Abstract
Rehabilitative training drives plasticity in the ipsilesional (injured) motor cortex that is believed to support recovery of motor function after either stroke or traumatic brain injury (TBI). In addition, adaptive plasticity in the contralesional (uninjured) motor cortex has been well-characterized in the context of stroke. While similar rehabilitation-dependent plasticity in the intact hemisphere may occur after TBI, this has yet to be thoroughly explored. In this study, we investigated the effects of TBI and forelimb training on reorganization of movement representations in the intact motor cortex. Rats were trained to proficiency on the isometric pull task and then received a controlled cortical impact (CCI) in the left motor cortex to impair function of the trained right forelimb. After TBI, animals underwent forelimb training on the pull task for 2 months. At the end of training, intracortical microstimulation was used to document the organization of the intact motor cortex (the contralesional hemisphere). TBI significantly decreased the cortical area eliciting movements of the impaired forelimb in untrained animals. In the absence of TBI, training significantly increased forelimb map area, compared with in untrained controls. However, training of the impaired forelimb after TBI was insufficient to increase forelimb map area. These findings are consistent with other studies showing impaired rehabilitation-dependent plasticity after TBI and provide a novel characterization of TBI on rehabilitation-dependent plasticity in contralesional motor circuits.
Keywords: : contralesional hemisphere, map plasticity, motor recovery, traumatic brain injury
Introduction
Traumatic brain injury (TBI) affects 1.7 million people in the United States on an annual basis.1 Severe traumatic brain injury can lead to chronic impairments in both cognitive and motor function.2–4 Rehabilitation is a common strategy to improve recovery. However, despite extensive rehabilitative therapy, as many as 5.3 million Americans are currently living with long-term disabilities as a result of TBI.5 Improved rehabilitative therapies are needed to further enhance recovery in patients with long-term motor disabilities.
Rehabilitative therapy supports recovery in part by promoting reorganization of spared motor circuits.6–8 Studies in both stroke patients and animal models of stroke indicate that reorganization of networks occurs in the lesioned hemisphere.9–17 In addition to reorganization of the lesioned hemisphere, several studies also have observed that extensive rehabilitation after stroke drives transient reorganization within the intact hemisphere (opposite of the lesion) that is believed to support recovery.12,13,18–23 Despite the thorough characterization in the context of stroke, few studies have examined how plasticity in the intact hemisphere is related to motor recovery in animal models of TBI. It is possible that rehabilitative training drives reorganization in the intact motor cortex after TBI similar to that observed after stroke. Alternatively, as suggested in previous studies, recovery may be blunted after TBI due to limited cortical reorganization.24
In this study, we characterize map plasticity in the intact hemisphere in the context of both TBI and forelimb training. In the first experiment, we examined the effect of TBI on movement representations in the contralesional motor cortex. Rats were given a controlled-cortical impact (CCI), and intracortical microstimulation (ICMS) was used to investigate map reorganization within the intact hemisphere 2 months after CCI. In the second experiment, we evaluated the effect of forelimb training on contralesional motor cortex plasticity following TBI. Rats were trained to perform the isometric pull task, an automated measure of skilled forelimb function. After reaching proficiency, rats were then given a CCI, followed by forelimb training on the same task for 2 months. ICMS was then used to investigate map organization of the impaired forelimb within the intact hemisphere. The results of this study provide a novel characterization of map plasticity in the intact hemisphere after TBI and extensive training of the impaired forelimb.
Methods
Subjects
Twenty-five adult female Sprague-Dawley rats were used in this study. All rats were 4 to 5 months old at the beginning of the experiment. Rats weighed at least 250 g and were food deprived to no less than 85% of their normal body weight during behavioral testing. The rats were housed in a reverse 12:12 h light cycle to increase daytime activity. All handling, housing, surgical procedures, and behavioral training of the rats were approved by the University of Texas Institutional Animal Care and Use Committee.
Experimental design
In the first experiment, we examined the effect of TBI on organization of the intact motor cortex in untrained animals (Fig. 1A). Two groups of animals were used: untrained controls that received no CCI (Controls; n = 6) and untrained animals that received a CCI in the left motor cortex (TBI Only; n = 7). ICMS was performed in the right motor cortex (contralateral to the TBI) of each group, and both ipsilateral (right side) and contralateral (left side) movement responses were recorded (Fig. 1C). ICMS occurred approximately 2 months after the TBI in the TBI Only group.
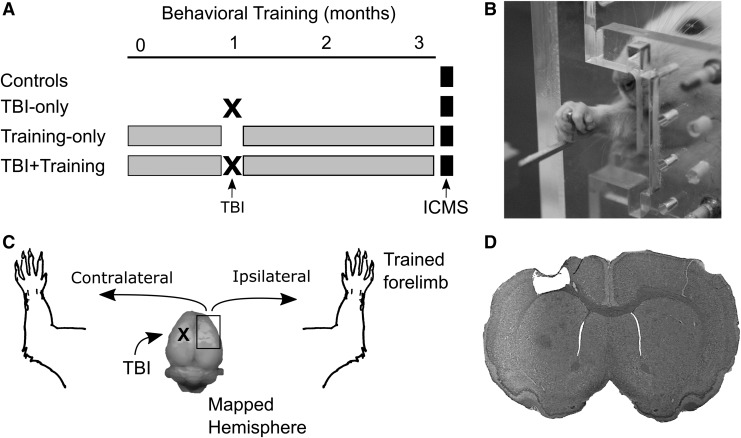
FIG. 1.
Experimental design. (A) Four groups of animals were used in this study. In the first experiment, animals were separated into a group of unlesioned controls and another group that received a traumatic brain injury (TBI). The second experiment consisted of two groups of animals, each receiving training on the pull task for 3 months, and one of these groups received a TBI after achieving proficiency at the task. Intracortical microstimulation (ICMS) was performed in all groups such that the time since the controlled cortical impact (CCI) and the total amount of training was matched between groups. (B) An example of a rat performing the isometric pull task. (C) Rats were given a CCI in the left motor cortex, which primarily affects usage of the opposite limb. ICMS was performed in the hemisphere ipsilateral to the affected limb. (D) A picture illustrating an exemplar TBI lesion.
