Delirium is a common and costly problem, affecting up to 50% of hospitalized older adults, with estimated annual costs of $164 billion in the United States.1,2 Delirium is associated with cognitive decline, loss of functional independence, and increased mortality.1 Therefore, identifying individuals at risk for developing delirium, differentiating episodes of delirium likely to lead to lasting deleterious consequences, and minimizing the impact of delirium has assumed heightened importance. One central limitation preventing the achievement of these clinical goals is that current clinical definitions, such as the widely used Confusion Assessment Method (CAM3), are based on cognitive tests and bedside observations, and it is not completely clear how these signs and symptoms relate to the underlying brain dysfunction. Thus, a critical step is to understand, at a neurophysiologic level, why delirium happens in specific individuals. To this end, we begin by briefly reviewing the current literature on neurophysiologic investigations of delirium and other conditions that affect cognition, such as Alzheimer’s Disease (AD). We present a conceptual model advancing the hypothesis that delirium is due to a breakdown of normal brain function reflecting impairments in brain connectivity and plasticity. We explain how this model can be tested using the combination of Transcranial Magnetic Stimulation (TMS) and electroencephalograpy (EEG), and discuss some of the clinical implications of the model.
Neurophysiological Investigations of Delirium to Date
Neurophysiology during delirium has traditionally been studied using EEG, which measures the electrical fields produced by synchronized synaptic activity of cortical neurons. EEG activity is often divided into different spectral frequency bands, and changes in spectral band power (signal strength) have been reported in different disease states. The traditional spectral frequency bands evaluated during EEG recordings include delta (1 – 4 Hz), theta (4 – 8 Hz), alpha (8 – 13 Hz) and beta (13 – 30 Hz) bands; alpha activity is the most prominent rhythm in the resting awake state (eyes-closed). More recently, techniques have been developed to assess statistical correlations in the EEG signals recorded from different electrodes, that indicate connectivity between different brain regions. Typically, these measures fall into two broad categories: measures of functional connectivity, which identify correlations in the statistical properties of brain signals from two or more regions, or measures of effective connectivity, which attempt to identify causal interactions between regions.
Changes in EEG spectral power and connectivity have been identified in patients with diseases that affect cognition such as Mild Cognitive Impairment (MCI) and AD. In these conditions, loss of frontoparietal EEG connectivity is correlated with cognitive test results and disease progression over time.4 Extending this work in MCI and AD, EEG measures may be useful in characterizing cerebral dysfunction in patients at risk for delirium. EEG is also useful in understanding the physiologic changes that occur during delirium, when the most consistent neurophysiological abnormality is a relative slowing of resting-state EEG rhythms, with abnormally decreased background alpha power and increased theta- and delta-frequency activity.5,6 The degree of EEG slowing in delirium correlates with decline in performance on cognitive tests,6 and both EEG slowing and cognitive dysfunction normalize when metabolic derangements leading to delirium (e.g. hypoxemia, hypoglycemia) resolve.5 More recently, increased spectral variability,7 decreased complexity of EEG activity,7 and decreased EEG connectivity in the alpha band8 have also been reported during delirium. EEG changes can differentiate individuals with delirium from those without delirium with an estimated sensitivity of 83.3% and specificity of 77.8% for visual analysis of EEG features9, up to a sensitivity of 100% and specificity of 96% for a quantitative measure of EEG spectral power.10 Notably, EEG features may help differentiate patients with delirium and dementia from those with dementia alone with up to 83% accuracy.11 However, in these studies the specific measures were retrospectively identified. Prospective studies validating these measures against reference standard delirium ratings are needed. In current clinical practice, EEG is used to distinguish delirium from nonconvulsive status epilepticus or an underlying psychiatric condition.
