Abstract
Growth hormone (GH)/insulin-like growth factor (IGF)-1 axis abnormalities have been associated with body composition changes among HIV-infected persons with wasting or lipodystrophy. Little is known of GH/IGF-1 axis alterations with antiretroviral therapy (ART) initiation or differing ART therapies. The AIDS Clinical Trials Group Prospective Evaluation of Antiretrovirals in Resource-Limited Settings (PEARLS) study was a prospective, randomized clinical trial of ART initiation with emtricitabine/tenofovir + efavirenz (FTC/TDF+EFV) versus lamivudine/zidovudine + efavirenz (3TC/ZDV+EFV) in HIV-1-infected individuals from resource-diverse settings. IGF-1 was measured from baseline, week 48, and week 96 stored serum samples. Multivariate models were constructed. 415 participants were included: 170 (41%) were randomized to FTC/TDF+EFV and 245 (59%) to 3TC/ZDV+EFV. The mean age was 35 years, 60% were black, 42% women. The mean IGF-1 level did not change significantly from baseline to week 96 (−0.65 ng/ml; 95% confidence interval (CI) −5.18–3.87), p = .78 and there were no differences by treatment arm at week 96, p = .74. Lower baseline IGF-1 was associated with age, non-white race, greater waist–hip ratio (WHR), low CD4 count, and lower baseline albumin (all p < .01) but not plasma HIV-1 RNA, body mass index, or treatment arm. Greater change in IGF-1 from baseline to 96 weeks was associated with female sex, smaller WHR change, lower baseline albumin, and higher baseline HIV-1 RNA (all p < .01). ART initiation with either ZDV or TDF did not significantly impact overall IGF-1 levels. Baseline and on-treatment changes in IGF-1 with ART initiation may be related to the body composition changes that occur after ART initiation.
Keywords: : HIV, obesity, lipodystrophy, antiretroviral therapy, highly active, insulin-like growth factor-1
Introduction
Initiation of antiretroviral therapy (ART) among HIV-infected individuals is associated with changes in body composition, including gains in visceral adiposity, loss or gain of subcutaneous fat, and gains in lean body mass.1,2 These changes are typically most pronounced among HIV-infected persons with most severe disease (i.e., lowest CD4 lymphocyte counts and highest HIV-1 RNA levels) before ART initiation.3,4 The mechanisms that mediate these body composition changes are unclear, but may be, in part, regulated by changes in anabolic hormones: free testosterone levels increase significantly with ART initiation, particularly among HIV-infected individuals with the lowest CD4 lymphocyte counts before ART initiation.5 The role of ART on other anabolic hormones and the role of other anabolic hormones on these body composition changes are unclear.
The growth hormone (GH) and insulin-like growth hormone (IGF)-1 axis is an important regulator of adiposity, muscle mass, and bone in adults, and multiple studies have demonstrated strong associations between low levels of IGF-1 and obesity, metabolic syndrome, immune dysfunction, cardiovascular disease, physical and cognitive functional impairments, and increased mortality.6–12 Regulation of the GH/IGF-I axis is complex: hypothalamic GH-releasing hormone (GHRH) secretion triggers pulsatile pituitary secretion of GH, which subsequently results in hepatic and extrahepatic IGF-1 production. A link between HIV infection and abnormalities in the GH/IGF-1 axis was recognized in the early pre-ART era: HIV proteins were present in the hypothalamus of deceased subjects with advanced HIV13 and injection of the HIV envelope protein (gp120) into the third ventricle of rodents suppressed GH secretion compared to saline,14 suggesting HIV may have direct central effects on GH/IGF-1 dynamics. Subsequent cross-sectional studies have found numerous disruptions, including low GH and IGF-1 levels, blunted GH pulsatility, and a relative GH-resistant state in prior studies of lipodystrophy and wasting.15–17 Furthermore, therapies targeting the GH/IGF-1 axis such as the GHRH analogue, tesamorelin, are effective in reversing body composition changes in some but not all lipodystrophic patients.18–20
No prior studies have assessed changes in IGF-1 with ART initiation, and most studies examining the impact of any ART on IGF-1 are limited to small studies (N < 20) in the pre-ART era or with zidovudine (ZDV) monotherapy.21 ZDV use is associated with lipoatrophy but also achieves high concentrations in the central nervous system,22 and thus, the impact of ZDV on the GH/IGF-1 axis could be multifactorial. In a preliminary, unpublished analysis, we found significantly higher IGF-1 concentrations (185 ± 6 ng/ml) among participants on ZDV (N = 15) compared with participants using non-ZDV-based regimens (N = 65; 101 ± 4 ng/ml; p < .001; Erlandson unpublished data). Based on prior studies,17,21 we expected a decreased rather than increased IGF-I level in those taking ZDV. Our preliminary results may have been confounded by ART selection, body composition, gender, comorbidities, physical activity, and other lifestyle factors that were not controlled for in this small sample. Thus, the primary goal of the current study was to compare changes in IGF-1 with randomized ART initiation between a ZDV-containing or noncontaining regimen, as a potential mechanism in the development of lipodystrophy. Within this large sample, we also wanted to (1) explore clinical factors associated with baseline and change in IGF-1 levels and (2) determine whether baseline IGF-1 levels were predictive of change in body composition.
