Abstract
Background
The influence of socio-economic determinants on choice of infant male circumcision provider is not known in areas with high population coverage such as rural Africa. The overall aim of this study was to determine the key socio-economic factors which influence the choice of infant male circumcision provider in rural Ghana.
Methods
The study investigated the effect of family income, distance to health facility, and cost of the circumcision on choice of infant male circumcision provider in rural Ghana. Data from 2847 circumcised infant males aged under 12 weeks and their families were analysed in a population-based cross-sectional study conducted from May to December 2012 in rural Ghana. Multivariable logistic regression models were adjusted for income status, distance to health facility, cost of circumcision, religion, maternal education, and maternal age.
Results
Infants from the lowest income households (325, 84.0%) were more likely to receive circumcision from an informal provider compared to infants from the highest income households (260, 42.4%) even after adjusting for religious affiliation (adjusted odds ratio [aOR] 4.42, 95% CI 3.12–6.27 p = <0.001). There appeared to be a dose response with increasing risk of receiving a circumcision from an informal provider as distance to a health facility increased (aOR 1.25, 95 CI 1.30–1.38 P = <0.001). Only 9.0% (34) of families in the lowest socio-economic quintile received free circumcision services compared to 27.9% (171) of the highest income families.
Conclusions
The Government of Ghana and Non-Government Organisations should consider additional support to poor families so they can access high quality free infant male circumcision in rural Ghana.
Keywords: Socio-economic, Infant, Male, Circumcision, Community, Population-based, Ghana
Background
Globally, male infants are circumcised mostly for medical and religious reasons [1, 2]. Male circumcision has been reported in a number of high quality trials to reduce human immunodeficiency virus (HIV) infection in adult males who live in communities with high HIV prevalence such as South and East Africa [3, 4]. Other health benefits are less clear though some families feel that it reduces risks of urinary tract infection and balanitis [1, 2]. Approximately 91% of infant (age 0–11 months) males are circumcised in Ghana [5] and other West African countries [2]. We reported high risks of concerning health care practices and morbidities following infant male circumcision in our community based study in rural Ghana [6]. Fifty eight percent of circumcisions were performed by informal providers; including Wanzams (village based traditional circumcision providers), family members, and drug sellers.
Initiatives to improve the health care practices of Wanzams and other circumcision providers are underway [7, 8]. These include training on infection control, instruments to perform circumcision and hygiene. However, other strategies to influence family’s care seeking patterns, improve use of health facilities, and improve use of trained circumcision providers are also needed. This requires an understanding of the key factors which influence a family’s choice of circumcision provider. A recent systematic review reported that socio-economic factors such as income, location (rural and urban), and cost of the circumcision were key determinants of choice of health service provider for infant male circumcision [2]. Socio-economic status, cost, and geographical access are also key determinants of care seeking for antenatal and birthing care in sub-Saharan African populations [9–14]. However, to our knowledge, there have been no studies from rural Africa that have investigated the effect of these factors on choice of infant male circumcision provider.
Thus, the overall aim of this study was to determine the key socio-economic factors which influence the choice of infant male circumcision provider in rural Ghana. The primary objective was to determine if socio-economic status was an important determinant of choice of circumcision provider. The secondary objectives were to assess the associations between distance to health facilities and cost of circumcision on choice of circumcision provider.
Methods
Study design and setting
This was a community level population-based cross-sectional study conducted in the Brong Ahafo Region of central Ghana from 21st May 2012 to 31st December 2012. Data were collected during a large neonatal vitamin A supplementation trial (Neovita) and full details are published elsewhere [15]. At the time of the circumcision study, 80% of the study population lived in rural settlements and almost 20% of mothers did not have primary school education. Four major district hospitals and 80 small health facilities provided health care services to the population. There were approximately 60 Wanzams and 100 formal circumcision providers (doctors, nurses, and medical assistants) at the time of the study.
Data collection
All births in the Neovita study area were reported to the trial team via a network of fieldworkers and key informants. Fieldworkers visited all families at home between two hours and two days after birth and interviewed the mother of the infant, or the primary care giver. Fieldworkers weighed the baby and asked the mother or the primary care giver about: date of birth, site of birth, current address, distance to health facilities, socio-demographic characteristics, and socio-economic information (using an asset index). The fieldworkers also collected data on the vital status of the baby (including if the baby was alive, dead, or hospitalised).
Only male liveborn Neovita infants who were aged under 12 weeks were included to ensure the most accurate recall of circumcision related events. Infants were included in the Neovita trial if they were aged under three days, able to feed, were staying in the study area for at least six months after enrolment and their mother provided written informed consent. Follow-up visits were scheduled between eight to eleven weeks post birth and trained senior fieldworkers asked for consent to collect additional detailed data on: age at circumcision, site of circumcision, and type of circumcision provider. Infant male circumcision was supposed to be covered under the Ghana Health Insurance Scheme but it was well known that fees for circumcisions were charged by some formal and informal providers. So we also asked families if they had to pay any fees or “in-kind” contributions for the circumcision. Families were also asked if the study team could have access to the baby’s Neovita data including socio-economic, and socio-demographic data.
Fieldworkers were trained for two weeks in all study procedures prior to the commencement of the study. Interrater reliability was checked between all fieldworkers. During the study fieldworkers received scheduled and unscheduled supervisory visits from the study coordinator to assess data quality and consistency. The fieldworkers used standardised paper based data collection tools (including a standardised list of closed ended questions) for all interviews.
