–G, For each of the Massachusetts Institute of Technology (MIT)–Manus devices, a patient views a video screen and moves the end of the robotic arm. A cursor on a video screen represents the position, direction, and speed of the movement of the robotic arm. Computers connected to the robot record the position, speed, and forces or torques of a patient’s complete movement history during the training. The task is to make point-to-point movements, which the robot can also assist by correcting the path or increasing the speed of movement to the target. The MIT-Manus devices are back-drivable, which means the robot “gets out of the way” of a movement so that the patient experiences a device that moves easily even with weak forces.26 A spatial extension device expands an MIT-Manus shoulder/elbow device to train movements of the arm in the antigravity plane27 and to train rotator cuff and scapulation movements (E). A shoulder/elbow MIT-Manus device trains elbow and shoulder flexion and extension and shoulder abduction and adduction (F).26,28,29 The wrist device expansion of the shoulder/elbow MIT-Manus trains wrist pronation, supination, flexion, extension, ulnar, and radial movements (G).
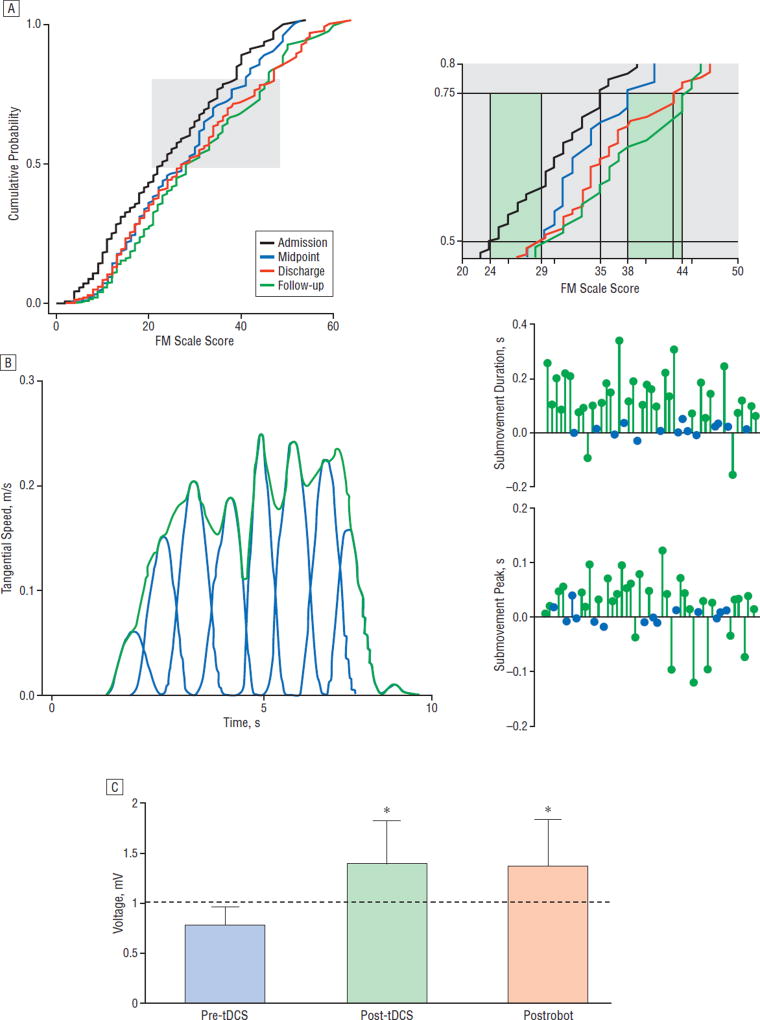
In our restorative neurology center at the Burke-Cornell Medical Research Institute, the treatment with robotic devices has included patients within days of their stroke, together with patients with chronic stroke (greater than 6 months from injury). Our ongoing results suggest that robotic training influences motor learning, a notion that is strengthened by the very rapid improvement that occurs at the treatment’s onset and, also, by the sustained improvement the patients exhibit even months after the training has ended. For example, we analyzed the performance of 248 patients (mean age, 62.3 years; range, 17–89 years; 5 days-11.3 years after stroke) who participated in robotic training for at least 18 sessions. The impairment level of the upper extremity was estimated by the Fugl-Meyer Motor (FM) scale (a reliable and standard clinical scale of movement performance; maximum score, 66; lower scores indicate more severe impairment) and the scores were used to generate cumulative probability distributions (CPDs) for the different points of the training (Figure 2A). On admission to the treatment, the patients’ degree of motor impairments spanned nearly the complete range of the upper extremity FM scale (range, 0–54). Inspection of the CPDs at the 0.5 level (Figure 2A) revealed an improvement of 5 points between the admission and midpoint evaluation. Crucially, a 3-point improvement in the FM scale score has been shown to have a significant impact on disability.34 Still at the 0.5 level, the CPDs showed little change from midpoint to treatment discharge, but the gain was maintained in the 3-month follow-up. Examining the CPDs at the 0.75 level (Figure 2A) revealed a 3-point improvement from admission to midpoint evaluation and yet a further improvement by discharge and again at follow-up. Remarkably, the total improvement from admission to follow-up was 9 points. The timing and degree of motor changes in response to robotic training may thus be instructive. Shorter robotic training periods might be productive for patients with lower admission FM scale scores and greater upper extremity impairment. Conversely, longer training periods resulted in stepwise improvements that continued after the training had ended for those with less severe impairment (higher admission scores). For some patients with chronic stroke, these overall results suggest that a plateau performance during standard therapy may belie a reserve brain recovery potential. Whether bursts of improvement, as demonstrated by some of the patients who improved after the robotic training ended, depend on some critical level of performance that effectively incorporates the affected limb more often in natural circumstances remains to be determined.