Another method useful for analysis of functional connectivity is resting-state functional magnetic resonance imaging (rs-fMRI). In this method, brain activity is measured while the subject sits in the MRI in a resting state (not doing any tasks, in contrast to active functional neuroimaging); different brain regions that show correlated changes in blood oxygenation are said to be “connected” into functional networks. rs-fMRI may play an important role in identifying abnormal brain networks during delirium. Patients with delirium show a positive correlation between activity in the dorsolateral prefrontal cortex (DLPFC) and the posterior cingulate, whereas a negative correlation between these regions is typically seen in patients without delirium.12 Importantly, such abnormal correlations resolve after the episode of delirium ends. Abnormalities in brain resting-state connectivity have also been identified in hepatic encephalopathy, with improvements in brain connectivity seen after clinical improvement.13 Furthermore, alterations in resting-state fMRI connectivity have been reported in many other neurocognitive disorders, ranging from AD to schizophrenia. Despite these intriguing findings, the neurophysiological relationship between pre-existing brain function, delirium risk, and the effects of delirium on brain activity and cognitive function have not been well-explored.
A Conceptual Neurophysiological Model of Delirium
Based on the above results, we propose a conceptual model (TABLE 1; FIGURE 1) that delirium is the consequence of the breakdown in brain network dynamics induced by insults or stressors in individuals with baseline low brain resilience due to low connectivity and/or deficient mechanisms of neuroplasticity, such as may be present in AD. Neuroplasticity, defined as the brain’s ability to reorganize itself by forming new neural connections throughout life, allows the brain to compensate for injury and disease, and is often considered necessary for neurologic resilience (the ability to accommodate to or recover from a stressor).14 Relevant brain stressors can include major surgery, general anesthesia, systemic inflammation, infections, and psychotropic drugs. Health conditions that might result in impaired plasticity include pre-existing neurodegenerative disorders, such as MCI and AD,15 and comorbid conditions such as diabetes16 or renal impairment. This model predicts that when individuals are confronted with acute insults, these stressors will alter brain connectivity (e.g. within the dorsal attention network17) and/or brain network dynamics (e.g. the relationship between dorsolateral prefrontal cortex activity and posterior cingulate activity12), resulting in symptoms of delirium such as inattention. This impact will be greater in individuals with pre-existing deficits in brain connectivity, specifically in the brain networks involved in resilience – which are linked to the construct of cognitive reserve.18 Such alterations in brain connectivity and dynamics will be inadequately compensated in a brain with impaired plasticity, manifesting as the clinical syndrome of delirium. Supporting this model is the finding of altered connectivity and impaired plasticity in AD, which is established as a major risk factor for delirium.1 This validity of this conceptual model can be assessed using the combination of Transcranial Magnetic Stimulation (TMS) and EEG.
Table 1.
Relationship between connectivity, plasticity and delirium.
| Plasticity | |||
|---|---|---|---|
| Preserved | Impaired | ||
| Connectivity | High | No delirium | Acute delirium followed by compensation |
| Low | Acute delirium followed by compensation | Complicated delirium with sustained deficit | |
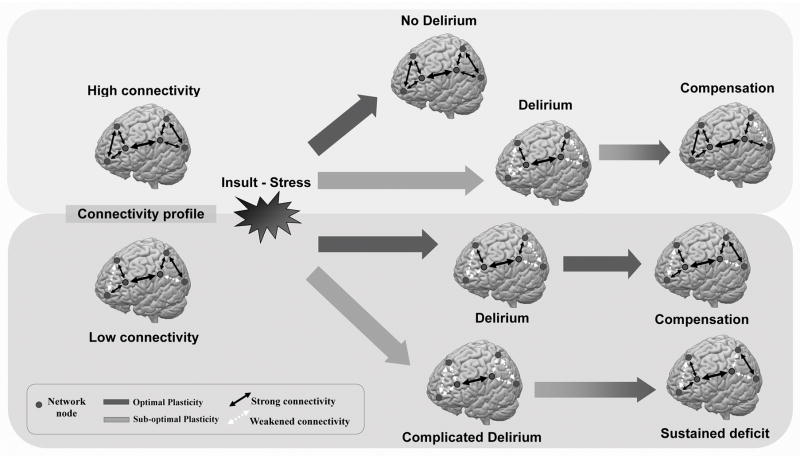
Figure 1. Conceptual Model of Brain Connectivity, Plasticity and Delirium.