Materials and Methods
The AIDS Clinical Trials Group (ACTG) Prospective Evaluation of Antiretrovirals in Resource-Limited Settings (PEARLS) study (ClinicalTrials.gov NCT00084136) was a phase IV, randomized, open-label comparison of the once-daily non-nucleoside reverse transcriptase inhibitor [efavirenz, (EFV)] with either lamivudine/zidovudine (3TC/ZDV) or emtricitabine/tenofovir disoproxil fumarate (FTC/TDF). The data and safety monitoring board recommended stopping treatment early with a third regimen (didanosine-EC +FTC + atazanavir) due to inferiority; these participants are not included here. The study included participants from nine countries: Brazil, Haiti, India, Malawi, Peru, South Africa, Thailand, United States, and Zimbabwe. As we have previously shown, the two study arms did not differ significantly in virologic, immunologic, or mortality outcomes.23 The EFV + FTC/TDF arm experienced significantly greater gains in body mass index (BMI), waist, arm, and thigh circumference, and significantly fewer cases of lipoatrophy than the EFV +3TC/ZDV arm.24 Written informed consent was obtained from all participants, and the human experimentation guidelines of the U.S. Department of Health and Human Services were followed. The study was approved by local ethics committees at each participating institution.
Briefly, participants were ≥18 years old, had documented HIV-1 infection, were ART naive, and had a CD4 cell count <300 cells/μl within 90 days before entry into the study enrollment began in 2005, and participants were followed through May 2010. Participants had received no more than 7 days of cumulative prior ART (prior use of single-dose nevirapine or ZDV for any duration to prevent mother-to-child transmission of HIV was allowed). BMI was categorized as underweight (<18.5 kg/m2), normal/overweight (18.5–29.9 kg/m2), and obese (≥30 kg/m2).25 Waist and hip circumferences were measured as previously described.24 IGF-1 levels were measured from stored serum samples using chemiluminescence (Immunodiagnostics Systems, Fountain Hills, AZ) in the Clinical Translational Research Center of the University of Colorado.
Statistical analyses
Two-sample t-tests were used to describe the differences between baseline, week 48, and week 96 IGF-1 levels, respectively, along with baseline to week 48 and baseline to week 96 change in IGF-1 levels, between study treatment arms. Linear regression analysis was used to explore the role of other a priori identified variables and potential confounders in baseline, week 48, week 96, and change in IGF-1 levels. Covariates considered during model building included study treatment arm, age, race/ethnicity, sex, region (North America, South/Central America, Africa), BMI, waist-to-hip circumference ratio, CD4 T lymphocyte count, and plasma HIV-1 RNA concentration; variables with a p-value <.10 were retained in models. Two sensitivity analyses restricted the analyses to participants who remained on the initial randomized ART and to participants who remained virologically suppressed. Additional regression analyses included IGF-1 as a predictor variable and BMI and waist–hip ratio (WHR) as outcome measures, adjusted for age, gender, region, treatment group, baseline HIV-1 RNA, and CD4 count (< or ≥200 cells/μl). Statistical analyses were conducted in SAS v. 9.4 (SAS Institute, Cary, NC) and assumed a two-sided significance level of 0.05. No adjustment was made for multiple comparisons.