Figure 2.
Results from treatments using robotic devices. A, Cumulative probability distributions of total Fugl-Meyer Motor (FM) scale scores (range, most severe=0 to less severe=54; maximum, 66) for patients evaluated at treatment admission, midpoint, treatment discharge, and follow-up 3 months after robotic training has ended. The curve representing this group of treated patients shifts to higher FM scores at the midpoint of training and it shifts to the right at discharge and follow-up indicating progressive increase of motor ability as the FM scores increase. The improvement after training is significant (Kolmogorov-Smirnov test, P<.001). Closer inspection of the cumulative probability values between 0.5 and 0.75 (right side of part A) are taken from the gray region depicted in the main graph. At the 0.5 level, admission to midpoint captures most of the improvement. At the 0.75 level, the improvement occurs after the midpoint evaluation and again at follow-up. For some patients with less severe impairment, even an intensive training experience did not define a performance optimum, as there was additional improvement after discharge. For other patients, the dose of training needs to be optimized. B, The left panel demonstrates the speed with which the patient executes an untrained movement at the start of training. The movement can be decomposed into submovements. The right panels depict an analysis of the individual submovements and, in particular, the peak speed and the duration of each submovement. A patient’s performance on this untrained task is represented by a line from the baseline performance connected to a green (P<.05) or a blue circle (P>.05), and each circle represents the discharge value. At discharge, most patients demonstrate longer submovements that are executed more quickly. These changes in submovements reflect smoother movement.10 C, Transcranial magnetic stimulation generates motor evoked potentials (MEPs) in the flexor carpi radialis (N=6 patients, bars represent mean [SEM]). The MEPs are recorded from the flexor carpi radialis muscle during a low-level isometric wrist flexion, before and immediately following 20-minute anodal transcranial direct current stimulation (tDCS), then again after 1 hour of robotic wrist therapy. Following tDCS, MEP amplitude is significantly elevated (*P<.05) and remains significantly elevated after robotic therapy (*P<.05), indicating integrity and potentially increased efficiency within the corticomotor pathways.33
Many investigators worry about the subjective nature of the FM scale, and the field of restorative neurology desperately needs objective outcome data. Among the objective parameters recorded by robotic devices, we offer submovements as a reasonable candidate that captures velocity and position information and represents an essential building block of motor performance. The robotic devices record speed and position constantly, and in this manner, they provide a longitudinal kinematic performance history. In a robotic study of stroke patients (N=47, all greater than 6 months after stroke), the submovement profiles were extracted (Figure 2B) from unassisted movements performed by each patient treated with interactive robotic devices. By discharge, the submovements grew taller and longer and became less numerous (Figure 2B). This submovement analysis was commensurate with improved task performance and suggested that the form of the movement contributed importantly to the function10 (Figure 2B, right panels). Improving smoothness of movement may begin to define objective criteria with which to track recovery or effectiveness of new treatment. The outliers who responded poorly, or not at all, suggest that a specific treatment approach, whether robotic treatment or standard therapy, might be better tailored to detailed movement failures. It would also be very useful to include correlative neuroimaging information.