The figure depicts a conceptual model illustrating how premorbid individual brain connectivity between brain regions (network nodes, grey circles) and the integrity of mechanism of brain plasticity (thick gray arrows) may relate to the susceptibility to delirium in response to exogenous (e.g. anesthesia) or endogenous (e.g. systemic infection) insults or stressors, and long-term outcome after recovery from delirium. Individuals with robust (high) baseline connectivity and preserved (optimal) cerebral plasticity (dark gray arrow, top) can accommodate stressors without changes in the integrity of brain networks, and thus do not experience delirium. Individuals with high baseline connectivity but impaired plasticity (light gray arrow, second row) cannot quickly accommodate to insults or stressors, and develop a significant decrease in connectivity and impairments in brain network integrity, which produce the symptoms of delirium. As the stressor resolves and normal brain connectivity is mostly reestablished, behavioral compensation occurs and normal cognitive function is restored. In individuals with low baseline connectivity but preserved plasticity (dark gray arrow, third row), insults/stressors acutely overwhelm normal plasticity processes, resulting in acute delirium. Over time, the baseline brain connectivity pattern is restored, and delirium resolves. In individuals with impairments in both baseline connectivity and plasticity (light gray arrow, bottom row), insults lead to severe disruptions in brain connectivity and function (complicated delirium). Brain network connectivity remains weakened even after resolution of the stressor, leading to sustained deficits.
Testing the Conceptual Model of Delirium with TMS-EEG
EEG and functional MRI passively record brain activity, and therefore are limited in their capacity for inferences about brain function. In contrast, TMS is a noninvasive brain stimulation technique that uses electromagnetic induction to produce changes in the activity of stimulated brain regions. When combined with simultaneous EEG recordings (TMS-EEG), TMS provides a powerful means to directly measure the cerebral response to an induced perturbation. TMS produces waves of activity that are reproducible and reliable19 and that reverberate throughout the cortex.20 To date, TMS-EEG has been used to assess cortical network properties in health and in a variety of neurologic and psychiatric diseases,21,22 and detect alterations in cortical excitation/inhibition balance in diseases such as epilepsy.23
TMS-EEG provides a powerful means to measure the fundamental brain properties of effective connectivity and neuroplasticity (defined above). While TMS directly stimulates a relatively localized brain region, the evoked response propagates across brain regions over time20 and can be used to determine the effective (causal) connectivity of the stimulated brain regions in individual subjects. When applied in repetitive trains, TMS produces persistent changes in cortical excitability that can be assessed using electromyography (EMG) and EEG, and can serve as measures of the integrity of neuroplasticity mechanisms.24 Such TMS measures of neuroplasticity have been shown to be altered in diseases such as AD25 and minimal hepatic encephalopathy.26
The conceptual model of delirium as a consequence of a breakdown in brain network dynamics is testable using TMS-EEG. Ideally, such a study should involve systematic and repeated assessments of brain structure, connectivity, neurophysiology and cognitive performance before patients enter the hospital (such as for scheduled elective major surgery), during hospitalization, and in the short- and long-term periods following hospitalization. The incorporation of neuroimaging and neurophysiologic approaches into longitudinal studies in vulnerable patients may ultimately help clarify the nature of the brain dysfunction that leads some patients to develop delirium in response to physiological stressors, and thus, identify physiological biomarkers for characterization of delirium risk. These biomarkers could also be assessed in animal models to enhance mechanistic insights and assess potential therapies.
Clinical Implications
The identification of specific features that mediate cerebral vulnerability to delirium may also lead to the development of brain-based interventions to reduce risk. For example, patients scheduled for major elective surgery who are found to have decreased cerebral connectivity might receive behavioral, pharmacologic or neurostimulatory interventions designed to increase connectivity27–29 prior to surgery. Furthermore, patients already suffering from delirium could receive interventions to restore normal brain connectivity in affected networks, thereby facilitating recovery from delirium in situations when prophylaxis is not possible (such as after emergency surgery, when delirium incidence is particularly high.30) Such studies will also lead to an improved understanding of how delirium impacts the vulnerable brain, and may thus lead to interventions to mitigate the long-term effects of delirium on cognitive function.