In the second experiment, we investigated how forelimb training affects organization of the intact hemisphere after TBI (Fig. 1A, 1B). Two additional cohorts of animals were used: trained animals that did not receive a CCI (Training Only; n = 6) and trained animals that received a CCI (TBI+Training; n = 6). After 1 week of recovery following the TBI, all animals performed forelimb training for 2 months. ICMS was then performed in the right motor cortex (contralateral to the TBI, and ipsilateral to the trained limb) after the completion of forelimb training.
Animals were matched such that the Training Only and TBI+Training groups each received an equal amount of training on the pull task, and the TBI Only and TBI+Training groups were each mapped at equivalent time-points after the CCI. Figure 1A shows a detailed timeline for each group of animals. Data from a subset of these rats were published in a previous study.25
Controlled cortical impact procedure
Rats were anesthetized with a cocktail of ketamine hydrochloride (50 mg/kg), xylazine (20 mg/kg), and acepromazine (5 mg/kg) injected intramuscularly, and given supplemental doses as needed. After placing the rat in a stereotaxic frame, a craniotomy was performed to expose motor cortex in the left hemisphere. A controlled-cortical impact device was then centered over the forelimb area of exposed motor cortex (Fig. 1D). In Experiment 1, rats in the TBI Only group were impacted using a spring-loaded impactor26,27 with a calculated velocity of 3 m/sec and an impact depth of 2 mm. As in our previous studies, the impactor was allowed to dwell for 5 sec before it was removed from the brain. In Experiment 2, rats in the TBI+Training group were impacted using a commercially acquired controlled-cortical impact device (Impact One; Leica). Standard impact parameters used in the TBI+Training group were an impact velocity of 5–6 m/sec, an impact depth of 2 mm, a dwell time of 0.3 sec, and an impact tip with a 3 mm diameter. Table 1 details the exact impact parameters used for each rat in this study. After the impactor was positioned over the exposed cortex, an impact was delivered. Following the impact, the craniotomy was covered with a thin layer of bone cement.28 After surgery, rats received subcutaneous administration of antibiotics (Baytril, 10 mg/kg), analgesic (buprenorphine, 0.3 mg/kg), and 4 mL of 50:50 0.9% saline and 5% dextrose. Two days after surgery, each rat was given one additional Baytril tablet (0.5 mg, BioServ, Frenchtown, NJ).
Table 1.
CCI Parameters Used for Each Rat in the Study
| Rat | Impactor type | Impact velocity (m/sec) | Tip diameter (mm) | Dwell time (sec) | Depth (mm) |
|---|---|---|---|---|---|
| TBI-only group (Experiment 1) | |||||
| 1 | Spring | 3 | 3 | 5 | 2 |
| 2 | Spring | 3 | 3 | 5 | 2 |
| 3 | Spring | 3 | 3 | 5 | 2 |
| 4 | Spring | 3 | 3 | 5 | 2 |
| 5 | Spring | 3 | 3 | 5 | 2 |
| 6 | Spring | 3 | 3 | 5 | 2 |
| 7 | Spring | 3 | 3 | 5 | 2 |
| TBI+training group (Experiment 2) | |||||
| 1 | Leica | 5 | 3 | 0.3 | 2 |
| 2 | Leica | 5 | 3 | 5 | 2 |
| 3 | Leica | 5 | 3 | 0.3 | 2 |
| 4 | Leica | 6 | 3 | 0.3 | 2 |
| 5 | Leica | 6 | 3 | 0.3 | 2 |
| 6 | Leica | 6 | 3 | 0.3 | 4 |
CCI, controlled cortical impact; TBI, traumatic brain injury.
Isometric pull task
The behavioral apparatus and software were used as described in previous studies.26,27,29–31 The isometric pull task is designed to assess skilled forelimb function and volitional forelimb strength. The apparatus (Base Cage; Vulintus, Inc., Dallas, TX) consisted of an acrylic box (25.4 × 30.5 × 12.1 cm). The box contained a slot in the front right corner that the rat could reach through to access an aluminum pull handle (Pull Behavior Module; Vulintus, Inc.). The slot was sized and positioned such that rats could only reach and pull using their right forelimb (Fig. 1B). The pull handle was centered in the slot at a height of 6.4 cm from the cage floor and 1.9 cm outside the cage relative to the inner cage wall for fully trained animals. The aluminum handle was connected to a force transducer that could measure pull force with sub-gram accuracy. The force transducers were inspected daily and recalibrated using a system of standard weights and linear interpolation when the baseline drifted by ±5 g. Matlab software was used to control the behavioral apparatus. A microprocessor controller (Controller; Vulintus, Inc.) sampled the force transducer at 100 Hz and sent the information to the Matlab software, which displayed the data on screen, controlled the behavior session, and saved the data to permanent files.
Behavioral training
All animals in Experiment 2 underwent identical forelimb training, consisting of two 30-min sessions per day, 5 days per week, with at least 2 h between daily sessions. Shaping procedures were similar to those previously described.26,27,29,30 Animals were trained to reach through a narrow slot in the cage with the right forelimb, grasp a handle, and apply at least 120 g of force. A trial was initiated when at least 10 g of force were applied to the pull handle. After initiation, the force on the pull handle was sampled for 4 sec. If the force threshold required for a hit was met within 2 sec after trial initiation, the trial was recorded as a success and a reward pellet was delivered (45 mg dustless precision pellet; BioServ). If the force threshold was not reached within 2 sec, the trial was recorded as a failure and no reward was delivered. Success rate for each day was calculated as the number of hits for that day divided by the total number of trials for the day. After achieving a stable baseline, defined as 10 consecutive sessions over which the average success rate on the pull task was 85% or higher, half of the animals received a CCI. Forelimb training commenced 1 week after the CCI and continued for 2 months. At the completion of 2 months of post-lesion training, the ICMS procedure was performed (Fig. 1A).
ICMS procedure
At the prescribed time (Fig. 1A), ICMS was performed using standard procedures to investigate right-sided (ipsilateral) and left-sided (contralateral) movement representations in the right hemisphere (the hemisphere ipsilateral to the trained forelimb; Fig. 1C).25 Rats were anesthetized with a cocktail of ketamine hydrochloride (50 mg/kg), xylazine (20 mg/kg), and acepromazine (5 mg/kg) injected intramuscularly, and given supplemental doses as needed.