Results
Four hundred fifteen participants of the 1045 individuals who were randomized to receive EFV with either 3TC/ZDV or FTC/TDF had samples available for IGF-1 measurement from baseline, week 48, and week 96. Among participants, 245 (59%) were randomized to EFV +3TC/ZDV and 170 (41%) to EFV + FTC/TDF. The median age was 35 [intraquartile range (IQR) 29–43 years], and 42% were women. Between-group differences were similar with the exception of race (Table 1).
Table 1.
Baseline Participant Characteristics
| Characteristics | Overalla n = 415 | 3TC/ZDV+EFV n = 245 | FTC/TDF+EFV n = 170 | p |
|---|---|---|---|---|
| Age, years (median, IQR) | 35 (29–43) | 35 (29–43) | 35 (29–44) | .52 |
| Male | 239 (57.6) | 141 (57.6) | 98 (57.7) | .98 |
| Female | 176 (42.4) | 104 (42.4) | 72 (42.3) | |
| Race | ||||
| White | 97 (23.4) | 70 (28.6) | 27 (15.9) | .01 |
| Black | 247 (59.5) | 134 (54.7) | 113 (66.5) | |
| Other | 71 (17.1) | 41 (16.7) | 30 (17.6) | |
| Region | ||||
| United States | 71 (17.1) | 39 (15.9) | 32 (18.8) | .16 |
| Africa (Malawi, South Africa) | 128 (30.8) | 69 (28.2) | 59 (34.7) | |
| South American/Caribbean (Brazil, Peru, Haiti) | 216 (52.1) | 137 (55.9) | 79 (46.5) | |
| BMI (kg/m2) | 23.2 (20.8–26.1) | 23.4 (20.8–26.0) | 22.9 (20.8–26.3) | .36 |
| WHR | 0.88 (0.83–0.92) | 0.88 (0.83–0.93) | 0.88 (0.83–0.92) | .97 |
| CD4+ count (cells/μl) | 168 (84–233) | 172 (89–244) | 164 (83–220) | .35 |
| HIV-1 RNA (log10 copies/ml) | 5.03 (4.64–5.47) | 5.05 (4.65–5.48) | 5.02 (4.61–5.44) | .83 |
| Albumin (g/dl) | 4.0 (3.6–4.3) | 4.0 (3.6–4.3) | 4.0 (3.6–4.3) | .46 |
Results presented as n (frequency) or median (intraquartile range).
BMI, body mass index; EFV, efavirenz; FTC, emtricitabine; IQR, intraquartile range; 3TC, lamivudine; TDF, tenofovir; ZDV, zidovudine; WHR, waist–hip ratio.
At baseline, mean IGF-1 level was similar between treatment groups [156.7 ng/ml (95% CI 149.0–164.4 ng/ml) in 3TC/ZDV+EFV versus 158.5 ng/ml (95% CI 149.1–168.0 ng/ml) in FTC/TDF+EFV; p = .77]. IGF-1 level was below the age/gender-specific reference range among 32 (8%) participants. The baseline IGF-1 level was significantly lower among underweight participants [131.5 ng/ml (95% CI 109.3–153.7 ng/ml)] compared with normal/overweight participants [160.9 ng/ml (95% CI 154.4–167.4 ng/ml); p = .03], but not significantly different between normal/overweight and obese participants [143.1 ng/ml (95% CI 123.6–162.6 ng/ml; p = .10)]. In multivariate models, lower baseline IGF-1 level was significantly associated with increased age, black and other nonwhite race/ethnicity, greater WHR, lower CD4 count, and lower baseline albumin (all p ≤ .02) (Table 2).
Table 2.
Multivariate Analyses of Covariate Associations with IGF-1 Serum Levels at Baseline and Change in Insulin-Like Growth Factor -1 from Baseline to 96 Weeks
| Covariate | Coefficient | SE | p |
|---|---|---|---|
| Baseline IGF-1 level associations with baseline covariatesa | |||
| Age, years | −1.61 | 0.30 | <.0001 |
| Race (white, reference) | 0.00 | ||
| Black | −16.77 | 7.42 | .02 |
| Other race | −28.14 | 9.03 | .002 |
| Baseline WHR (per 0.1 change) | −11.68 | 4.17 | .005 |
| CD4 count (>200 cells/μl) | 13.47 | 5.88 | .02 |
| Albumin (g/dl) | 24.60 | 5.51 | <.0001 |
| Baseline to week 96 change IGF-1 level associations with baseline and change covariatesb | |||
| Female sex | 13.33 | 5.16 | .01 |
| Change in WHR (per 0.1 change) | −10.56 | 3.66 | .004 |
| HIV-1 RNA level (>log5) | −11.39 | 4.86 | .02 |
| Albumin (g/dl) | −10.80 | 4.50 | .02 |
Also included sex, HIV-1 RNA, BMI, treatment group.