More broadly, physiological stressors such as surgery or systemic infection can be viewed as a “stress test” for the brain. By systematically identifying the brain features related to connectivity and plasticity that lead some patients to “fail” this test and develop delirium, we may be able to operationalize and meaningfully test the concepts of brain health, brain vulnerability and brain reserve, with significant implications across a broad range of neuropsychiatric and cognitive diseases. As such, delirium provides a window of opportunity warranting detailed study not only in its own right, but for what it can teach us about brain function more generally.
Acknowledgments
The authors gratefully acknowledge the contributions of the patients, family members, nurses, physicians, staff members, and members of the Executive Committee who participated in the Successful Aging after Elective Surgery (SAGES) Study. This work is dedicated to the memory of Joshua Bryan Inouye Helfand.
Funding: This manuscript was funded by P01AG031720 and K07AG041835 from the National Institute on Aging (SKI). Dr. Marcantonio’s effort was also supported in part by National Institute of Aging grant K24AG035075. Dr. Inouye holds the Milton and Shirley F. Levy Family Chair at Hebrew SeniorLife/Harvard Medical School. APL was supported in part by grants from the Sidney R. Baer Jr. Foundation, the National Institutes of Health (R01 HD069776, R01 NS073601, R21 MH099196, R21 NS082870, R21 NS085491, R21 HD07616), Harvard Catalyst/The Harvard Clinical and Translational Science Center (NCRR and the NCATS, NIH UL1 RR025758), and the Football Players Health Study at Harvard.
Sponsor’s Role: This manuscript was funded by P01AG031720 and K07AG041835 from the National Institute on Aging (SKI). Dr. Marcantonio’s effort was also supported in part by National Institute of Aging grants R01AG030618 and K24AG035075. Dr. Inouye holds the Milton and Shirley F. Levy Family Chair at Hebrew SeniorLife/Harvard Medical School. APL was supported in part by grants from the Sidney R. Baer Jr. Foundation, the National Institutes of Health (R01 HD069776, R01 NS073601, R21 MH099196, R21 NS082870, R21 NS085491, R21 HD07616), Harvard Catalyst/The Harvard Clinical and Translational Science Center (NCRR and the NCATS, NIH UL1 RR025758), and the Football Players Health Study at Harvard. The funding sources had no role in the design, conduct, or preparation of this paper.
Conflict of Interest
| Elements of Financial/Personal Conflicts | Mouhsin M. Shafi | Emiliano Santarnecchi | Tamara G. Fong | Richard N. Jones | ||||
|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | X | ||||
| Grants/Funds | X | X | X | X | ||||
| Honoraria | X | X | X | X | ||||
| Speaker Forum | X | X | X | X | ||||
| Consultant | X | X | X | X | ||||
| Stocks | X | X | X | X | ||||
| Royalties | X | X | X | X | ||||
| Expert Testimony | X | X | X | X | ||||
| Board Member | X | X | X | X | ||||
| Patents | X | X | X | X | ||||
| Personal Relationship | X | X | X | X | ||||
| Elements of Financial/Personal Conflicts | Edward R. Marcantonio | Alvaro Pascual-Leone | Sharon K. Inouye | |||||
| Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | |||||
| Grants/Funds | X | X | X | |||||
| Honoraria | X | X | X | |||||
| Speaker Forum | X | X | X | |||||
| Consultant | X | X | X | |||||
| Stocks | X | X | X | |||||
| Royalties | X | X | X | |||||
| Expert Testimony | X | X | X | |||||
| Board Member | X | X | X | |||||
| Patents | X | X | X | |||||
| Personal Relationship | X | X | X | |||||
Footnotes
Conflict of Interest: APL serves on the scientific advisory boards for Nexstim, Neuronix, Starlab Neuroscience, Neuroelectrics, and Neosync, and is listed as an inventor on several issued and pending patents on the real-time integration of transcranial magnetic stimulation (TMS) with electroencephalography (EEG) and magnetic resonance imaging (MRI). None of the other authors report any conflicts of interest. All the other co-authors fully disclose they have no financial interests, activities, relationships and affiliations. The other co-authors also declare they have no potential conflicts in the three years prior to submission of this manuscript.
