Abstract
Histone H1 accumulates in the nucleus after injection into the cytoplasm of Xenopus oocytes. A proteolytic fragment of 89 amino acids encompassing the carboxy-terminal domain also accumulates in the nucleus. Lysine, alanine and proline compose 84% of this domain. Accumulation is not due solely to the high lysine content since poly-L-lysine does not accumulate in the nucleus when injected into the cytoplasm of Xenopus oocytes. Proteolytic fragments encompassing other domains of the molecule are degraded in the oocyte after injection. In these instances degradation is more rapid in the cytoplasm than in the nucleus giving the false impression of accumulation in the nucleus, an artefact which is likely to confuse other studies of protein migration. Susceptibility to rapid degradation is a dominant feature, thus the globular domain destabilises the contiguous carboxy-terminal domain. The properties of the carboxy-terminal domain of H1 and the possible involvement of the amino acids lysine, proline and alanine in migration are discussed and compared with those of a domain that specifies migration of nucleoplasmin into the oocyte nucleus.
Full text
PDF
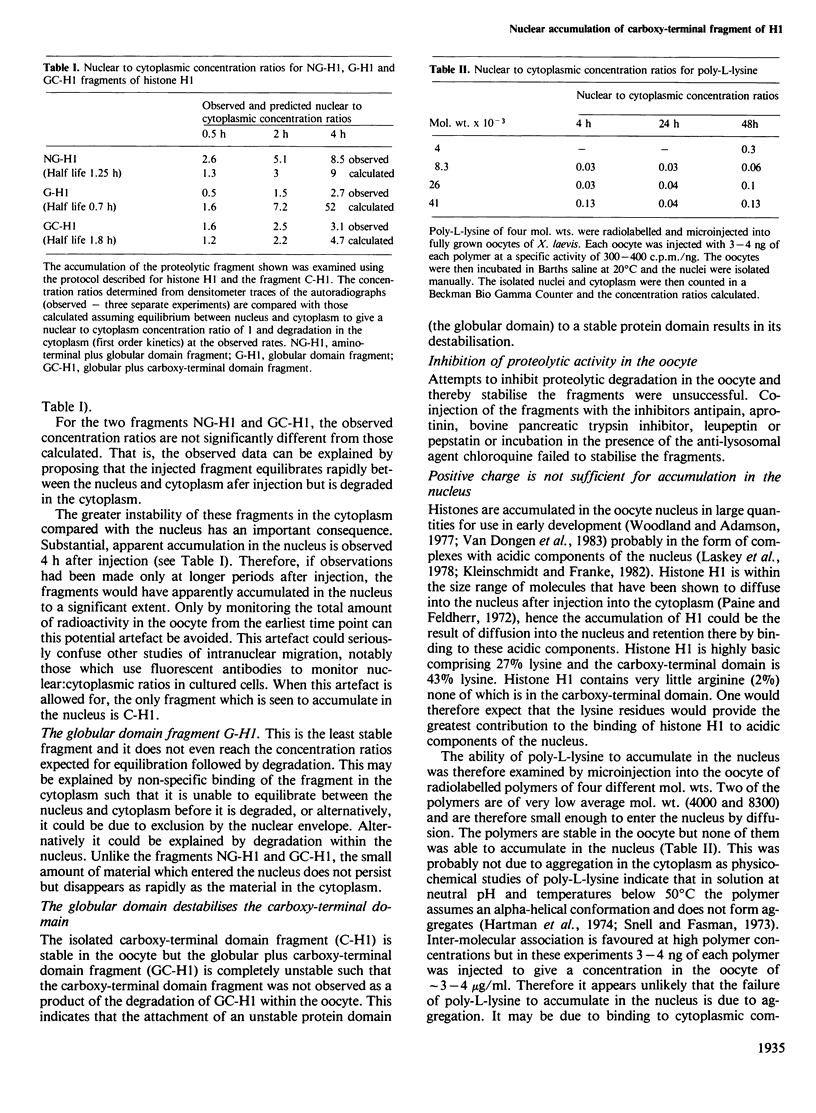

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bigelow S., Hough R., Rechsteiner M. The selective degradation of injected proteins occurs principally in the cytosol rather than in lysosomes. Cell. 1981 Jul;25(1):83–93. doi: 10.1016/0092-8674(81)90233-6. [DOI] [PubMed] [Google Scholar]
- Bolton A. E., Hunter W. M. The labelling of proteins to high specific radioactivities by conjugation to a 125I-containing acylating agent. Biochem J. 1973 Jul;133(3):529–539. doi: 10.1042/bj1330529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M. Protein migration into nuclei. I. Frog oocyte nuclei in vivo accumulate microinjected histones, allow entry to small proteins, and exclude large proteins. J Cell Biol. 1975 Feb;64(2):421–430. doi: 10.1083/jcb.64.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M. Protein migration into nuclei. II. Frog oocyte nuclei accumulate a class of microinjected oocyte nuclear proteins and exclude a class of microinjected oocyte cytoplasmic proteins. J Cell Biol. 1975 Feb;64(2):431–437. doi: 10.1083/jcb.64.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
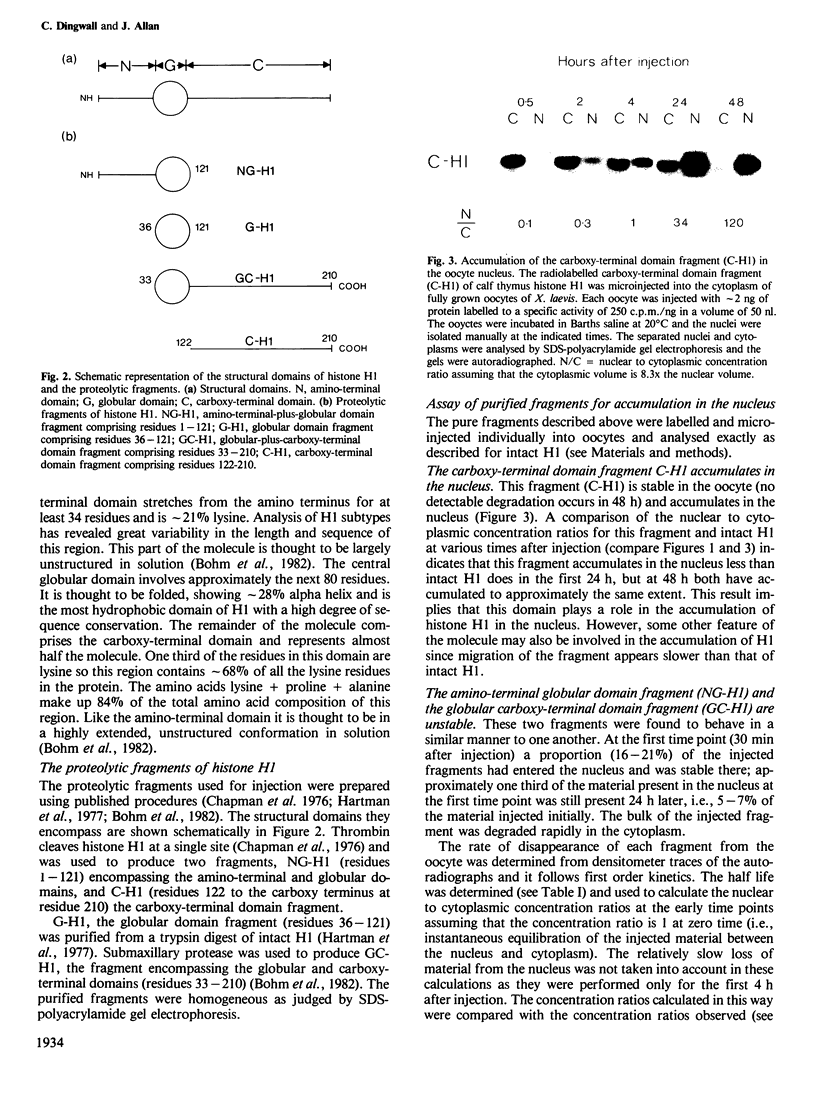