Also included age, race, BMI week 96, CD4+ count week 96, baseline to week 96 change in CD4+ count, baseline to week 96 change in BMI, WHR week 96, and treatment group.
IGF-1, insulin-like growth factor-1.
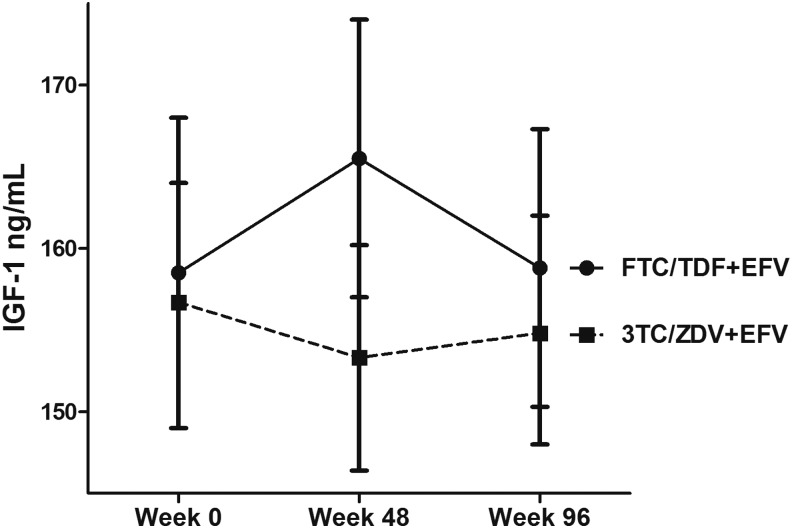
Following treatment initiation, mean IGF-1 levels at 96 weeks were not significantly different than baseline [−0.65 ng/ml (95% CI–5.18, 3.87); p = .78]. The mean difference in IGF-1 level from baseline to week 48 was significantly greater in the FTC/TDF+EFV arm (7.0 ng/ml) compared to 3TD/ZDV + EFV (−3.4 ng/ml; p = .04), but was not significantly different between arms from week 0 to week 96 (0.3 and −1.3 ng/ml, respectively; p = .74; Fig. 1). In one sensitivity analysis restricted to participants who remained on the initial randomized ART at week 48 (n = 376) and week 96 (n = 370), these differences were similar (week 48: 6.7 and −3.4 ng/ml, respectively, p = .05; week 96: −0.3 and −0.7 ng/ml; p = .93). In another sensitivity analysis restricted to participants who remained virologically suppressed at week 48 (n = 376) and week 96 (n = 370), these differences were also similar (week 48; 6.5 and −3.5 ng/ml; p = .06 and week 96; −0.1 and −0.7; p = .91). In multivariate analyses, female sex, higher baseline HIV-1 RNA, less change in WHR, and lower baseline albumin were associated with a greater increase in IGF-1 level from baseline to week 96 (all p < .01) (Table 2).
FIG. 1.
Treatment arm differences in mean (95% CI) IGF-1 levels at weeks 0, 48, and 96. FTC/TDF+EFV, emtricitabine/tenofovir + efavirenz; IGF-1, insulin-like growth factor; 3TC/ZDV+EFV, lamivudine/zidovudine + efavirenz.
To account for known age and gender differences in the expected “normal” range of IGF-1, we investigated whether a baseline IGF-1 below the specific reference range for age and gender might serve as a simple predictor for change in weight with ART initiation. In an unadjusted comparison, participants with low baseline IGF-1 levels had a significantly greater increase in BMI at 96 weeks compared with participants with normal or high baseline IGF-1 (9.9 vs 5.7% increase in BMI, p = .03).