After placing the rat in the stereotaxic frame, a craniotomy and duratomy were performed to expose motor cortex in the right hemisphere (4 mm to −3 mm anteroposterior [AP], and 0.25 mm to 5 mm mediolateral [ML]). A tungsten electrode (impedance less than 1 MΩ) was inserted to a depth of 1.8 mm into the cortex. Electrode penetrations were performed along a grid with each site spaced 500 μm apart. Stimulation sites were chosen randomly, with an effort made to ensure that each site was at least 1 mm in distance from the immediately previous penetration. Each stimulation consisted of a 40 msec pulse train of ten 200-μsec monophasic cathodal pulses delivered at 286 Hz. A maximum stimulation intensity of 300 μA was used to determine the presence of any ipsilateral forelimb response to stimulation. The maximum stimulation intensity was chosen based on previous studies that indicate ipsilateral motor responses are evoked at higher currents than contralateral responses.25,32,33 The presence of ipsilateral forelimb movement was first determined, and the current was then lowered to find the lowest threshold at which ipsilateral movement was reliably evoked. After determining the threshold current for ipsilateral responses, the current was again lowered until a threshold was found for contralateral movement. If no ipsilateral or contralateral movement was observed at the maximal stimulation, then the site was deemed nonresponsive. The borders of motor cortex were defined based on nonresponsive sites on all sides.
All ICMS experiments were performed blinded by two experimenters, as in previous studies.25,34,35 The first experimenter would place the electrode. The second experimenter, blinded to experimental condition and electrode location, would deliver stimulation and classify responses. Each site was classified based on the part of the body that moved at the lowest threshold stimulation current. Jaw, vibrissae, and neck movement were classified as “head” responses. Any movement of the digits, wrist, elbow, and shoulder was considered a “forelimb” response. Any hindlimb movements were classified as “hindlimb” responses. Cortical area for each movement representation was calculated by multiplying the number of sites eliciting a response by 0.25 mm2 (0.5 mm × 0.5 mm). At the completion of the ICMS procedure, all animals that had received CCI lesions were perfused with 4% paraformaldehyde and standard histological techniques were used to confirm the presence of a lesion.26,27
Statistical analysis
Behavioral data obtained with the isometric pull task were analyzed using Matlab software similar to previous descriptions.26,27,29,36 Groups were compared across time using a mixed-model repeated-measures analysis of variance (ANOVA) followed by post hoc paired or unpaired t-tests as statistically merited.
The primary outcome measure of the ICMS procedures was the number of ipsilateral forelimb responses observed from each animal, while other responses on the contralateral side of the body were secondary measures. Lilliefors test was used to determine whether the ICMS data was normally distributed. Because the ICMS data of the TBI Only group was not normally distributed, a Wilcoxon rank-sum test was used to compare the TBI Only group with control rats in the first experiment. Due to an absence of ipsilateral forelimb responses in animals receiving a TBI, a Chi-squared test was used to investigate differences in the proportion of animals that had responses between the Control and TBI Only groups. In the second experiment, the data was normally distributed and an unpaired t-test was used to compare ICMS data between the Training Only and TBI+Training groups.
When comparing ICMS data from both experiments together, a two-way ANOVA was used for a complete analysis of all groups, followed by Tukey-Kramer post hoc tests to compare individual group differences. Complete maps from all animals used in this study have been included in the Supplementary Material (see online supplementary material at www.liebertpub.com).
Where appropriate, corresponding effect sizes were calculated for each statistical test. The effect size measurements used were Cohen's U1 (to complement the Wilcoxon rank-sum test), Hedge's g (to complement t-tests), risk difference (to complement Chi-squared), and partial eta-squared (ηp2, to complement repeated-measures ANOVA and two-way ANOVA).
Lesion size within TBI animals from both experiments was measured by four separate raters blinded to experimental group. The median lesion size from the four raters was calculated for each brain. Lesion size was then compared between the two groups of TBI animals using an unpaired t-test. A Spearman's rho was calculated to investigate whether a relationship exists between lesion size and ICMS ipsilateral forelimb area.