Study definitions and categories
In our study a ‘formal circumcision provider’ was defined as a professionally trained, licensed, and regulated provider of circumcision services. This included: doctors, medical assistants, or nurses [2]. An ‘informal circumcision provider’ was an untrained, unlicensed, unregulated private provider of circumcision services including: Wanzams (village based traditional circumcision providers), drug sellers, and family members [2, 8, 16]. To assess ‘income status’ an asset index was constructed based on data collected on household assets (ownership of animals, television, motorcycle, etc) and housing material (walls, floor, windows, and roof). The index was analysed using principal component analysis (PCA) in Stata version 13 and categorised into five income quintiles [17]. ‘Distance to a health facility’ was measured in kilometres using Geographic Information System (GIS) software and the most commonly used roads from each village to the nearest health facility. It was categorised into four levels (<1 km (kilometre), 1–4.9 km, 5–9.9 km, 10 km or more). Many of the families in our study had limited recall about the exact cash amounts they paid for their circumcision but could categorise their responses. Thus information on the exact cash amounts for ‘cost of the circumcision’ was not collected and data were collected in the following categories: free, not free but less than 10 Ghana Cedis (Ghs), between 10 and 20 Ghs, 20 Ghs or more (at the time of conducting the study 1 Ghs = 0.6 United States dollars ($US)) [18]. ‘In kind contributions’ were defined as any non-cash payment to the formal or informal provider for the circumcision (e.g. bars of soap, chickens, kola nuts, and corn).
Statistical analysis
Crude logistic regression models were used to examine the effect of income status on type of circumcision provider (informal vs formal). Odds ratios (ORs) and 95% confidence intervals (95% CI) were calculated. Multivariable logistic regression models were constructed apriori to adjust for the effect of important explanatory variables (income status, cost of circumcision, religion, maternal education, maternal age and distance to health facility). Model one assessed each of the infant and maternal characteristics as determinants of choice of informal provider, adjusting for income status, cost of circumcision, religion, maternal education and maternal age. Model two is the same as model one with an additional adjustment for distance to health facility. All analyses were conducted using STATA version 13.
We calculated that the 2800 infants included in this study would provide 80% power to detect at least a 20% effect due to income status on choice of circumcision provider. We assumed a 5% significance level and a baseline 60% risk of receiving circumcision from an informal circumcision provider [6].
Ethical issues
Ethical approvals were obtained from Ghana Health Service Ethical Review Committee, the Institutional Ethics Committee of Kintampo Health Research Centre (KHRC), the Research Ethics Committee of London School of Hygiene and Tropical Medicine, and the Human Research Ethics Committee of the University of Western Australia. Written informed consent was obtained from all the families of the circumcised male infants.
Role of funding source
The funders had no role in data gathering, data analysis, or writing of the report. The corresponding author had full access to all the data in the study, and for the decision to submit for publication.
Results
There were 9100 live births in the Neovita trial study area from 21st May to 31st December 2012 (Fig. 1). A total of 8110 (89%) liveborn infants were recruited into the Neovita study. Forty nine percent (4005) were male infants and 78% (3141) were aged under 12 weeks. Of the 3141 eligible male infants, 2850 (90.7%) were circumcised. Two hundred and ninety one (9.3%) infants were not circumcised within 12 weeks after birth. Of these, 153 (52.6%) were circumcised at a later date, 84 (28.9%) were never circumcised and 54 (18.6%) died. Three circumcised babies (0.1%) had no socio-economic or demographic data collected and were excluded in the statistical analysis of associations between socio-economic or demographic factors and choice of circumcision provider. Of the remaining 2847 circumcised infants, 1670 (59%) were circumcised by informal providers and 1177 (41%) by formal health service providers (Table 1). Three hundred and eighty seven (13.6%) were in the lowest socio-economic quintile, 186 (6.7%) lived 10 km or more from a health facility, and 512 (18.0%) mothers of circumcised infants had no primary school education (Table 1). A total of 666 (23.4%) mothers of circumcised infants were Muslim, and 549 (19.3%) delivered at home (Table 1). Five hundred and thirty nine (18.9%) infants received their circumcision free of charge (Table 2). A total of 2229 (78.3%) families paid some form of cash currency (between 1 and 100 Ghana Cedis (Ghs) [approximately 0.60 to 55 $US]) for their infant’s circumcision and 87 (3.1%) families paid in-kind contributions in the form of bars of soap, chickens, kola nuts, and corn (Table 3).
Fig. 1.
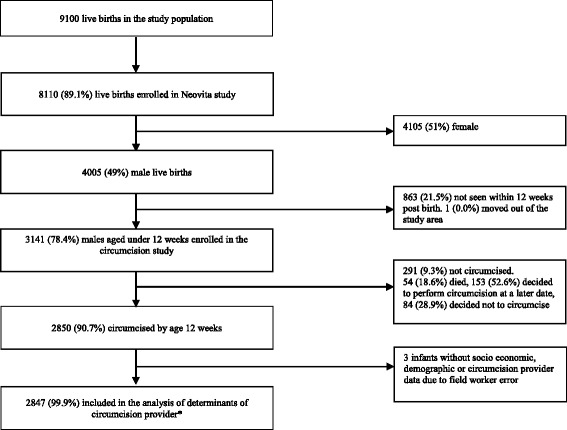
Flow diagram for the circumcision study. *Includes the 54 (18.6%) who died. These families were still interviewed and provided full information about circumcision thus their data were included
Table 1.