In a recent study, we treated patients with chronic stroke (N=6; on average 4.7 years from stroke) with tDCS (anode over the affected hemisphere, 1 mA) for 20 minutes prior to robotic training. A motor evoked potential (by transcranial magnetic stimulation) in the flexor carpi radialis of the affected limb remained facilitated for the entire treatment34 (Figure 2C). Although a recent pilot experiment that exposed stroke patients (4–8 weeks of stroke) to robotic training (20-minute sessions for 30 trials) and tDCS (1.5mAfor the initial 7 minutes of training) was not successful,22 further studies are needed to determine whether prior tDCS, or simultaneous tCDS of longer duration, may have an effect on outcome.
This selected glimpse into the confluence of bioengineering and restorative neurology suggests that the opportunity to reduce impairment lasts longer than formerly thought. It is also obvious that therapists should consider arming themselves with some new tools. Robotic devices create the possibility for objective kinematic and dynamic metrics in a view of stroke recovery that includes the measurement of form and structure of movement. These measurements, in turn, can render a richer and more complete phenotype for stroke recovery.
Acknowledgments
Financial Disclosure: Drs Hogan and Krebs are coin-ventors of the Massachusetts Institute of Technology– held patent for the robotic devices used to treat patients in this work. They hold equity positions in Interactive Motion Technologies, Inc, a company that manufactures this type of technology under license to the Massachusetts Institute of Technology.
Funding/Support: This work was supported by National Institutes of Health grant HD043343, Skirball Foundation, and Burke Medical Research Institute.
Footnotes
Author Contributions: Study concept and design: Volpe, Huerta, Edwards, Hogan, and Krebs. Acquisition of data: Volpe, Zipse, Rykman, Hogan, and Krebs. Analysis and interpretation of data: Volpe, Huerta, Rykman, Dipietro, Hogan, and Krebs. Drafting of the manuscript: Volpe, Huerta, Zipse, and Edwards. Critical revision of the manuscript for important intellectual content: Volpe, Huerta, Rykman, Dipietro, Hogan, and Krebs. Statistical analysis: Volpe, Huerta, and Dipietro. Obtained funding: Volpe, Huerta, and Hogan. Administrative, technical, and material support: Huerta, Zipse, Rykman, Edwards, Dipietro, and Hogan. Study supervision: Volpe, Hogan, and Krebs.
References
- 1.Lynch D, Ferraro M, Krol J, Trudell CM, Christos P, Volpe BT. Continuous passive motion improves shoulder joint integrity following stroke. Clin Rehabil. 2005;19(6):594–599. doi: 10.1191/0269215505cr901oa. [DOI] [PubMed] [Google Scholar]
- 2.Lo A, Guarino P, Krebs HI, et al. Multi-center randomized trial of robot-assisted rehabilitation for chronic stroke: methods and entry characteristics for VA ROBOTICS. Neurorehabil Neural Repair. doi: 10.1177/1545968309338195. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kwakkel G, Kollen BJ, Krebs HI. Effects of robot-assisted therapy on upper limb recovery after stroke: a systematic review. Neurorehabil Neural Repair. 2008;22(2):111–121. doi: 10.1177/1545968307305457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prange GB, Jannink MJ, Groothuis-Oudshoorn CG, Hermens HJ, Ijzerman MJ. Systematic review of the effect of robot-aided therapy on recovery of the hemiparetic arm after stroke. J Rehabil Res Dev. 2006;43(2):171–184. doi: 10.1682/jrrd.2005.04.0076. [DOI] [PubMed] [Google Scholar]