Results
Experiment 1: Effects of TBI on impaired forelimb representation in the intact hemisphere
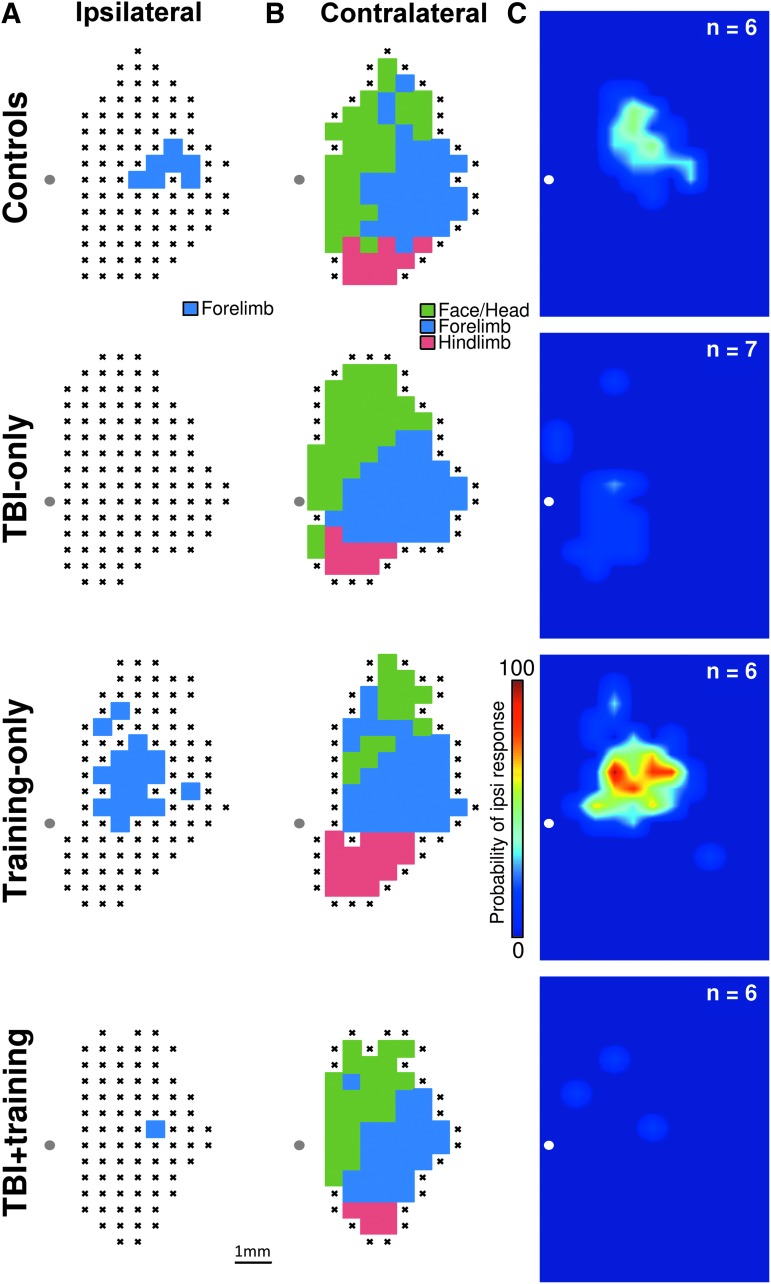
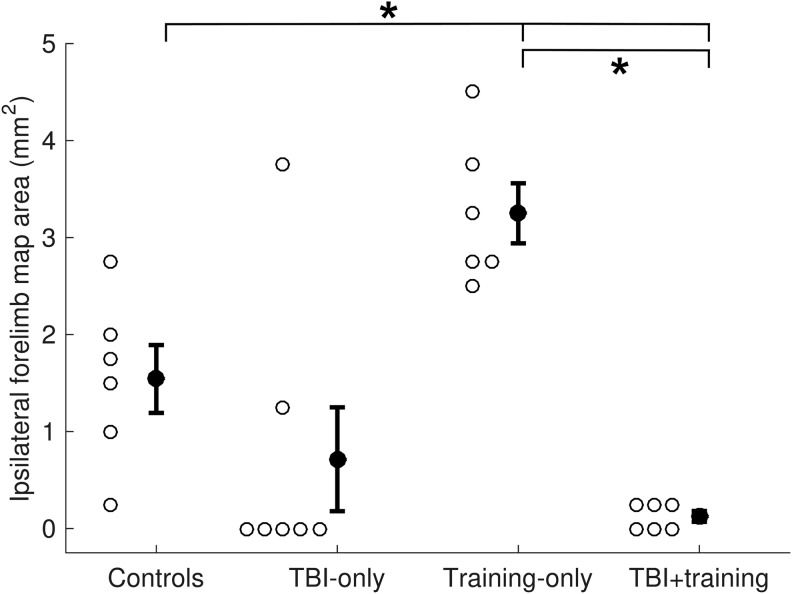
Previous research suggests unilateral TBI results in cortical reorganization of the intact hemisphere.33,37 To test this, we used ICMS to investigate how representation of the ipsilateral (right) forelimb in the intact motor cortex was affected after a unilateral TBI (Fig. 2). The resulting counts of ipsilateral forelimb responses in the TBI Only group failed to match a normal distribution (Lilliefors test; p < 0.001). Therefore, a Wilcoxon rank-sum test was used to analyze differences in the number of ipsilateral forelimb responses between the TBI Only and control groups. While a trend towards reduction in forelimb representation was observed after TBI, the comparison failed to reach statistical significance (Fig. 3; Controls = 1.54 ± 0.35 mm2, TBI Only = 0.71 ± 0.54 mm2, Wilcoxon rank-sum test p = 0.067; effect size U1 = 0.46). Due to the abnormality of the data and an observed lack of forelimb responses in the TBI Only group, we followed our initial analysis with a Chi-squared assessment to analyze the proportion of animals in each group that had ipsilateral forelimb movements elicited during ICMS. Movement of the ipsilateral forelimb could be elicited in all (six of six) unlesioned control animals by stimulating the right motor cortex. Responses of the ipsilateral forelimb could be elicited in only two of seven animals with TBIs, significantly fewer than observed in unlesioned animals (χ2 = 6.96, p = 0.0083, risk difference = 0.71). Despite the failure of the Wilcoxon rank-sum test to reach statistical significance, the trend observed in combination with the result of Chi-squared test suggest that TBI may influence representation of the ipsilateral forelimb in the contralesional hemisphere.
FIG. 2.
Representative motor maps. (A) Representative ipsilateral forelimb maps from a single animal in each group. This forelimb was the trained forelimb in groups that received pull tasks training, and the affected forelimb in groups that received a traumatic brain injury (TBI). (B) Representative contralateral motor maps (showing representation of the untrained/unaffected forelimb) from the same animals as shown in panel A. (C) Group data in heat plots representing the probability of observing an ipsilateral (trained) forelimb response at any site within right motor cortex. The TBI Only and TBI+training groups had significantly reduced probabilities of observing ipsilateral forelimb responses.
FIG. 3.
Cortical area representing the ipsilateral forelimb, derived by intracortical microstimulation. There was a significant reduction of ipsilateral forelimb representation within the traumatic brain injury (TBI) Only and TBI+training groups, compared with controls and training-only animals.
Experiment 2: TBI reduces impaired forelimb representation in the intact hemisphere of trained animals
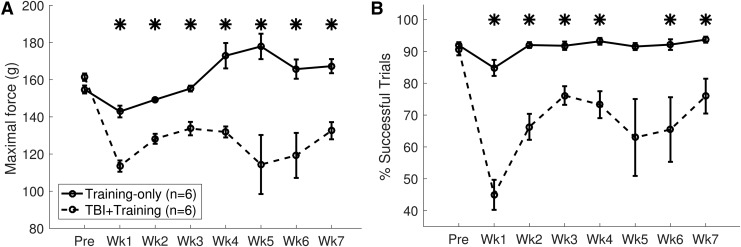
Rehabilitative training results in adaptive plasticity in the intact motor cortex after stroke.6 Although similar reorganization may occur after TBI, previous studies suggest more limited plasticity after TBI, which may impair training-dependent reorganization of map representations.24 Therefore, we sought to evaluate the effects of training on motor representation of the impaired forelimb in the intact hemisphere after TBI. Rats were trained to proficiency on the isometric pull task and received a TBI to impair performance of the trained forelimb. As expected, TBI resulted in a significant impairment in function of the trained forelimb (TBI+Training, pre-lesion vs. Week 1 post-lesion; percent successful trials, unpaired t-test, p = 6.45 × 10−5, effect size g = 4.39).26,27 Examining performance after the lesion, a repeated measures ANOVA failed to reveal any significant interaction of time and TBI on forelimb pull performance during post-lesion training (Fig. 4; Training Only vs TBI+Training, percent successful trials, F[1, 60] = 1.41, p = 0.23, ηp2 = 0.12). A main effect of TBI was observed (F[1, 10] = 29.96, p = 0.00027, ηp2 = 0.75). Post hoc tests revealed that success rate on the pull task was significantly impaired on most weeks over the duration of the study (unpaired t-tests of each post-lesion time-point of TBI+Training vs Training Only, all weeks p < 0.05, except Week 5 p = 0.057). Similar results were observed for maximal pull force, consistent with a reduction in volitional forelimb strength (Fig. 4).