Infant and maternal characteristics in the study population
| Characteristics | Uncircumcised infants n = 291 | Circumcised infants included in the analysis n = 2847 (99.9%) | Total circumcised infants n = 2850a |
|---|---|---|---|
| Income status of household (quintile) | |||
| 1 (Lowest) | 115 (39.5%) | 387 (13.6%) | 387 (13.6%) |
| 2 | 75 (25.8%) | 532 (18.7%) | 532 (18.7%) |
| 3 | 47 (16.2%) | 628 (22.1%) | 628 (22.1%) |
| 4 | 38 (13.0%) | 687 (21.1%) | 687 (24.1%) |
| 5 (Highest) | 16 (5.5%) | 613 (21.5%) | 613 (21.5%) |
| Missing data | – | – | 3 (0.1%) |
| Distance to health facility | |||
| < 1 km | 101 (34.7%) | 1444 (50.7%) | 1444 (50.7%) |
| 1–4.9 km | 60 (20.6%) | 741 (26.0%) | 741 (26.0%) |
| 5–10 km | 84 (2.9%) | 400 (14.0%) | 400 (14.0%) |
| 10 km or more | 45 (15.5%) | 186 (6.5%) | 186 (6.5%) |
| Missing data | 1 (0.3%) | 76 (2.7%) | 79 (2.8%) |
| Cost of circumcisionb | |||
| Free | – | 539 (18.9%) | 539 (18.9%) |
| Less than 10 Ghs | – | 145 (5.1%) | 145 (5.1%) |
| Between 10 and 20 Ghs | – | 1530 (53.7%) | 1530 (53.7%) |
| 20 Ghs or more | – | 554 (19.5) | 554 (19.4) |
| Missing data | – | 79 (2.8%) | 82 (2.9%) |
| Maternal occupation | |||
| Gov’t/private employed | 4 (1.4%) | 105 (3.7%) | 105 (3.7%) |
| Self-employed | 53 (18.2%) | 927 (32.6%) | 927 (32.5%) |
| Farming | 101 (34.7%) | 589 (20.7%) | 589 (20.7%) |
| Not working | 49 (16.8%) | 484 (17.0%) | 484 (17.0%) |
| Missing data | 84 (28.9%) | 742 (26.0%) | 745 (26.4%) |
| Maternal highest educational level | |||
| None | 89 (30.6%) | 512 (18.0%) | 512 (18.0%) |
| Primary | 138 (47.2%) | 1481 (52.0%) | 1482 (52.0%) |
| Secondary | 63 (21.6%) | 850 (29.9%) | 852 (29.9%) |
| Missing data | 1 (0.3%) | 4 (0.1%) | 4 (0.1%) |
| Religion | |||
| Christian | 186 (8.3%) | 2048 (71.9%) | 2048 (71.9%) |
| Muslim | 61 (8.4%) | 666 (23.4%) | 666 (23.4%) |
| Traditional African/none | 42 (24.6%) | 129 (4.5%) | 129 (4.5%) |
| Missing data | 2 (0.7%) | 4 (0.1%) | 7 (0.2%) |
| Maternal age (years) | |||
| Less than 20 | 41 (14.1%) | 319 (11.2%) | 319 (11.2% |
| 20–29 | 145 (49.8%) | 1458 (51.2%) | 1458 (51.2%) |
| 30 or more | 104 (35.7%) | 1066 (37.4%) | 1066 (37.4%) |
| Missing data | 1 (0.3%) | 4 (0.1%) | 7 (0.2%) |
| Site of delivery | |||
| Health facility | 187 (64.3%) | 2292 (80.5%) | 2292 (80.4%) |
| Home | 101 (34.7%) | 549 (19.3%) | 549 (19.3%) |
| Missing data | 3 (1.0%) | 6 (0.2%) | 9 (0.3%) |
| Birth weight | |||
| Less than 2.5 kg | 41 (14.1%) | 214 (7.5%) | 214 (7.5%) |
| 2.5 kg or greater | 250 (85.9%) | 2633 (92.5%) | 2636 (92.5%) |
| Missing data | – | – | – |
| Type of circumcision provider | – | ||
| Formal provider | – | 1177 (41.3%) | 1177 (41.3%) |
| Informal provider | – | 1670 (58.7%) | 1670 (58.6%) |
| Missing data | – | 0 (0.0%) | 3 (0.1%) |
| Age at circumcision | |||
| 0–6 days | – | 117 (4.1%) | 117 (4.1%) |
| 7–20 days | – | 2556 (89.8%) | 2556 (89.7%) |
| > 20 days | – | 172 (6.0%) | 172 (6.0%) |
| Missing data | – | 2 (0.1%) | 5 (0.2%) |
aThree circumcised infants had no socioeconomic and demographic data due to field worker error
b1 Ghs = 0.6 $US (2012)
Table 2.
Determinants of choice of informal provider for infant male circumcision
| Total number of infants | Number (%) of infants who received circumcision from an informal provider | ||||
|---|---|---|---|---|---|
| n = 2847 | n = 1670 (58.7%) | Unadjusted odds ratio (95% CI) | Adjusted odds ratio (95% CI) model 1a | Adjusted odds ratio (95% CI) model 2b | |
| Income status of household (quintile) | |||||
| 1 (Lowest) | 387 | 325 (84.0%) | 7.12 (5.19–9.76) | 5.77 (4.15–8.02) | 4.42 (3.12–6.27) |
| 2 | 532 | 349 (65.6%) | 2.59 (2.04–3.29) | 2.26 (1.76–2.91) | 1.89 (1.45–2.47) |
| 3 | 628 | 355 (56.5%) | 1.77 (1.41–2.21) | 1.67 (1.32–2.11) | 1.49 (1.17–1.90) |
| 4 | 687 | 381 (55.5%) | 1.69 (1.36–2.11) | 1.58 (1.26–1.98) | 1.48 (1.17–1.87) |
| 5 (Highest) | 613 | 260 (42.4%) | Ref | Ref | Ref |
| Missing data | 0 | – | – | - | - |
| Distance to health facility | |||||
| < 1 km | 1444 | 776 (53.8%) | Ref | Ref | Ref |
| 1–4.9 km | 741 | 433 (58.4%) | 1.21 (1.01–1.45) | 1.20 (1.00–1.45) | 1.20 (1.00–1.45) |
| 5–9.9 km | 400 | 286 (71.5%) | 2.16 (1.70–2.74) | 1.28 (1.00–1.67) | 1.28 (1.00–1.67) |
| 10 km or more | 186 | 154 (83.4%) | 4.41 (2.94–6.61) | 2.70 (1.76–4.12) | 2.70 (1.76–4.12) |
| Missing data | 76 | 21 (26.6%) | – | - | - |
| Mean 3.2 km sd 3.7 | – | – | - | ||
| Cost of circumcisionc | |||||
| Free | 539 | 269 (49.9%) | Ref | Ref | Ref |
| Less than 10 Ghs | 145 | 114 (78.6%) | 3.69 (2.40–5.68) | 2.43 (1.54–3.84) | 2.41 (1.53–3.79) |
| Between 10 and 20 Ghs | 1530 | 888 (58.0%) | 1.39 (1.14–1.69) | 1.22 (0.91–1.38) | 1.14 (0.92–1.41) |
| 20 Ghs or more | 554 | 337 (60.8%) | 1.56 (1.23–1.98) | 1.32 (1.03–1.69) | 1.26 (0.97–1.63) |
| Missing data | 79 | 62 (78.5%) | – | – | – |
| Maternal occupation | |||||