Finally, the ability of IGF-1 to predict changes in BMI or WHR was explored in multivariate models with age, gender, region, treatment group, baseline HIV-1 RNA, CD4 count (< or ≥200 cells/μl), and baseline albumin level (g/dl). A greater percent increase in BMI from baseline to week 96 was associated with lower baseline HIV-1 RNA (<100,000 copies/ml; β 4.07%, SE 1.01; p < .0001), lower CD4 count (<200 cells/μl; β 4.23%, SE 1.04; p < .0001), lower baseline albumin level (g/dl; 3.77%, SE 1.06; p = .0004), assignment to FTC/TDF (β 2.16%, SE 0.99; p = .03), and younger age (β 0.12%, SE 0.05; p = .02), but not baseline IGF-1 (continuous). In contrast, in similar multivariate models, a greater percent increase in WHR was associated with higher baseline IGF-1 level (β 0.03%, SE 0.01; p = .01) and assignment to 3TC/ZDV (β2.45%, SE 1.25; p = .05).
Discussion
In this study, we have shown the IGF-1 trajectories over 96 weeks of ART initiation, in a cohort of participants from low-, middle-, and high-income countries. Although prior literature in the pre-ART era support abnormalities in the GH/IGF-1 axis associated with HIV and ZDV-based regimens,16,21,26 we found no significant changes in IGF-1 with ART initiation, and no significant differences between ZDV-based versus TDF-based regimens. Baseline and change in IGF-1 levels were, however, associated with albumin and WHR.
Due to differing treatment toxicities of the ART regimens, we anticipated that IGF-1 levels would differ by the two comparative treatment arms in our study. In a small eastern European study, IGF-1 was significantly higher among HIV-infected patients on a protease inhibitor-based regimen (N = 39) compared to an non-nucleoside reverse transcriptase inhibitor-based regimen (N = 17, both regimens with 2 nucleoside reverse transcriptase inhibitor [NRTI]).27 In contrast, other studies have not found differences: peak GH concentration and GH area under the curve were not significantly different among HIV-infected men and women taking tenofovir, lamivudine, or efavirenz.21 Similarly, GH, IGF-1, and IGF-binding protein-3 (IGFBP-3) concentrations among ZDV-treated patients with AIDS (N = 8) or asymptomatic HIV (N = 2) were not significantly different from patients not on ZDV (n = 6).17 To the best of our knowledge, this analysis is the first report of differences in IGF-1 in a large cohort with randomized ART, minimizing bias that may influence treatment choice and differences in HIV severity.
The associations between IGF-1, body composition, and the changes in these measures are complex: (1) a low baseline IGF-1 was associated with greater baseline WHR, (2) greater change in IGF-1 from baseline to week 96 was associated with smaller WHR changes, and (3) greater change in WHR was associated with lower baseline IGF-1. How can we make sense of these relationships? First, the low baseline IGF-1 was associated with greater baseline WHR as well as lower CD4 count, higher HIV-1 viral load, and lower albumin. This combination of findings suggests that low IGF-1 may serve as a marker for HIV disease severity and wasting with a higher baseline WHR reflecting more loss of hip girdle musculature than abdominal girth. Several prior studies have found a strong association between IGF-1 and AIDS or AIDS wasting: low IGF-1 has been associated with skeletal muscle wasting among both older adults without HIV infection28 and with AIDS wasting.29 Other studies have described AIDS wasting as a GH-resistant state with increased basal GH, but decreased IGF-1 and decreased IGF-binding proteins, resulting in further decreased systemic effects of bioactive IGF-1.15,30,31 Finally, although seldom used in the current ART era, growth hormone-based therapies previously demonstrated marked improvement in AIDS wasting32,33 and an increase in CD4+ T cells among HIV-infected patients on ART with an incomplete immune response.34
Second, a greater change in IGF-1 from baseline to week 96 was associated with smaller WHR changes. In contrast to the baseline associations, these findings likely reflect the relationship between adiposity and IGF-1. Among both HIV-infected and HIV-uninfected populations, IGF-1 is suppressed in the setting of central abdominal fat or visceral adipose tissue.35–37 Thus, the greater gains in central adiposity following ART initiation24 may have attenuated expected increases in IGF-1 with a “return to health.” This concept is also illustrated in the third point above: greater increases in WHR were associated with both lower baseline IGF-1 and randomized ZDV, independent of HIV disease severity. Together, these findings suggest that a low baseline IGF-1 in addition to 3TC/ZDV may be risk factors for the development of WHR-estimated lipodystrophy. Although directionality cannot be assumed with the data, our findings suggest that low baseline IGF-1 and blunted IGF-1 increases with ART are associated with a greater central fat accumulation and/or subcutaneous fat loss.