FIG. 4.
Pull task performance. (A) Controlled cortical impact (CCI) resulted in chronic impairment of volitional pull force within the traumatic brain injury (TBI)+training group. (B) The percentage of successful trials also was reduced after CCI.
We hypothesized that forelimb training after TBI would result in increased contralesional plasticity and support functional recovery. To test this hypothesis, we used ICMS to investigate representation of the impaired forelimb within the intact hemisphere after the completion of forelimb training (Fig. 1). In unlesioned animals, pull training results in increased representation of the trained (ipsilateral) forelimb compared to untrained controls.25 An unpaired t-test revealed a significant reduction of ipsilateral forelimb representation in trained rats after TBI, compared with unlesioned rats that were also pull trained (Fig. 2A and Fig. 3; Training Only = 3.25 ± 0.31 mm2, TBI+Training = 0.13 ± 0.06 mm2, unpaired t-test p = 1.69 × 10−6, effect size g = 5.29). Additionally, no significant difference was observed in the number of trials performed by the TBI+Training and the Training Only groups during forelimb training, suggesting that a reduction of engagement in the motor task cannot account for the differences in forelimb map representation (TBI+Training = 10,975 ± 1242 total trials, Training Only = 9927 ± 766 total trials, unpaired t-test p = 0.49, effect size g = 0.41). This indicates that TBI results in a significant reduction of map area eliciting responses of the impaired forelimb within the intact hemisphere despite extensive training of the impaired forelimb on a skilled motor task.
TBI resulted in reduced ipsilateral forelimb responses in both trained and untrained animals
To analyze whether TBI and forelimb training interacted to affect cortical map organization, a two-way ANOVA of the ICMS data across all groups of animals was performed. A significant interaction of training and TBI was observed (F[1, 21] = 9.22, p = 0.0063, ηp2 = 0.31) as well as a significant main effect of TBI (F[1, 21] = 27.29, p = 3.54 × 10−5, ηp2 = 0.57) on representation of the ipsilateral (impaired) forelimb within the contralesional hemisphere. Tukey-Kramer post hoc analysis confirmed that there were no significant differences in the number of observed ipsilateral forelimb responses elicited during ICMS between the two groups of TBI animals (TBI Only vs TBI+Training, p = 0.68). These results indicate that TBI reduces contralesional map representation of the impaired forelimb and that training is insufficient to overcome this effect.
Motor representations of the unimpaired forelimb within the intact hemisphere have been shown to be reduced following unilateral TBI.38 To determine whether TBI resulted in reorganization of motor maps of the unimpaired forelimb within the intact hemisphere (i.e., left forelimb representation within the right hemisphere), we investigated overall motor map area that elicited contralateral responses (Fig. 2B). No reduction in the number of contralateral forelimb responses was observed (F[1, 21] = 0.03, p = 0.86, ηp2 = 0.0015). Therefore, we did not observe any effect of TBI on contralateral forelimb responses.
No differences in ICMS threshold motor responses occurred due to TBI
TBI alters excitability in cortical circuits, which may influence map representations.39 To examine whether differences in cortical excitability could account for the observed differences in maps, a two-way ANOVA was performed on ICMS thresholds. No effect of TBI was observed on response thresholds of the ipsilateral forelimb in contralesional motor cortex (Controls = 193 ± 24 μA; TBI Only = 202 ± 9 μA; Training Only = 188 ± 7 μA; TBI+Training = 140 ± 16 μA; F[1, 13] = 0.74, p = 0.41, ηp2 = 0.054). Additionally, no effect of TBI was observed on thresholds of contralateral responses (Controls = 129 ± 4 μA; TBI Only = 133 ± 8 μA; Training Only = 123 ± 5 μA; TBI+Training = 108 ± 6 μA; F(1, 21) = 0.72, p = 0.41, ηp2 = 0.033), nor specifically on contralateral forelimb responses (Controls = 134 ± 5 μA; TBI Only = 142 ± 12 μA; Training Only = 123 ± 5 μA; TBI+Training = 125 ± 6 μA; F[1, 21] = 0.34, p = 0.57, ηp2 = 0.016). We also investigated whether excitability of contralateral responses was altered at sites that also generated ipsilateral responses, but observed no significant differences across groups (Controls = 90 ± 11 μA; TBI Only = 116 ± 11 μA; Training Only = 78 ± 8 μA; TBI+Training = 75 ± 2 μA; F[1, 12] = 0.58, p = 0.46, ηp2 = 0.046). These results suggest that TBI does not significantly influence excitability in the contralesional cortex. As expected, ipsilateral responses were generated at consistently higher threshold currents than contralateral responses at equivalent cortical sites (all sites from all animals with ipsilateral forelimb responses; ipsilateral response thresholds = 194 ± 5 μA; contralateral responses thresholds at same sites = 86 ± 3 μA; paired t-test, p = 1.63 × 10−51, effect size g = 2.06).
Histological analysis
Histological analysis was performed on a subset of animals from the TBI Only (n = 7) and TBI+Training (n = 4) groups. There was a significant difference in lesion size between the two groups (TBI Only = 2.6 ± 0.8 mm3; TBI+Training = 23.8 ± 7.0 mm3; unpaired t-test, p = 0.0028, effect size g = 2.33), likely due to differences between the impactors used for each group of animals. Within the TBI+Training group, no relationship was observed between maximal force used on the pull task during the last week of post-lesion training and the lesion size of animals (Spearman rho, r = 0.66; p = 0.34). Additionally, there was no correlation between lesion size and total ipsilateral forelimb responses across all TBI animals from both impactor types (Spearman rho, r = −0.0053; p = 0.99).
Discussion
In this study, we demonstrate that TBI significantly reduces representation of the impaired forelimb in the intact motor cortex. This reduction is observed in spite of extensive forelimb training, suggesting that TBI occludes typical map reorganization.