Author Contributions: Dr. Shafi, Dr. Inouye, and Dr. Pascual-Leone drafted the manuscript. All coauthors contributed significantly to the preparation of this manuscript and approve this final version to be published. All authors participated in interpretation of data, and provided critical review of, intellectual contributions to, and approval of the final manuscript.
References
- 1.Fong TG, Davis D, Growdon ME, et al. The interface between delirium and dementia in elderly adults. Lancet Neurol. 2015;14:823–32. doi: 10.1016/S1474-4422(15)00101-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leslie DL, Inouye SK. The Importance of Delirium: Economic and Societal Costs. J Am Geriatr Soc. 2011;59:S241–S243. doi: 10.1111/j.1532-5415.2011.03671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–8. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 4.Babiloni C, Lizio R, Marzano N, et al. Brain neural synchronization and functional coupling in Alzheimer’s disease as revealed by resting state EEG rhythms. Int J Psychophysiol Off J Int Organ Psychophysiol. 2016;103:88–102. doi: 10.1016/j.ijpsycho.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Engel GL, Romano J, Goldman L. Delirium; quantitative electroencephalographic study of a case of acute arsenical encephalopathy. Arch Neurol Psychiatry. 1946;56:659–64. [PubMed] [Google Scholar]
- 6.Jacobson SA, Leuchter AF, Walter DO, et al. Serial quantitative EEG among elderly subjects with delirium. Biol Psychiatry. 1993;34:135–40. doi: 10.1016/0006-3223(93)90382-n. [DOI] [PubMed] [Google Scholar]
- 7.Van der Kooi AW, Slooter AJC, van Het Klooster MA, et al. EEG in delirium: Increased spectral variability and decreased complexity. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2014;125:2137–9. doi: 10.1016/j.clinph.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 8.Van Dellen E, van der Kooi AW, Numan T, et al. Decreased functional connectivity and disturbed directionality of information flow in the electroencephalography of intensive care unit patients with delirium after cardiac surgery. Anesthesiology. 2014;121:328–35. doi: 10.1097/ALN.0000000000000329. [DOI] [PubMed] [Google Scholar]
- 9.Trzepacz PT, Brenner RP, Coffman G, et al. Delirium in liver transplantation candidates: discriminant analysis of multiple test variables. Biol Psychiatry. 1988;24:3–14. doi: 10.1016/0006-3223(88)90116-3. [DOI] [PubMed] [Google Scholar]
- 10.Van der Kooi AW, Zaal IJ, Klijn FA, et al. Delirium detection using EEG: what and how to measure. Chest. 2015;147:94–101. doi: 10.1378/chest.13-3050. [DOI] [PubMed] [Google Scholar]
- 11.Thomas C, Hestermann U, Walther S, et al. Prolonged activation EEG differentiates dementia with and without delirium in frail elderly patients. J Neurol Neurosurg Psychiatry. 2008;79:119–25. doi: 10.1136/jnnp.2006.111732. [DOI] [PubMed] [Google Scholar]
- 12.Choi S-H, Lee H, Chung T-S, et al. Neural network functional connectivity during and after an episode of delirium. Am J Psychiatry. 2012;169:498–507. doi: 10.1176/appi.ajp.2012.11060976. [DOI] [PubMed] [Google Scholar]
- 13.Zhang LJ, Wu S, Ren J, et al. Resting-state functional magnetic resonance imaging in hepatic encephalopathy: current status and perspectives. Metab Brain Dis. 2014;29:569–82. doi: 10.1007/s11011-014-9504-9. [DOI] [PubMed] [Google Scholar]
- 14.Pascual-Leone A, Amedi A, Fregni F, et al. The plastic human brain cortex. Annu Rev Neurosci. 2005;28:377–401. doi: 10.1146/annurev.neuro.27.070203.144216. [DOI] [PubMed] [Google Scholar]
- 15.Di Lorenzo F, Ponzo V, Bonnì S, et al. Long-term potentiation–like cortical plasticity is disrupted in Alzheimer’s disease patients independently from age of onset. Ann Neurol. 2016;80:202–10. doi: 10.1002/ana.24695. [DOI] [PubMed] [Google Scholar]