Several limitations of this study should be noted. Most circulating IGF-1 is bound to one of six different binding proteins, which were not assessed in this study.26 IGF-1 is highly influenced by many different confounders such as nutritional status, alcohol consumption, and physical activity, or by additional comorbidities, including hypothyroidism, diabetes, renal function, and liver function. Furthermore, the study population is diverse, with marked differences in nutrition and diet and physical activity, in addition to unmeasured genetic differences. Although we were unable to evaluate many of these additional factors in this setting, we do not expect that confounders such as nutritional status or physical activity would differ significantly by the ART regimen. Ideally, measures of GH stimulation could be assessed to determine the responsiveness of the GH/IGF-1 axis. Anthropomorphic measures such as WHR may not accurately distinguish subcutaneous adiposity and VAT. However, use of measurements such as BMI and WHR allows for translation of findings in the clinical setting where body composition imaging is not routinely available. The study has multiple notable strengths: the cohort includes nearly 50% women and a high proportion of persons of African race, populations with very limited data in HIV and IGF-1 previously. We provide the first data on the effect of ART initiation on IGF-1, and the first comparison of two randomized ART regimens differing only by the NRTI backbone.
In summary, IGF-1 changes did not differ by randomized ART treatment arm. Clinically, an important next question is whether a biomarker, such as IGF-1, before or during ART initiation can predict development of body composition changes and inform subsequent clinical care or treatment decisions. The association between IGF-1 and body composition suggests that baseline IGF-1 may be a marker of HIV disease severity, while on-treatment changes in IGF-1 may be associated with the heterogeneity of body composition changes seen following ART initiation. Attention should be directed toward limiting excessive weight gains among underweight and obese persons initiating ART. Finally, changes in the GH/IGF-1 axis should be investigated with more modern ART regimens, as body composition changes are still common with ART initiation and predict increased morbidity and mortality.
Acknowledgments
The authors thank the study sites, study participants, and support of the AIDS Clinical Trials Group. Research reported in this publication was supported by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under award number UM1 AI068634, UM1 AI068636, and UM1 AI106701. K.M.E. received support by the National Institute of Aging of the National Institutes of Health under award number K23AG050260 and NIH/NCATS Colorado CTSA grant number UL1 TR001082; T.T.B. is supported, in part, by NIH/NIAID K24 AI120834. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The pharmaceutical sponsors (Boehringer Ingelheim, Bristol-Myers Squibb, Gilead Sciences, and GlaxoSmithKline) provided study drug and Gilead Sciences provided funding to purchase study drug that was not otherwise available. Bristol-Myers Squibb provided atazanavir, didanosine-EC, and efavirenz (with consent of Merck); Gilead Sciences, Inc., provided emtricitabine, tenofovir-DF, emtricitabine/tenofovir-DF; GlaxoSmithKline provided lamivudine, zidovudine, and lamivudine/zidovudine; and Boehringer Ingelheim Pharmaceuticals, Inc., provided nevirapine. Representatives of the pharmaceutical company sponsors had no role in study design, data collection and analysis, and decision to publish or preparation of the manuscript.
Author Disclosure Statement
K.M.E. has received research funding from Gilead Sciences and has served as a medical advisor for Theratechnologies. T.T.B. has served as a consultant to Gilead Sciences, Merck, BMS, EMD Serono, and Theratechnologies. All other authors declair no conflicts of interest.