Previous research indicates that the motor cortex ipsilateral to a limb supports functionally relevant motor control that is likely mediated by interhemispheric fibers, and loss of this interhemispheric connectivity leads to a near complete loss of ipsilateral motor control.32 It has been demonstrated, however, that remaining direct descending ipsilateral projections can mediate some degree of motor control.32 The CCI model used in the present study resulted in a large contusion that damages interhemispheric fibers crossing through the corpus callosum,26 while likely leaving descending fibers within the contralesional hemisphere largely intact. The increase of ipsilateral forelimb representation observed in unlesioned trained animals could be mediated by plasticity of interhemispheric callosal projections that subsequently excite descending contralateral projections. Alternatively, the increased ipsilateral forelimb representation in unlesioned animals may be due to plasticity of the small population of direct descending ipsilateral connections. The reduction of ipsilateral forelimb responses within the TBI+Training group, however, suggests that the relevant plasticity occurs specifically in callosal projections, as TBI disrupts the callosal connections while leaving descending ipsilateral connections in the unlesioned hemisphere intact. In this case, these findings suggest that plasticity of the descending ipsilateral corticospinal projections is insufficient to mediate restoration of forelimb function after TBI with extensive training alone. Therefore, it is possible that adjunctive strategies that boost adaptive plasticity beyond that which is conferred by extensive rehabilitative training after TBI may support greater recovery.40–42
Recent studies demonstrate a transient increase of cortical activation and altered motor map representations within the contralesional hemisphere after stroke, suggesting that such ipsilateral corticospinal pathways within the contralesional hemisphere may be potentiated after brain injury to support functional recovery.12,13,18,20–23,43 Indeed, Axelson and colleagues33 observed an increase in contralesional motor map representation of the impaired limb five weeks after a unilateral motor cortex TBI. In our study, however, we observed a reduction of responses of the impaired forelimb during ICMS 2 months after TBI. One possible explanation of this difference in experimental results may be a difference in lesion size between this study and the study conducted by Axelson and colleagues.33 In light of previous results by Brus-Ramer and colleagues,32 if the TBI lesion in the study by Axelson and colleagues33 left a portion of callosal fibers intact, these fibers could potentially mediate map expansion, whereas the map reduction in our study can be explained by substantial damage to the callosal fibers.
Another plausible explanation for the differences between studies may relate to the timing when cortical organization is assessed. The study by Axelson and colleagues33 may reflect a transient increase in plasticity in the intact cortex after TBI, whereas the present study measured cortical representations several weeks later, after which the transient expansion may have reversed. Indeed, evidence from studies evaluating motor map plasticity in the lesioned hemisphere after stroke and in the intact somatosensory after TBI provides support for transient reorganization. Activation of ipsilateral cortex in response to sensory stimulation demonstrates a transient increase after TBI, which eventually normalizes by 30 days after the injury.37 A similar dynamic expansion/renormalization within motor circuits may explain the dynamic increase and decrease in ipsilateral motor cortex observed in the current study and the study by Axelson and colleagues.33 This hypothesis of a transient increase in contralesional activation followed by a later normalization also may explain the outlier data in the TBI Only group of this study in which two animals had distinctly higher ipsilateral forelimb representation than all other TBI animals. It may be that this normalization process had not yet occurred for these animals, although a conclusion regarding this cannot be made because motor maps were not performed at an earlier time-point.
Traumatic brain injury results in numerous alterations in plasticity, including changes in cortical excitation, limited dendritic regrowth, and a suppression of hippocampal LTP.24,39,44–47 This reduction in plasticity may limit the effectiveness of plasticity-based therapies to treat motor dysfunction after TBI. One previous study, however, has demonstrated that cortical stimulation paired with motor training after TBI results in significant reorganization of the injured hemisphere that is associated with modest improvements in functional recovery.48 Additionally, application of pro-plasticity vagus nerve stimulation paired with rehabilitative training improves motor recovery after TBI.27 These studies indicate that therapies directed at boosting plasticity in conjunction with rehabilitative training may overcome TBI-dependent reductions in plasticity to promote recovery.
We have demonstrated that TBI reduces representation of the impaired forelimb within the contralesional hemisphere ipsilateral to that limb. TBI also occludes training-dependent expansion of forelimb representation within ipsilateral motor cortex, consistent with the notion that TBI negatively influences plasticity.24 The results of this study reveal novel insights into the interaction between training-dependent plasticity and traumatic brain injury, and may help guide further investigation of plasticity-based therapies to treat brain trauma.
Supplementary Material
Acknowledgments
We would like to acknowledge the assistance of Jenny Trieu, Caroline Abe, Priyanka Reddy, and Nishi Patel who assisted with behavioral experiments and tissue analysis.
Funding: This work was sponsored by the Defense Advanced Research Projects Agency (DARPA) Biological Technologies Office (BTO) ElectRx program under the auspices of Dr. Doug Weber through the Space and Naval Warfare Systems Center, Pacific Cooperative Agreement No. HR0011-15-2-0017 (RLR, MPK, and SAH) and by NIH NINDS R01 NS094384-01 (SAH) and R01 NS085167-01 (RLR and MPK).
Author Contributions: DTP led the project, assisted with behavioral experiments, performed the surgeries, analyzed the data, and wrote the manuscript. TTD and ANS assisted with motor mapping procedures, surgical procedures, and behavioral experiments. RAM assisted with motor mapping procedures and behavioral experiments. MPK and RLR helped review the manuscript and provided feedback throughout the writing process. SAH assisted in writing the manuscript and also provided feedback throughout the writing process.
Author Disclosure Statement
DTP is an employee of and RLR is the owner of Vulintus, Inc. Authors supported by Vulintus, Inc. participated in study design, data collection and analysis, and preparation of the manuscript. Vulintus, Inc. did not have any additional role in the decision to publish.
For the other authors, no competing financial interests exist.