- 5.Barker RN, Brauer SG, Carson RG. Training of reaching in stroke survivors with severe and chronic upper limb paresis using a novel nonrobotic device: a randomized clinical trial. Stroke. 2008;39(6):1800–1807. doi: 10.1161/STROKEAHA.107.498485. [DOI] [PubMed] [Google Scholar]
- 6.Feys H, De Weerdt W, Verbeke G, et al. Early and repetitive stimulation of the arm can substantially improve the long-term outcome after stroke: a 5-year follow- up study of a randomized trial. Stroke. 2004;35(4):924–929. doi: 10.1161/01.STR.0000121645.44752.f7. [DOI] [PubMed] [Google Scholar]
- 7.McCombe Waller S, Whitall J. Bilateral arm training: why and who benefits? NeuroRehabilitation. 2008;23(1):29–41. [PMC free article] [PubMed] [Google Scholar]
- 8.Hogan N, Krebs HI, Rohrer B, et al. Motions or muscles? some behavioral factors underlying robotic assistance of motor recovery. J Rehabil Res Dev. 2006;43(5):605–618. doi: 10.1682/jrrd.2005.06.0103. [DOI] [PubMed] [Google Scholar]
- 9.Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol. 2006;19(1):84–90. doi: 10.1097/01.wco.0000200544.29915.cc. [DOI] [PubMed] [Google Scholar]
- 10.Dipietro L, Krebs HI, Fasoli SE, Volpe BT, Hogan N. Submovement changes characterize generalization of motor recovery after stroke. Cortex. 2009;45(3):318–324. doi: 10.1016/j.cortex.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Dipietro L, Krebs HI, Fasoli SE, et al. Changing motor synergies in chronic stroke. J Neurophysiol. 2007;98(2):757–768. doi: 10.1152/jn.01295.2006. [DOI] [PubMed] [Google Scholar]
- 12.Krebs HI, Aisen ML, Volpe BT, Hogan N. Quantization of continuous arm movements in humans with brain injury. Proc Natl Acad Sci U S A. 1999;96(8):4645–4649. doi: 10.1073/pnas.96.8.4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prabhakaran S, Zarahn E, Riley C, et al. Inter-individual variability in the capacity for motor recovery after ischemic stroke. Neurorehabil Neural Repair. 2008;22(1):64–71. doi: 10.1177/1545968307305302. [DOI] [PubMed] [Google Scholar]
- 14.Rohrer B, Fasoli S, Krebs HI, et al. Movement smoothness changes during stroke recovery. J Neurosci. 2002;22(18):8297–8304. doi: 10.1523/JNEUROSCI.22-18-08297.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lazar RM, Speizer AE, Festa JR, Krakauer JW, Marshall RS. Variability in language recovery after first-time stroke. J Neurol Neurosurg Psychiatry. 2008;79(5):530–534. doi: 10.1136/jnnp.2007.122457. [DOI] [PubMed] [Google Scholar]
- 16.Kleim JA, Chan S, Pringle E, et al. BDNF val66met polymorphism is associated with modified experience-dependent plasticity in human motor cortex. Nat Neurosci. 2006;9(6):735–737. doi: 10.1038/nn1699. [DOI] [PubMed] [Google Scholar]
- 17.Bolton DA, Cauraugh JH, Hausenblas HA. Electromyogram-triggered neuromuscular stimulation and stroke motor recovery of arm/hand functions: a meta-analysis. J Neurol Sci. 2004;223(2):121–127. doi: 10.1016/j.jns.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 18.Daly JJ, Roenigk K, Holcomb J, et al. A randomized controlled trial of functional neuromuscular stimulation in chronic stroke subjects. Stroke. 2006;37(1):172–178. doi: 10.1161/01.STR.0000195129.95220.77. [DOI] [PubMed] [Google Scholar]
- 19.Edwards DJ. On the understanding and development of modern physical neurorehabilitation methods: robotics and non-invasive brain stimulation. J Neuroeng Rehabil. 2009;6:3. doi: 10.1186/1743-0003-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacDonald E, Van der Lee H, Pocock D, et al. A novel phosphodiesterase type 4 inhibitor, HT-0712, enhances rehabilitation-dependent motor recovery and cortical reorganization after focal cortical ischemia. Neurorehabil Neural Repair. 2007;21(6):486–496. doi: 10.1177/1545968307305521. [DOI] [PubMed] [Google Scholar]