| Gov’t/private employed | 105 | 49 (46.7%) | 0.63 (0.41–0.96) | 1.19 (0.75–1.88) | 1.41 (0.87–2.27) |
| Self-employed | 927 | 514 (55.4%) | 0.89 (0.71–1.11) | 1.19 (0.92–1.54) | 1.21 (0.93–1.57) |
| Farming | 589 | 380 (64.6%) | 1.31 (1.02–1.68) | 0.91 (0.67–1.23) | 0.87 (0.63–1.19) |
| Not working | 484 | 282 (58.3%) | Ref | Ref | Ref |
| Missing data | 742 | 445 (59.9%) | – | - | - |
| Maternal educational level | |||||
| None | 512 | 359 (70.1%) | 2.31 (1.83–2.92) | 1.36 (1.05–1.77) | 1.30 (1.00–1.70) |
| Primary | 1481 | 882 (59.6%) | 1.45 (1.23–1.72) | 1.24 (1.04–1.48) | 1.21 (1.01–1.45) |
| Secondary | 850 | 427 (50.4%) | Ref | Ref | Ref |
| Missing data | 4 | 2 (28.6%) | – | - | - |
| Maternal religion | |||||
| Christian | 2048 | 1081 (52.9%) | Ref | Ref | Ref |
| Muslim | 666 | 496 (74.5%) | 2.60 (2.14–3.16) | 2.26 (1.84–2.78) | 2.40 (1.93–2.98) |
| Traditional African/None | 129 | 91 (70.5%) | 2.13 (1.45–3.15) | 1.36 (0.90–2.04) | 1.33 (0.88–1.99) |
| Missing data | 4 | 2 (0.0%) | – | – | – |
| Maternal age (years) | |||||
| Less than 20 | 319 | 192 (60.4%) | Ref | Ref | Ref |
| 20–29 | 1458 | 854 (58.6%) | 0.93 (0.72–1.19) | 1.10 (0.85–1.42) | 1.05 (0.80–1.37) |
| 30 or more | 1066 | 622 (58.7%) | 0.92 (0.72–1.19) | 0.98 (0.74–1.28) | 0.94 (0.71–1.23) |
| Missing data | 4 | 2 (28.6%) | – | – | – |
| Site of delivery | |||||
| Health facility | 2291 | 1241 (54.2%) | Ref | Ref | Ref |
| Home | 549 | 425 (77.7%) | 2.95 (2.37–3.66) | 2.04 (1.62–2.59) | 1.89 (1.49–2.41) |
| Missing data | 6 | 4 (40.0%) | – | – | – |
| Birth weight | |||||
| Less than 2.5 kg | 214 | 136 (63.6%) | 1.25 (0.94–1.67) | 1.24 (0.91–1.69) | 1.24 (0.91–1.70) |
| 2.5 kg or greater | 2633 | 1534 (58.2%) | Ref | Ref | Ref |
| Missing data | 0 | 0 (0.0%) | – | – | – |
| Age at circumcision | |||||
| 0–6 days | 117 | 68 (58.1%) | Ref | Ref | Ref |
| 7–20 days | 2556 | 1483 (58.3%) | 0.99 (0.68–1.45) | 1.09 (0.74–1.62) | 1.08 (0.71–1.64) |
| > 20 days | 172 | 119 (69.2%) | 1.62 (0.99–2.64) | 1.31 (0.78–2.21) | 1.29 (0.75–2.22) |
| Missing data | 2 | 0 (0.0%) | – | – | – |
Ref Reference group, CI Confidence interval, sd Standard deviation
a Model 1. Adjusted for income status, cost of circumcision, religion, maternal education and maternal age
bModel 2. Further adjusted for distance to health facility
c1 Ghs = 0.6 $US (2012)
Table 3.
Details of cash payments and in-kind payments by provider type
| Cost of circumcisiona | Provider Type | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All providers | Doctor | Medical assistant | Nurse | Drug seller | Domestic helper | Wanzam | Otherb | |||||||||
| Total | Included in-kind payment | Total | Included in-kind payment | Total | Included in-kind payment | Total | Included in-kind payment | Total | Included in-kind payment | Total | Included in-kind payment | Total | Included in-kind payment | Total | Included in-kind payment | |
| n(%) | n(%) | n(%) | n(%) | n(%) | n(%) | n(%) | n(%) | n(%) | n(%) | n(%) | n(%) | n(%) | n(%) | n(%) | n(%) | |
| Total | 2847 | 87 (3.1%) | 136 (4.8%) | 1 (0.7%) | 83 (2.9%) | 0 (0.0%) | 958 (33.6%) | 2 (0.2%) | 175 (6.1%) | 6 (3.4%) | 454 (15.9%) | 7 (1.5%) | 979 (34.4%) | 72 (7.4%) | 62 (2.2%) | 0 (0.0%) |
| Free | 539 (18.9%) | 16 (3.0%) | 81 (59.5%) | 1 (1.2%) | 34 (41.1%) | 0 (0.0%) | 155 (16.2%) | 1 (0.6%) | 16 (9.1%) | 2 (12.5) | 183 (40.3%) | 3 (1.6%) | 68 (6.9%) | 10 (14.0%) | 2 (3.2%) | 0 (0.0%) |
| 1.00–9.9 Ghs | 145 (5.1%) | 4 (2.9%) | 4 (2.9%) | 0 (0.0%) | 3 (3.6%) | 0 (0.0%) | 24 (2.5%) | 0 (0.0%) | 11 (6.2%) | 0 (0.0%) | 13 (2.9%) | 0 (0.0%) | 84 (8.6%) | 4 (4.6%) | 1 (1.6%) | 0 (0.0%) |
| Between 10 and 20 Ghs | 1530 (53.7%) | 42 (2.8%) | 17 (12.5%) | 0 (0.0%) | 29 (34.9%) | 0 (0.0%) | 596 (62.2%) | 0 (0.0%) | 122 (69.0%) | 2 (1.6%) | 154 (33.9%) | 2 (1.3%) | 579 (59.0%) | 38 (6.6%) | 33 (53.2%) | 0 (0.0%) |
| 20.00–100.00 Ghs | 554 (19.5%) | 23 (4.2%) | 31 (22.8%) | 0 (0.0%) | 16 (19.3%) | 0 (0.0%) | 170 (17.7%) | 1 (0.6%) | 23 (13.1%) | 2 (8.7%) | 100 (22.0%) | 2 (2.0%) | 188 (19.2%) | 18 (9.6%) | 26 (41.9%) | 0 (0.0%) |
| Missing data | 79 (2.8%) | 2 (2.5%) | 3 (2.2%) | 0 (0.0%) | 1 (1.2%) | 0 (0.0%) | 13 (1.4%) | 0 (0.0%) | 3 (1.7%) | 0 (0.0%) | 4 (0.9%) | 0 (0.0%) | 55 (5.6%) | 2 (3.6%) | 0 (0.0%) | 0 (0.0%) |
a1 Ghs = 0.6 $US (2012)
bOther: These included family members and friends
Infants from the lowest income households (quintile 1) (325, 84.0%) were four times more likely to receive a circumcision from an informal provider compared to infants from the highest income households (260, 42.4%) (adjusted odds ratio [aOR] 4.42, 95% CI 3.12–6.27 p = <0.001) (Table 2). There also appeared to be a ‘dose response’ with increasing risk of receiving a circumcision from an informal provider as income status decreased (Table 2) (aOR 1.34, 95% CI 1.25–1.43 p = <0.001).