- Böhm L., Sautière P., Cary P. D., Crane-Robinson C. Precise elimination of the N-terminal domain of histone H1. Biochem J. 1982 Jun 1;203(3):577–582. doi: 10.1042/bj2030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R., Capecchi N. E., Hughes S. H., Wahl G. M. Selective degradation of abnormal proteins in mammalian tissue culture cells. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4732–4736. doi: 10.1073/pnas.71.12.4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman G. E., Hartman P. G., Bradbury E. M. Studies on the role and mode of operation of the very-lysine-rich histone H1 in eukaryote chromatin. The isolation of the globular and non-globular regions of the histone H1 molecule. Eur J Biochem. 1976 Jan 2;61(1):69–75. doi: 10.1111/j.1432-1033.1976.tb09998.x. [DOI] [PubMed] [Google Scholar]
- Colman A., Morser J., Lane C., Besley J., Wylie C., Valle G. Fate of secretory proteins trapped in oocytes of Xenopus laevis by disruption of the cytoskeleton or by imbalanced subunit synthesis. J Cell Biol. 1981 Dec;91(3 Pt 1):770–780. doi: 10.1083/jcb.91.3.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingwall C., Sharnick S. V., Laskey R. A. A polypeptide domain that specifies migration of nucleoplasmin into the nucleus. Cell. 1982 Sep;30(2):449–458. doi: 10.1016/0092-8674(82)90242-2. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Honda B. M., Laskey R. A., Thomas J. O. Assembly of nucleosomes: the reaction involving X. laevis nucleoplasmin. Cell. 1980 Sep;21(2):373–383. doi: 10.1016/0092-8674(80)90474-2. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B. Injected nuclei in frog oocytes: fate, enlargement, and chromatin dispersal. J Embryol Exp Morphol. 1976 Dec;36(3):523–540. [PubMed] [Google Scholar]
- Gurdon J. B. Nuclear transplantation and the control of gene activity in animal development. Proc R Soc Lond B Biol Sci. 1970 Dec 1;176(1044):303–314. doi: 10.1098/rspb.1970.0050. [DOI] [PubMed] [Google Scholar]
- Hall M. N., Hereford L., Herskowitz I. Targeting of E. coli beta-galactosidase to the nucleus in yeast. Cell. 1984 Apr;36(4):1057–1065. doi: 10.1016/0092-8674(84)90055-2. [DOI] [PubMed] [Google Scholar]
- Hartman P. G., Chapman G. E., Moss T., Bradbury E. M. Studies on the role and mode of operation of the very-lysine-rich histone H1 in eukaryote chromatin. The three structural regions of the histone H1 molecule. Eur J Biochem. 1977 Jul 1;77(1):45–51. doi: 10.1111/j.1432-1033.1977.tb11639.x. [DOI] [PubMed] [Google Scholar]