References
- 1.Faul M., Xu L., Wald M., and Coronado V. (2010). Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths 2002–2006. Centers for Disease Control and Prevention, National Center for Injury Prevention and Control: Atlanta, GA [Google Scholar]
- 2.Pickelsimer E.E., Selassie A.W., Sample P.L.W., Heinemann A., Gu J.K., and Veldheer L.C. (2007). Unmet service needs of persons with traumatic brain injury. J. Head Trauma Rehabil. 22, 1–13 [DOI] [PubMed] [Google Scholar]
- 3.Thurman D.J., Alverson C., Dunn K.A., Guerrero J., and Sniezek J.E. (1999). Traumatic brain injury in the United States: a public health perspective. J. Head Trauma Rehabil. 14, 602–615 [DOI] [PubMed] [Google Scholar]
- 4.Selassie A.W., Zaloshnja E., Langlois J.A., Miller T., Jones P., and Steiner C. (2008). Incidence of long-term disability following traumatic brain injury hospitalization, United States, 2003. J. Head Trauma Rehabil. 23, 123–131 [DOI] [PubMed] [Google Scholar]
- 5.Langlois J.A., Rutland-Brown W., and Wald M.M. (2006). The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 21, 375–378 [DOI] [PubMed] [Google Scholar]
- 6.Kopp B., Kunkel A., Munickel W., Villringer K., Taub E., and Flor H. (1999). Plasticity in the motor system related to therapy induced improvement of movement after stroke. Neuroreport 10, 807–810 [DOI] [PubMed] [Google Scholar]
- 7.Kleim J.A. and Jones T.A. (2008). Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J. Speech. Lang. Hear. Res. 51, S225–S239 [DOI] [PubMed] [Google Scholar]
- 8.Combs H.L., Jones T.A., Kozlowski D.A., and Adkins D.L. (2015). Combinatorial motor training results in functional reorganization of remaining motor cortex after controlled cortical impact in rats. J. Neurotrauma 33, 741–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fridman E.A., Hanakawa T., Chung M., Hummel F., Leiguarda R.C., and Cohen L.G. (2004). Reorganization of the human ipsilesional premotor cortex after stroke. Brain 127, 747–758 [DOI] [PubMed] [Google Scholar]
- 10.Delvaux V., Alagona G., Gérard P., De Pasqua V., Pennisi G., and de Noordhout A.M. (2003). Post-stroke reorganization of hand motor area: a 1-year prospective follow-up with focal transcranial magnetic stimulation. Clin. Neurophysiol. 114, 1217–1225 [DOI] [PubMed] [Google Scholar]
- 11.Calautti C., Leroy F., Guincestre J.Y., and Baron J.C. (2003). Displacement of primary sensorimotor cortex activation after subcortical stroke: a longitudinal PET study with clinical correlation. Neuroimage 19, 1650–1654 [DOI] [PubMed] [Google Scholar]
- 12.Pineiro R., Pendlebury S., Johansen-Berg H., and Matthews P.M. (2001). Functional MRI detects posterior shifts in primary sensorimotor cortex activation after stroke: evidence of local adaptive reorganization? Stroke 32, 1134–1139 [DOI] [PubMed] [Google Scholar]
- 13.Weiller C., Chollet F., Friston K.J., Wise R.J.S., and Frackowiak R.S.J. (1992). Functional reorganization of the brain in recovery from striatocapsular infarction in man. Ann. Neurol. 31, 463–472 [DOI] [PubMed] [Google Scholar]
- 14.Biernaskie J., Szymanska A., Windle V., and Corbett D. (2005). Bi-hemispheric contribution to functional motor recovery of the affected forelimb following focal ischemic brain injury in rats. Eur. J. Neurosci. 21, 989–999 [DOI] [PubMed] [Google Scholar]
- 15.Hagemann G., Redecker C., Neumann-Haefelin T., Freund H.J., and Witte O.W. (1998). Increased long-term potentiation in the surround of experimentally induced focal cortical infarction. Ann. Neurol. 44, 255–258 [DOI] [PubMed] [Google Scholar]
- 16.Dijkhuizen R.M., Ren J., Mandeville J.B., Wu O., Ozdag F.M., Moskowitz M.A., Rosen B.R., and Finklestein S.P. (2001). Functional magnetic resonance imaging of reorganization in rat brain after stroke. Proc. Natl. Acad. Sci. U. S. A. 98, 12766–12771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dijkhuizen R.M., Singhal A.B., Mandeville J.B., Wu O., Halpern E.F., Finklestein S.P., Rosen B.R., and Lo E.H. (2003). Correlation between brain reorganization, ischemic damage, and neurologic status after transient focal cerebral ischemia in rats: a functional magnetic resonance imaging study. J. Neurosci. 23, 510–517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lotze M., Markert J., Sauseng P., Hoppe J., Plewnia C., and Gerloff C. (2006). The role of multiple contralesional motor areas for complex hand movements after internal capsular lesion. J. Neurosci. 26, 6096–6102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansen-Berg H., Rushworth M.F.S., Bogdanovic M.D., Kischka U., Wimalaratna S., and Matthews P.M. (2002). The role of ipsilateral premotor cortex in hand movement after stroke. Proc. Natl. Acad. Sci. U. S. A. 99, 14518–14523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossini P.M., Caltagirone C., Castriota-Scanderbeg A., Cicinelli P., Del Gratta C., Demartin M., Pizzella V., Traversa R., and Romani G.L. (1998). Hand motor cortical area reorganization in stroke: a study with fMRI, MEG and TCS maps. Neuroreport 9, 2141–2146 [DOI] [PubMed] [Google Scholar]
- 21.Turton A., Wroe S., Trepte N., Fraser C., and Lemon R.N. (1996). Contralateral and ipsilateral EMG responses to transcranial magnetic stimulation during recovery of arm and hand function after stroke. Electroencephalogr. Clin. Neurophysiol. Mot. Control 101, 316–328 [DOI] [PubMed] [Google Scholar]
- 22.Honda M., Nagamine T., Fukuyama H., Yonekura Y., Kimura J., and Shibasaki H. (1997). Movement-related cortical potentials and regional cerebral blood flow change in patients with stroke after motor recovery. J. Neurol. Sci. 146, 117–126 [DOI] [PubMed] [Google Scholar]
- 23.Muellbacher W., Artner C., and Mamoli B. (1999). The role of the intact hemisphere in recovery of midline muscles after recent monohemispheric stroke. J. Neurol. 246, 250–256 [DOI] [PubMed] [Google Scholar]