A total of 2229 (78.3%) families paid to receive circumcision services from both formal and informal circumcision providers (Tables 2 and 3). Five hundred and thirty nine (18.9%) infants received their circumcision free of charge (50.1% formal and 49.9% informal) (Tables 2 and 3). Only 6.9% (68) of Wanzams provided free circumcisions. In contrast, 59.5% of circumcisions were provided free by doctors, 16.2% by nurses, 41.1% by medical assistants, 9.1% by drug sellers, and 40.3% by domestic helpers (Table 3).
Families in the lowest income quintile also appeared to be the least likely to receive free circumcision services (Table 4). Only 9.0% of families in the lowest quintile received free circumcision services compared to 27.9% in the highest quintile (aOR 0.40, 95% CI 0.28–0.58 p = <0.001). There also appeared to be a ‘dose response’ where the likelihood of receiving a free circumcision decreased as income status decreased (aOR 0.35, 95% CI 0.23–0.53 p = <0.001). 58.7% of families in the lowest quintile paid between 10 and 20 Ghana Cedis for their circumcision and 20.2% paid 20–100 Ghana Cedis.
Table 4.
Cost of circumcision by household income status
| Cost of circumcisionb | ||||||
|---|---|---|---|---|---|---|
| Household income statusa | Total | Free | Less than 10 Ghs | Between 10 and 20 Ghs | 20.00–100 Ghs | Missing data |
| n = 2847 | n = 539 | n = 145 | n = 1530 | n = 554 | n = 79 | |
| n (%) | n (%) | n (%) | n (%) | n (%) | ||
| 1 (Lowest) | 387 | 34 (9.0%) | 41 (10.6%) | 227 (58.7%) | 78 (20.2%) | 7 (1.8%) |
| 2 | 532 | 55 (10.3%) | 27 (5.0%) | 319 (60.0%) | 118 (22.2%) | 13 (2.4%) |
| 3 | 628 | 114 (18.2%) | 25 (4.0%) | 367 (58.4%) | 104 (16.6%) | 18 (2.9%) |
| 4 | 687 | 165 (24.0%) | 34 (4.9%) | 336 (48.9%) | 132 (19.2%) | 20 (2.9%) |
| 5 (Highest) | 613 | 171 (27.9%) | 18 (2.9%) | 281 (45.8%) | 122 (19.9) | 21 (3.4%) |
aWeath quintile calculated using principal components analysis
b1 Ghs = 0.6 $US (2012)
Eighty seven (3.1%) families paid in-kind contributions in the form of bars of soap, chickens, kola nuts, and corn (Table 3). The payment of in-kind contributions was more common with Wanzams (7.4%) than doctors (0.7%), nurses (0.2%), medical assistants (0.0%), drug sellers (3.4%), and domestic helpers (1.5%) (Table 3). Families of low socio-economic status appeared to be more likely to pay additional in-kind contributions (31.0%) compared to highest income families (11.5%) (aOR 0.41, 95% CI 0.25–0.67 p = <0.001).
Infants who lived 10 km or more from a health facility (154, 83.4%) were two times more likely to receive their circumcision from an informal provider compared to infants who lived less than 1 km from a health facility (776, 53.8%) (aOR 2.70, 95% CI 1.76–4.12 p = <0.001) (Table 2). There also appeared to be a dose response with increasing risk of receiving a circumcision from an informal provider as distance to a health facility increased (Table 2) (aOR 1.25, 95 CI 1.30–1.38 P = <0.001).
Household income status was closely associated with distance to a health facility (Table 5). Families in the lowest socio-economic quintile lived an average of 6.1 km (standard deviation [sd] 4.4 km) from a health facility (median 6.9 km, interquartile range [IQR] 1–15.9 km) compared to an average of 1.1 km in families in the highest socio-economic quintile (sd 1.6 km, median 0.6 km, IQR 0–10.4 km) (Table 5). Families in the lowest socio-economic quintile (79, 42.5%) were 22 times more likely to live more than 10 km from a health facility compared to families in the highest socio-economic quintile (5, 2.7%) (aOR 22.35 95% CI 8.84–56.54 p = <0.001) (Table 5). However, both socio-economic status (aOR 1.32, 95 CI 1.23–1.41 P = <0.001) and distance to health facilities (aOR 1.28, 95 CI 1.14–1.43 P = <0.001) had independent effects on the choice of circumcision provider.
Table 5.