References
- 1.McComsey GA, Kitch D, Sax PE, et al. : Peripheral and central fat changes in subjects randomized to abacavir-lamivudine or tenofovir-emtricitabine with atazanavir-ritonavir or efavirenz: ACTG Study A5224s. Clin Infect Dis 2011;53:185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McComsey GA, Moser C, Currier J, et al. : Body composition changes after initiation of Raltegravir or protease inhibitors: ACTG A5260s. Clin Infect Dis 2016;62:853–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erlandson KM, Kitch D, Tierney C, et al. : Weight and lean body mass change with antiretroviral initiation and impact on bone mineral density. AIDS 2013;27:2069–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grant PM, Kitch D, McComsey GA, et al. : Long-term body composition changes in antiretroviral-treated HIV-infected individuals. AIDS 2016;30:2805–2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dube MP, Parker RA, Mulligan K, et al. : Effects of potent antiretroviral therapy on free testosterone levels and fat-free mass in men in a prospective, randomized trial: A5005s, a substudy of AIDS Clinical Trials Group Study 384. Clin Infect Dis 2007;45:120–126 [DOI] [PubMed] [Google Scholar]
- 6.Fried LP, Xue QL, Cappola AR, et al. : Nonlinear multisystem physiological dysregulation associated with frailty in older women: Implications for etiology and treatment. J Gerontol A Biol Sci Med Sci 2009;64:1049–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leng SX, Cappola AR, Andersen RE, et al. : Serum levels of insulin-like growth factor-I (IGF-I) and dehydroepiandrosterone sulfate (DHEA-S), and their relationships with serum interleukin-6, in the geriatric syndrome of frailty. Aging Clin Exp Res 2004;16:153–157 [DOI] [PubMed] [Google Scholar]
- 8.Onder G, Liperoti R, Russo A, et al. : Body mass index, free insulin-like growth factor I, and physical function among older adults: Results from the ilSIRENTE study. Am J Physiol Endocrinol Metab 2006;291:E829–E834 [DOI] [PubMed] [Google Scholar]
- 9.Cappola AR, Xue QL, Ferrucci L, et al. : Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J Clin Endocrinol Metab 2003;88:2019–2025 [DOI] [PubMed] [Google Scholar]
- 10.Cappola AR, Bandeen-Roche K, Wand GS, et al. : Association of IGF-I levels with muscle strength and mobility in older women. J Clin Endocrinol Metab 2001;86:4139–4146 [DOI] [PubMed] [Google Scholar]
- 11.Faupel-Badger JM, Berrigan D, Ballard-Barbash R, et al. : Anthropometric correlates of insulin-like growth factor 1 (IGF-1) and IGF binding protein-3 (IGFBP-3) levels by race/ethnicity and gender. Ann Epidemiol 2009;19:841–849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roubenoff R, Parise H, Payette HA, et al. : Cytokines, insulin-like growth factor 1, sarcopenia, and mortality in very old community-dwelling men and women: The Framingham Heart Study. Am J Med 2003;115:429–435 [DOI] [PubMed] [Google Scholar]
- 13.Langford D, Baron D, Joy J, et al. : Contributions of HIV infection in the hypothalamus and substance abuse/use to HPT dysregulation. Psychoneuroendocrinology 2011;36:710–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mulroney SE, McDonnell KJ, Pert CB, et al. : HIV gp120 inhibits the somatotropic axis: A possible GH-releasing hormone receptor mechanism for the pathogenesis of AIDS wasting. Proc Natl Acad Sci U S A 1998;95:1927–1932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frost RA, Fuhrer J, Steigbigel R, et al. : Wasting in the acquired immune deficiency syndrome is associated with multiple defects in the serum insulin-like growth factor system. Clin Endocrinol (Oxf) 1996;44:501–514 [DOI] [PubMed] [Google Scholar]
- 16.Geffner ME, Yeh DY, Landaw EM, et al. : In vitro insulin-like growth factor-I, growth hormone, and insulin resistance occurs in symptomatic human immunodeficiency virus-1-infected children. Pediatr Res 1993;34:66–72 [DOI] [PubMed] [Google Scholar]
- 17.Heijligenberg R, Sauerwein HP, Brabant G, et al. : Circadian growth hormone secretion in asymptomatic human immune deficiency virus infection and acquired immunodeficiency syndrome. J Clin Endocrinol Metab 1996;81:4028–4032 [DOI] [PubMed] [Google Scholar]
- 18.Falutz J, Allas S, Mamputu JC, et al. : Long-term safety and effects of tesamorelin, a growth hormone-releasing factor analogue, in HIV patients with abdominal fat accumulation. AIDS 2008;22:1719–1728 [DOI] [PubMed] [Google Scholar]