- 21.Rose GM, Hopper A, De Vivo M, Tehim A. Phosphodiesterase inhibitors for cognitive enhancement. Curr Pharm Des. 2005;11(26):3329–3334. doi: 10.2174/138161205774370799. [DOI] [PubMed] [Google Scholar]
- 22.Hesse S, Werner C, Schonhardt EM, Bardeleben A, Jenrich W, Kirker SG. Combined transcranial direct current stimulation and robot-assisted arm training in subacute stroke patients: a pilot study. Restor Neurol Neurosci. 2007;25(1):9–15. [PubMed] [Google Scholar]
- 23.Kahn LE, Zygman ML, Rymer WZ, Reinkensmeyer DJ. Robot-assisted reaching exercise promotes arm movement recovery in chronic hemiparetic stroke: a randomized controlled pilot study. J Neuroeng Rehabil. 2006;3:12. doi: 10.1186/1743-0003-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hesse S, Schulte-Tigges G, Konrad M, Bardeleben A, Werner C. Robot-assisted arm trainer for the passive and active practice of bilateral forearm and wrist movements in hemiparetic subjects. Arch Phys Med Rehabil. 2003;84(6):915–920. doi: 10.1016/s0003-9993(02)04954-7. [DOI] [PubMed] [Google Scholar]
- 25.Lum PS, Burgar CG, Van der Loos M, Shor PC, Majmundar M, Yap R. MIME robotic device for upper-limb neurorehabilitation in subacute stroke subjects: a follow-up study. J Rehabil Res Dev. 2006;43(5):631–642. doi: 10.1682/jrrd.2005.02.0044. [DOI] [PubMed] [Google Scholar]
- 26.Hogan N, Krebs HI, Charnnarong J, Srikrishna P, Sharon A, editors. Proceedings, IEEE International Workshop on Robot and Human Communication, 1992. Tokyo, Japan: IEEE; 1992. MIT-MANUS: a workstation for manual therapy and training; pp. 161–165. [Google Scholar]
- 27.Krebs HI, Ferraro M, Buerger SP, et al. Rehabilitation robotics: pilot trial of a spatial extension for MIT-Manus. J Neuroeng Rehabil. 2004;1(1):5. doi: 10.1186/1743-0003-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aisen ML, Krebs HI, Hogan N, McDowell F, Volpe BT. The effect of robot-assisted therapy and rehabilitative training on motor recovery following stroke. Arch Neurol. 1997;54(4):443–446. doi: 10.1001/archneur.1997.00550160075019. [DOI] [PubMed] [Google Scholar]
- 29.Krebs HI, Hogan N, Aisen ML, Volpe BT. Robot-aided neurorehabilitation. IEEE Trans Rehabil Eng. 1998;6(1):75–87. doi: 10.1109/86.662623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burgar CG, Lum PS, Shor PC, Machiel Van der Loos HF. Development of robots for rehabilitation therapy: the Palo Alto VA/Stanford experience. J Rehabil Res Dev. 2000;37(6):663–673. [PubMed] [Google Scholar]
- 31.Lum PS, Burgar CG, Shor PC, Majmundar M, Van der Loos M. Robot-assisted movement training compared with conventional therapy techniques for the rehabilitation of upper-limb motor function after stroke. Arch Phys Med Rehabil. 2002;83(7):952–959. doi: 10.1053/apmr.2001.33101. [DOI] [PubMed] [Google Scholar]
- 32.Reinkensmeyer DJ, Kahn LE, Averbuch M, McKenna-Cole A, Schmit BD, Rymer WZ. Understanding and treating arm movement impairment after chronic brain injury: progress with the ARM guide. J Rehabil Res Dev. 2000;37(6):653–662. [PubMed] [Google Scholar]
- 33.Edwards DJ, Krebs HI, Rykman A, et al. Raised corticomotor excitability of M1 forearm area following anodal tDCS is sustained during robotic wrist therapy in chronic stroke. J Neuroeng Rehabil. doi: 10.3233/RNN-2009-0470. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duncan PW, Lai SM, Tyler D, Perera S, Reker DM, Studenski S. Evaluation of proxy responses to the Stroke Impact Scale. Stroke. 2002;33(11):2593–2599. doi: 10.1161/01.str.0000034395.06874.3e. [DOI] [PubMed] [Google Scholar]