Distance to health facility by household income status
| Household income status | Total | Mean distance (sd) | Median distance (interquartile range) | Min. & max. Values | < I Km | 1–4.9 Km | 5–9.9 Km | 10 Km or more | Not known/missing |
|---|---|---|---|---|---|---|---|---|---|
| n (%) | km | Km | km | n (%) | n (%) | n (%) | n (%) | n (%) | |
| Total | 2847 | 3.1 (3.6) | 1.1 (0–12.8) | 0 & 18.9 | 1444 | 741 | 400 | 186 | 76 |
| 1 (Lowest) | 387 (13.6%) | 6.1 (4.4) | 6.9 (1–15.9) | 0 & 18.5 | 106 (7.3%) | 49 (6.1%) | 153 (38.3%) | 79 (42.5%) | 0 (0.0%) |
| 2 | 532 (18.7%) | 3.9 (3.8) | 2.1 (0–12.3) | 0 & 13.1 | 218 (15.1%) | 117 (15.8%) | 132 (33.0%) | 55 (29.6%) | 10 (13.2%) |
| 3 | 628 (22.1%) | 2.4 (3.0) | 0.9 (0–11.3) | 0 & 12.3 | 321 (22.2%) | 203 (27.4%) | 62 (15.5%) | 35 (18.8%) | 7 (9.2%) |
| 4 | 687 (24.1%) | 1.7 (2.2) | 0.8 (0–10.9) | 0 & 12.7 | 387 (26.8%) | 228 (30.8%) | 42 (10.5%) | 12 (6.5%) | 18 (23.7%) |
| 5 (Highest) | 613 (21.5%) | 1.1 (1.6) | 0.6 (0–10.4) | 0 & 11.4 | 412 (28.5%) | 144 (19.4%) | 11 (2.8%) | 5 (2.7%) | 41 (53.9%) |
There was no statistical evidence of modification of the effect of distance from health facility on the choice of provider for circumcision by income status of the household (p-value for the interaction, 0.188).
Infants were two times more likely to receive circumcision from an informal provider if the families were Muslim (496, 74.5%) compared to Christian (1081, 52.9%) (aOR 2.40, 95% CI 1.93–2.98 p = <0.001) (Table 2). Mothers with no formal education (359, 70.1%) were 30% more likely to receive an informal circumcision provider compared to mothers with secondary level education (427, 50.4%) (aOR 1.30, 95% CI 1.00–1.70 p = <0.049) even after adjusting for other variables. There were no obvious differences associated with other socio demographic characteristics (Table 2).
Discussion
In our population-based study in rural Ghana, infant male circumcision was almost universal (91%) and was performed by both formal (41%) and informal (59%) circumcision providers. Both socio-economic status and geographic access to health facilities had important and independent effects on the choice of circumcision provider. The risk of receiving a circumcision from an informal provider increased with each level of deprivation and with the distance that families lived from health facilities. We also found that families with the lowest household income were the most likely to pay for their circumcision. Poor families were also most likely to pay additional in-kind contributions.
The relationship between socio-economic status [2, 19–21], geographic access [2, 22, 23], and choice of informal provider for infant male circumcision has been reported in many studies in low and middle income countries. However, our study is the first to report data from a rural area in Africa with high population level coverage of infant male circumcision. This is also the first study to report the double burden that circumcision places on families of low socio-economic status. In our study poor families were more likely to receive a circumcision from an untrained informal provider and also more likely to incur a significant economic cost.
In 2008, the “Free Maternal Care Policy” [24] was introduced into the Ghana Health Insurance Scheme [25]. Under the policy, all pregnant women and their infants up to 90 days postpartum and all children aged 90 days to 18 years are meant to receive free care in accredited public and private healthcare facilities. The services that are covered include antenatal care, delivery care, postnatal care, and infant male circumcision. Mothers and children just have to be registered and receive a registration card. The registration process is free and there are meant to be no out of pocket expenses. However, there have been difficulties in enrolling many families into the scheme. This has been attributed to difficulties in accessing many areas of Ghana, especially the poorest and most disadvantaged areas [26, 27]. In 2011, close to the time of conducting this study, only 33% of Ghana’s population were registered with 4.2% coverage for the poorest [27]. The most recent data from 2013 indicate that the national coverage still remains limited with only 38% registered [28]. Inequity in health insurance coverage is likely to be an important driver of the costs of circumcision incurred by poor families that we reported. Our study area is located in central rural Ghana in the Brong Ahafo region and health insurance coverage in the Brong Ahafo region was 45.9% in 2011 [27]. However, there are no data on coverage of health insurance in the poorest families in our study area.
Antenatal care and delivery services are also meant to be free under the Ghana health insurance scheme [24, 25] and similar inequities are also reported for these services. There are reports of poor women being charged unofficial and non-legitimate fees for delivery and postnatal care services [29] (https://www.ghanabusinessnews.com/2016/04/23/ghanas-free-maternal-healthcare-policy-not-workingresearch/). Reports of poor women and their babies being forcibly kept in birthing hospitals until their bills are settled have also been published [29]. Poor women have also been charged unofficial fees for antenatal (http://vibeghana.com/2012/01/18/free-maternal-health-policy-is-it-really-working/), delivery, and postnatal care services (http://www.ghanavoice.com/2016/04/23/ghanas-free-maternal-healthcare-policy-not-working-research/) in accredited facilities because they were unable to confront authority figures [30, 31]. Poor women are also less likely to be insured for delivery care compared to richer women in Ghana [32, 33].
Additional economic costs of circumcision include the payment of ‘in-kind’ contributions. The payment of in-kind contributions was more common with Wanzams (7%) than formal providers (3%) (doctors, nurses, and medical assistants) in our study. The poorest families also paid more in-kind contributions (31%) than the highest income families (12%). Two rural Kenyan studies have reported the payment of in-kind contributions (chickens, sheep, food and medical supplies) by families for circumcision [21, 22]. In these studies medical practitioners (49%) and informal traditional providers (51%) received similar in-kind contributions. However, these studies did not provide any information on the in-kind contributions paid by poor and richer families within the same study area.
We also reported that families of the Muslim religion were two-fold more likely to choose an informal provider than families with other religious affiliations. The Muslim religion is a well-known determinant of use of informal providers for circumcision in urban and rural Africa [2, 19, 34] and many Wanzams are Muslim themselves [8]. Approximately, 70% of Wanzams who performed circumcisions in our rural study area were Muslims. We also reported that mothers with no formal education were more likely to choose an informal circumcision provider compared to mothers with secondary level education. These data are also consistent with other African studies [35]. There were no obvious differences in choice of circumcision provider associated with other socio-demographic characteristics in our study.