- 19.Falutz J, Mamputu JC, Potvin D, et al. : Effects of tesamorelin (TH9507), a growth hormone-releasing factor analog, in human immunodeficiency virus-infected patients with excess abdominal fat: A pooled analysis of two multicenter, double-blind placebo-controlled phase 3 trials with safety extension data. J Clin Endocrinol Metab 2010;95:4291–4304 [DOI] [PubMed] [Google Scholar]
- 20.Falutz J, Potvin D, Mamputu JC, et al. : Effects of tesamorelin, a growth hormone-releasing factor, in HIV-infected patients with abdominal fat accumulation: A randomized placebo-controlled trial with a safety extension. J Acquir Immune Defic Syndr 2010;53:311–322 [DOI] [PubMed] [Google Scholar]
- 21.Koutkia P, Eaton K, You SM, et al. : Growth hormone secretion among HIV infected patients: Effects of gender, race and fat distribution. AIDS 2006;20:855–862 [DOI] [PubMed] [Google Scholar]
- 22.Letendre S, Marquie-Beck J, Capparelli E, et al. : Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol 2008;65:65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Campbell TB, Smeaton LM, Kumarasamy N, et al. : Efficacy and safety of three antiretroviral regimens for initial treatment of HIV-1: A Rrndomized clinicaltrial in diverse multinational settings. PLoS Med 2012;9:e1001290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erlandson KM, Taejaroenkul S, Smeaton L, et al. : A randomized comparison of anthropomorphic changes with preferred and alternative efavirenz-based antiretroviral regimens in diverse multinational settings. Open Forum Infect Dis 2015;2:ofv095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishida C, Ko GT, Kumanyika S: Body fat distribution and noncommunicable diseases in populations: Overview of the 2008 WHO expert consultation on waist circumference and Waist-Hip Ratio. Eur J Clin Nutr 2010;64:2–5 [DOI] [PubMed] [Google Scholar]
- 26.Holly JM, Perks CM: Insulin-like growth factor physiology: What we have learned from human studies. Endocrinol Metab Clin North Am 2012;41:249–263, v [DOI] [PubMed] [Google Scholar]
- 27.Parfieniuk-Kowerda A, Czaban SL, Grzeszczuk A, et al. : Assessment of serum IGF-1 and adipokines related to metabolic dysfunction in HIV-infected adults. Cytokine 2013;64:97–102 [DOI] [PubMed] [Google Scholar]
- 28.Perrini S, Laviola L, Carreira MC, et al. : The GH/IGF1 axis and signaling pathways in the muscle and bone: Mechanisms underlying age-related skeletal muscle wasting and osteoporosis. J Endocrinol 2010;205:201–210 [DOI] [PubMed] [Google Scholar]
- 29.Grinspoon S, Corcoran C, Stanley T, et al. : Effects of androgen administration on the growth hormone-insulin-like growth factor I axis in men with acquired immunodeficiency syndrome wasting. J Clin Endocrinol Metab 1998;83:4251–4256 [DOI] [PubMed] [Google Scholar]
- 30.Falutz J: Growth hormone and HIV infection: Contribution to disease manifestations and clinical implications. Best Pract Res Clin Endocrinol Metab 2011;25:517–529 [DOI] [PubMed] [Google Scholar]
- 31.Congote LF: Monitoring insulin-like growth factors in HIV infection and AIDS. Clin Chim Acta 2005;361:30–53 [DOI] [PubMed] [Google Scholar]
- 32.Krentz AJ, Koster FT, Crist DM, et al. : Anthropometric, metabolic, and immunological effects of recombinant human growth hormone in AIDS and AIDS-related complex. J Acquir Immune Defic Syndr 1993;6:245–251 [PubMed] [Google Scholar]
- 33.Schambelan M, Mulligan K, Grunfeld C, et al. : Recombinant human growth hormone in patients with HIV-associated wasting. A randomized, placebo-controlled trial. Serostim Study Group. Ann Intern Med 1996;125:873–882 [DOI] [PubMed] [Google Scholar]
- 34.Smith K, Zheng L, Bosch R, et al. : Treatment with recombinant growth hormone is associated with modest improvement in CD4 lymphocyte reconstitution in HIV-infected persons on antiretroviral therapy: Results of ACTG A5174. AIDS Res Hum Retroviruses 2010;26:425–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grinspoon S, Gelato M: Editorial: The rational use of growth hormone in HIV-infected patients. J Clin Endocrinol Metab 2001;86:3478–3479 [DOI] [PubMed] [Google Scholar]
- 36.Blackman MR: Manipulation of the growth hormone axis in patients with HIV infection. N Engl J Med 2007;357:2397–2399 [DOI] [PubMed] [Google Scholar]
- 37.Di Somma C, Ciresi A, Amato MC, et al. : Alteration of the growth hormone axis, visceral fat dysfunction, and early cardiometabolic risk in adults: The role of the visceral adiposity index. Endocrine 2015;49:492–502 [DOI] [PubMed] [Google Scholar]