- 16.Fried PJ, Schilberg L, Brem A-K, et al. Humans with Type-2 Diabetes Show Abnormal Long-Term Potentiation-Like Cortical Plasticity Associated with Verbal Learning Deficits. J Alzheimers Dis JAD. doi: 10.3233/JAD-160505. Published Online First: 13 September 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qi R, Zhang LJ, Xu Q, et al. Selective impairments of resting-state networks in minimal hepatic encephalopathy. PloS One. 2012;7:e37400. doi: 10.1371/journal.pone.0037400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Santarnecchi E, Rossi S, Rossi A. The smarter, the stronger: intelligence level correlates with brain resilience to systematic insults. Cortex J Devoted Study Nerv Syst Behav. 2015;64:293–309. doi: 10.1016/j.cortex.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 19.Lioumis P, Kicić D, Savolainen P, et al. Reproducibility of TMS-Evoked EEG responses. Hum Brain Mapp. 2009;30:1387–96. doi: 10.1002/hbm.20608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Massimini M, Ferrarelli F, Huber R, et al. Breakdown of cortical effective connectivity during sleep. Science. 2005;309:2228–32. doi: 10.1126/science.1117256. [DOI] [PubMed] [Google Scholar]
- 21.Casarotto S, Määttä S, Herukka S-K, et al. Transcranial magnetic stimulation-evoked EEG/cortical potentials in physiological and pathological aging. Neuroreport. 2011;22:592–7. doi: 10.1097/WNR.0b013e328349433a. [DOI] [PubMed] [Google Scholar]
- 22.Rogasch NC, Daskalakis ZJ, Fitzgerald PB. Cortical inhibition, excitation, and connectivity in schizophrenia: a review of insights from transcranial magnetic stimulation. Schizophr Bull. 2014;40:685–96. doi: 10.1093/schbul/sbt078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shafi MM, Vernet M, Klooster D, et al. Physiological consequences of abnormal connectivity in a developmental epilepsy: Cortical Connectivity. Ann Neurol. 2015;77:487–503. doi: 10.1002/ana.24343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pascual-Leone A, Freitas C, Oberman L, et al. Characterizing brain cortical plasticity and network dynamics across the age-span in health and disease with TMS-EEG and TMS-fMRI. Brain Topogr. 2011;24:302–15. doi: 10.1007/s10548-011-0196-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koch G, Di Lorenzo F, Bonnì S, et al. Impaired LTP- but not LTD-like cortical plasticity in Alzheimer’s disease patients. J Alzheimers Dis JAD. 2012;31:593–9. doi: 10.3233/JAD-2012-120532. [DOI] [PubMed] [Google Scholar]
- 26.Golaszewski S, Langthaler PB, Schwenker K, et al. Abnormal cortical synaptic plasticity in minimal hepatic encephalopathy. Brain Res Bull. 2016;125:200–4. doi: 10.1016/j.brainresbull.2016.07.011. [DOI] [PubMed] [Google Scholar]
- 27.Plewnia C, Rilk AJ, Soekadar SR, et al. Enhancement of long-range EEG coherence by synchronous bifocal transcranial magnetic stimulation. Eur J Neurosci. 2008;27:1577–83. doi: 10.1111/j.1460-9568.2008.06124.x. [DOI] [PubMed] [Google Scholar]
- 28.Meinzer M, Antonenko D, Lindenberg R, et al. Electrical brain stimulation improves cognitive performance by modulating functional connectivity and task-specific activation. J Neurosci Off J Soc Neurosci. 2012;32:1859–66. doi: 10.1523/JNEUROSCI.4812-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pa J, Berry AS, Compagnone M, et al. Cholinergic enhancement of functional networks in older adults with mild cognitive impairment. Ann Neurol. 2013;73:762–73. doi: 10.1002/ana.23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koebrugge B, van Wensen RJA, Bosscha K, et al. Delirium after emergency/elective open and endovascular aortoiliac surgery at a surgical ward with a high-standard delirium care protocol. Vascular. 2010;18:279–87. doi: 10.2310/6670.2010.00052. [DOI] [PubMed] [Google Scholar]