Our study had some limitations. Investigators from Egypt have reported a lack of confidence in the formal health care system as a reason for the use of informal circumcision providers who charge fees [2, 36]. These studies also reported that traditional providers were perceived as more experienced and better in providing healthcare than formal health service providers [36]. However, we were not able to conduct indepth qualitative interviews to explore perspectives and experiences of families and health service providers in our study. We were also unable to assess family’s perceptions of quality of care. We were also unable to collect data on transport costs and other opportunity costs incurred by the families. Our study was observational and cross-sectional and does not provide proof of causation. However, we controlled for a wide range of individual, household and community level confounders and strengths of our study included its large community and population-based data collection system. In addition 22% of babies were not able to be visited within a 12 week period after birth. Anecdotal information from the study area indicated that these families needed to travel more for employment and they were of lower socio economic status and educational levels. The omission of these infants reduces the generalisability of our study a little but is unlikely to have introduced any systematic bias.
Conclusions
Our study appears to be the first to analyse the “on the ground” “community level” influence of socioeconomic factors on choice of infant male circumcision provider in an area with almost total population coverage. It also appears to be the first study that has described the high and inequitable costs paid by the poorest families in rural Africa for infant male circumcision. The Government of Ghana and other Non-Government Organisations should provide additional support to poor families so they can access high quality free infant male circumcision in rural Ghana. This includes improved coverage of Ghana’s free maternal care policy and health insurance scheme for the poorest families.
Acknowledgements
This study was supported by Kintampo Health Research Centre, London School of Hygiene and Tropical Medicine and the University of Western Australia. We also thank the families and infants who participated in this study, the staff of KHRC particularly Oscar Agyei, a data manager and the staff of Division of Paediatrics, University of Western Australia. The views expressed are those of the authors and not to be taken to represent the views of their institutions or the funders.
Funding
This research was funded by the University of Western Australia Scholarship fund. The funders had no role in data gathering, data analysis, or writing of the report. The corresponding author had full access to all the data in the study, and for the decision to submit for publication.
Availability of data and materials
The dataset analysed during the current study available from the corresponding author on reasonable request and with permission of KE.
Abbreviations
- $US
United States dollars
- AOR
Adjusted odds ratio
- CI
Confidence interval
- Ghs
Ghana Cedis
- GIS
Geographic information system
- HIV
Human immunodeficiency virus
- IQR
Interquartile range
- KHRC
Kintampo Health Research Centre
- KM
Kilometre
- ORs
Odds ratios
- PCA
Principal component analysis
- Ref
Reference group
- SD
Standard deviation
Authors’ contributions
TG drafted the report. KE, TG, KM and SN designed the study. TG, KE, SN and SO-A were responsible for the study conduct. KM, KE, NS, SN, SO-A participated in the statistical analyses, interpretation and report revisions. All the authors approved the final version and agreed to be accountable for the study.
Ethics approval and consent to participate
Ethical approvals were obtained from Ghana Health Service Ethical Review Committee, the Institutional Ethics Committee of Kintampo Health Research Centre, the Research Ethics Committee of London School of Hygiene and Tropical Medicine, and the Human Research Ethics Committee of the University of Western Australia. Written informed consent was obtained from all the families of the circumcised male infants.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Thomas Gyan, Email: thomas.gyan@uwa.edu.au, Email: kgyan8@gmail.com.
Kimberley McAuley, Email: kimberley.mcauley@uwa.edu.au.
Natalie Strobel, Email: natalie.strobel@uwa.edu.au.
Sam Newton, Email: samkofinewton@yahoo.com.
Seth Owusu-Agyei, Email: seth.owusu-agyei@kintampo-hrc.org.
Karen Edmond, Email: kedmond@unicef.org.
References
- 1.Alanis MC, Lucidi RS. Neonatal circumcision: a review of the world’s oldest and most controversial operation. Obstet Gynecol Surv. 2004;59(5):379–395. doi: 10.1097/00006254-200405000-00026. [DOI] [PubMed] [Google Scholar]
- 2.Weiss H, Larke N, Halperin D, Schenker I. Neonatal and child male circumcision: a global review. UNAIDS Technical Bulletin. UNAIDS, Geneva Switzerland, UNAIDS/10.07E – JC1672E (English original, April 2010).
- 3.Bailey RC, et al. Male circumcision for HIV prevention in young men in Kisumu, Kenya: a randomised controlled trial. Lancet. 2007;369(9562):643–656. doi: 10.1016/S0140-6736(07)60312-2. [DOI] [PubMed] [Google Scholar]
- 4.Bailey RC, Plummer FA, Moses S. Male circumcision and HIV prevention: current knowledge and future research directions. Lancet Infect Dis. 2001;1(4):223–231. doi: 10.1016/S1473-3099(01)00117-7. [DOI] [PubMed] [Google Scholar]
- 5.Gyan, T., et al., Determinants of morbidity associated with infant male circumcision: community-level population-based study in rural Ghana. Trop Med Int Health. 2017. [DOI] [PubMed]
- 6.Gyan T., McAuley, K., Strobel, A. N., Shannon, C., Newton, S., Tawiah-Agemang, C., Amenga-Etego, S., Owusu-Agyei, S., Kirkwood, B.R., Edmond K. Determinants of morbidity associated with infant male circumcision; community level population-based study in rural Ghana. Trop Med Int Health. 2016; In Press. [DOI] [PubMed]
- 7.World Health Organisation . Report on neonatal, infant and pre-pubertal male circumcision practices in traditional and clinical settings in Ghana. Accra: World Health Organisation; 2008. [Google Scholar]