- 24.Jones T.A., Liput D.J., Maresh E.L., Donlan N., Parikh T.J., Marlowe D., and Kozlowski D.A. (2012). Use-dependent dendritic regrowth is limited after unilateral controlled cortical impact to the forelimb sensorimotor cortex. J. Neurotrauma 29, 1455–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pruitt D.T., Schmid A.N., Danaphongse T.T., Flanagan K.E., Morrison R.A., Kilgard M.P., Rennaker R.L., and Hays S.A. (2016). Forelimb training drives transient map reorganization in ipsilateral motor cortex. Behav. Brain Res. 313, 10–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pruitt D., Hays S., Schmid A., Choua C., Kim L., Trieu J., Kilgard M.P., and Rennaker R.L. (2014). Controlled-cortical impact reduces volitional forelimb strength in rats. Brain Res. 1582, 91–98 [DOI] [PubMed] [Google Scholar]
- 27.Pruitt D.T., Schmid A.N., Kim L.J., Abe C.M., Trieu J.L., Choua C., Hays S.A., Kilgard M.P., and Rennaker R.L. (2015). Vagus nerve stimulation delivered with motor training enhances recovery of function after traumatic brain injury. J. Neurotrauma 33, 871–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodriguez L.C., Palmer K., Montagner F., and Rodrigues D.C. (2015). A novel chlorhexidine-releasing composite bone cement: characterization of antimicrobial effectiveness and cement strength. J. Bioact. Compat. Polym. 30, 34–47 [Google Scholar]
- 29.Hays S.A., Khodaparast N., Sloan A.M., Hulsey D.R., Pantoja M., Ruiz A.D., Kilgard M.P., and Rennaker R.L. (2013). The isometric pull task: a novel automated method for quantifying forelimb force generation in rats. J. Neurosci. Methods 212, 329–337 [DOI] [PubMed] [Google Scholar]
- 30.Khodaparast N., Hays S.A., Sloan A.M., Hulsey D.R., Ruiz A., Pantoja M., Rennaker R.L., and Kilgard M.P. (2013). Vagus nerve stimulation during rehabilitative training improves forelimb strength following ischemic stroke. Neurobiol. Dis. 60, 80–88 [DOI] [PubMed] [Google Scholar]
- 31.Sloan A.M., Fink M.K., Rodriguez A.J., Lovitz A.M., Khodaparast N., Rennaker R.L., and Hays S.A. (2015). A within-animal comparison of skilled forelimb assessments in rats. PLoS One 10, e0141254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brus-Ramer M., Carmel J.B., and Martin J.H. (2009). Motor cortex bilateral motor representation depends on subcortical and interhemispheric interactions. J. Neurosci. 29, 6196–6206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Axelson H.W., Winkler T., Flygt J., Djupsjö A., Hånell A., and Marklund N. (2013). Plasticity of the contralateral motor cortex following focal traumatic brain injury in the rat. Restor. Neurol. Neurosci. 31, 73–85 [DOI] [PubMed] [Google Scholar]
- 34.Porter B.A., Khodaparast N., Fayyaz T., Cheung R.J., Ahmed S.S., Vrana W.A., Rennaker R.L., and Kilgard M.P. (2012). Repeatedly pairing vagus nerve stimulation with a movement reorganizes primary motor cortex. Cereb. Cortex 22, 2365–2374 [DOI] [PubMed] [Google Scholar]
- 35.Hulsey D.R., Hays S.A., Khodaparast N., Ruiz A., Das P., Rennaker R.L., and Kilgard M.P. (2016). Reorganization of motor cortex by vagus nerve stimulation requires cholinergic innervation. Brain Stimul. 9, 174–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khodaparast N., Hays S.A., Sloan A.M., Fayyaz T., Hulsey D.R., Rennaker R.L., and Kilgard M.P. (2014). Vagus nerve stimulation delivered during motor rehabilitation improves recovery in a rat model of stroke. Neurorehabil. Neural Repair 28, 698–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris N.G., Chen S.F., and Pickard J.D. (2013). Cortical reorganization after experimental traumatic brain injury: a functional autoradiography study. J. Neurotrauma 30, 1137–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barbay S., Guggenmos D.J., Nishibe M., and Nudo R.J. (2013). Motor representations in the intact hemisphere of the rat are reduced after repetitive training of the impaired forelimb. Neurorehabil. Neural Repair 27, 381–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ding M.C., Wang Q., Lo E.H., and Stanley G.B. (2011). Cortical excitation and inhibition following focal traumatic brain injury. J. Neurosci. 31, 14085–14094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nudo R.J. (2003). Adaptive plasticity in motor cortex: implications for rehabilitation after brain injury. J. Rehabil. Med. 7–10 [DOI] [PubMed] [Google Scholar]
- 41.Bach-y-Rita P. (2003). Theoretical basis for brain plasticity after a TBI. Brain Inj. 17, 643–651 [DOI] [PubMed] [Google Scholar]
- 42.Hays S.A. (2015). Enhancing rehabilitative therapies with vagus nerve stimulation. Neurotherapeutics 13, 382–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johansen-Berg H., Rushworth M.F.S., Bogdanovic M.D., Kischka U., Wimalaratna S., and Matthews P.M. (2002). The role of ipsilateral premotor cortex in hand movement after stroke. Proc. Natl. Acad. Sci. U. S. A. 99, 14518–14523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bashir S., Vernet M., Yoo W.K., Mizrahi I., Theoret H., and Pascual-Leone A. (2012). Changes in cortical plasticity after mild traumatic brain injury. Restor. Neurol. Neurosci. 30, 277–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sanders M.J., Sick T.J., Perez-Pinzon M.A., Dietrich W.D., and Green E.J. (2000). Chronic failure in the maintenance of long-term potentiation following fluid percussion injury in the rat. Brain Res. 861, 69–76 [DOI] [PubMed] [Google Scholar]
- 46.Reeves T.M., Lyeth B.G., and Povlishock J.T. (1995). Long-term potentiation deficits and excitability changes following traumatic brain injury. Exp. Brain Res. 106, 248–56 [DOI] [PubMed] [Google Scholar]
- 47.Miyazaki S., Katayama Y., Lyeth B.G., Jenkins L.W., DeWitt D.S., Goldberg S.J., Newlon P.G., and Hayes R.L. (1992). Enduring suppression of hippocampal long-term potentiation following traumatic brain injury in rat. Brain Res. 585, 335–9 [DOI] [PubMed] [Google Scholar]
- 48.Jefferson S.C., Clayton E.R., Donlan N.A., Kozlowski D.A., Jones T.A., and Adkins D.L. (2015). Cortical stimulation concurrent with skilled motor training improves forelimb function and enhances motor cortical reorganization following controlled cortical impact. Neurorehabil. Neural Repair 30, 155–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.