- 8.Adu-Gyamfi S, Adjei P. Twentieth century Wanzams among the Asante people of Ghana: a historical study of the facts on male circumcision. Open J Prev Med. 2014;4(09):730. doi: 10.4236/ojpm.2014.49083. [DOI] [Google Scholar]
- 9.Gabrysch S, Campbell OM. Still too far to walk: literature review of the determinants of delivery service use. BMC Pregnancy Childbirth. 2009;9(1):34. doi: 10.1186/1471-2393-9-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berhan Y, Berhan A. A meta-analysis of socio-demographic factors predicting birth in health facility. Ethiop J Health Sci. 2014;24:81–92. doi: 10.4314/ejhs.v24i0.8S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moyer CA, Mustafa A. Drivers and deterrents of facility delivery in sub-Saharan Africa: a systematic review. Reprod Health. 2013;10(1):40. doi: 10.1186/1742-4755-10-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baral Y, et al. Determinants of skilled birth attendants for delivery in Nepal. Kathmandu Univ Med J. 2012;8(3):325–332. doi: 10.3126/kumj.v8i3.6223. [DOI] [PubMed] [Google Scholar]
- 13.Idris S, Gwarzo U, Shehu A. Determinants of place of delivery among women in a semi-urban settlement in Zaria, northern Nigeria. Ann Afr Med. 2007;5(2):68–72. [Google Scholar]
- 14.Aseweh Abor P, et al. The socio-economic determinants of maternal health care utilization in Ghana. Int J Soc Econ. 2011;38(7):628–648. doi: 10.1108/03068291111139258. [DOI] [Google Scholar]
- 15.Edmond KM, et al. Effect of early neonatal vitamin A supplementation on mortality during infancy in Ghana (Neovita): a randomised, double-blind, placebo-controlled trial. Lancet. 2015;385(9975):1315–1323. doi: 10.1016/S0140-6736(14)60880-1. [DOI] [PubMed] [Google Scholar]
- 16.Wilcken A, Keil T, Dick B. Traditional male circumcision in eastern and southern Africa: a systematic review of prevalence and complications. Bull World Health Organ. 2010;88(12):907–914. doi: 10.2471/BLT.09.072975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gwatkin DR, Rutstein S, Johnson K, Suliman E, Wagstaff A, Amouzou A. Socio-Economic Differences in Health, Nutrition, and Population within developing countries. Ghana: Health Nutrition and Population, The World Bank; 2007. [PubMed] [Google Scholar]
- 18.Ghana Living Standards Survey Round 6 (GLSSR6): Poverty Profile in Ghana 2005–2013, GLSSR6 Poverty Report Final 11.09.2014. Ghana Statistical service 2014. Available: http://catalog.ihsn.org/index.php/catalog/5350. Accessed 25th Aug 2016.
- 19.Weiss H, W. H. Organization, and J.U.N.P.o. HIV/AIDS . Male circumcision: global trends and determinants of prevalence, safety, and acceptability. Geneva: World Health Organization; 2008. [Google Scholar]
- 20.Douglas M. and C. Hongoro, The consideration of socioeconomic determinants in prevention of traditional male circumcision deaths and complications. Am J Mens Health. 2016. [DOI] [PMC free article] [PubMed]
- 21.Bailey RC, Egesah O, Rosenberg S. Male circumcision for HIV prevention: a prospective study of complications in clinical and traditional settings in Bungoma, Kenya. Bull World Health Organ. 2008;86(9):669–677. doi: 10.2471/BLT.08.051482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bailey RC, Egesah O. Complication rates and operational needs. Special report. Washington, DC: USAID, PSI AIDSMark; 2006. Assessment of clinical and traditional male circumcision services in Bungoma district, Kenya; pp. 1–39. [Google Scholar]
- 23.Weiss HA, et al. Circumcision among adolescent boys in rural northwestern Tanzania. Tropical Med Int Health. 2008;13(8):1054–1061. doi: 10.1111/j.1365-3156.2008.02107.x. [DOI] [PubMed] [Google Scholar]
- 24.Ministry of Health (2008–4) Implementation guidelines for financing free maternal health care. Accra, Ghana: MOH: 2008.
- 25.National Health Insurance Authority: http://www.nhis.gov.gh/nhia.aspx. Accessed 8 Sept 2016.
- 26.Ghana Statistical Service . Ghana multiple indicator cluster survey with an enhanced malaria module and biomarker, 2011, Final report. Accra: Ghana Statistical Service; 2011. [Google Scholar]
- 27.National Health Insurance Scheme, Annual Report 2011. Available http://www.nhis.gov.gh/files/annualreport2011.pdf. Accessed 8th Sept. 2016.
- 28.National Health Insurance Authority, Ghana: 2013 Annual Report. Available: http://www.nhis.gov.gh. Accessed 25th Aug 2016.
- 29.Barimah KB, Mensah J. Ghana's National Health Insurance Scheme: insights from members, administrators and health care providers. J Health Care Poor Underserved. 2013;24(3):1378–1390. doi: 10.1353/hpu.2013.0144. [DOI] [PubMed] [Google Scholar]
- 30.Kabeer N. Can the MDGS provide a pathway to social justice? The challenge of intersecting inequalities. Institute of Development Studies and MDG Achievement Fund, 2010. www.ids.ac.uk/files/dmfile/MDGreportwebsiteu2WC.pdf. Accessed 6th September 2016
- 31.Mumtaz Z, et al. Addressing invisibility, inferiority, and powerlessness to achieve gains in maternal health for ultra-poor women. Lancet. 2014;383(9922):1095–1097. doi: 10.1016/S0140-6736(13)61646-3. [DOI] [PubMed] [Google Scholar]
- 32.Ganle JK, et al. Inequities in accessibility to and utilisation of maternal health services in Ghana after user-fee exemption: a descriptive study. Int J Equity Health. 2014;13(1):1. doi: 10.1186/s12939-014-0089-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Houweling TA, et al. Huge poor-rich inequalities in maternity care: an international comparative study of maternity and child care in developing countries. Bull World Health Organ. 2007;85(10):745–754. doi: 10.2471/BLT.06.038588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rizvi S, et al. Religious circumcision: a Muslim view. BJU Int. 1999;83(S1):13–16. doi: 10.1046/j.1464-410x.1999.0830s1013.x. [DOI] [PubMed] [Google Scholar]
- 35.Alagoa PJ, Gbobo I. Complications of male infant circumcision in a semi-urban Niger Delta Town. International Journal of Tropical Diseases and Health 2013;3:217–23.
- 36.El Katsha S, et al. Informal health providers and the transmission of hepatitis C virus: pilot study in two Egyptian villages. 2006. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset analysed during the current study available from the corresponding author on reasonable request and with permission of KE.