- Hartman R., Schwaner R. C., Hermans J., Jr Beta poly(L-lysine): a model system for biological self-assembly. J Mol Biol. 1974 Dec 15;90(3):415–429. doi: 10.1016/0022-2836(74)90225-3. [DOI] [PubMed] [Google Scholar]
- Horowitz S. B., Paine P. L. Cytoplasmic exclusion as a basis for asymmetric nucleocytoplasmic solute distributions. Nature. 1976 Mar 11;260(5547):151–153. doi: 10.1038/260151a0. [DOI] [PubMed] [Google Scholar]
- Johns E. W. The electrophoresis of histones in polyacrylamide gel and their quantitative determination. Biochem J. 1967 Jul;104(1):78–82. doi: 10.1042/bj1040078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinschmidt J. A., Franke W. W. Soluble acidic complexes containing histones H3 and H4 in nuclei of Xenopus laevis oocytes. Cell. 1982 Jul;29(3):799–809. doi: 10.1016/0092-8674(82)90442-1. [DOI] [PubMed] [Google Scholar]
- Lane C., Shannon S., Craig R. Sequestration and turnover of guinea-pig milk proteins and chicken ovalbumin in Xenopus oocytes. Eur J Biochem. 1979 Nov;101(2):485–495. doi: 10.1111/j.1432-1033.1979.tb19743.x. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Honda B. M., Mills A. D., Morris N. R., Wyllie A. H., Mertz J. E., De Roberts E. M., Gurdon J. B. Chromatin assembly and transcription in eggs and oocytes of Xenopus laevis. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):171–178. doi: 10.1101/sqb.1978.042.01.019. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- McGarry T., Hough R., Rogers S., Rechsteiner M. Intracellular distribution and degradation of immunoglobulin G and immunoglobulin G fragments injected into HeLa cells. J Cell Biol. 1983 Feb;96(2):338–346. doi: 10.1083/jcb.96.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine P. L., Feldherr C. M. Nucleocytoplasmic exchange of macromolecules. Exp Cell Res. 1972 Sep;74(1):81–98. doi: 10.1016/0014-4827(72)90483-1. [DOI] [PubMed] [Google Scholar]
- Paine P. L. Nucleocytoplasmic movement of fluorescent tracers microinjected into living salivary gland cells. J Cell Biol. 1975 Sep;66(3):652–657. doi: 10.1083/jcb.66.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell C. R., Fasman G. D. Kinetics and thermodynamics of the helix leads to transconformation of poly(L-lysine) and L-leucine copolymers. A compensation phenomenon. Biochemistry. 1973 Mar 13;12(6):1017–1025. doi: 10.1021/bi00730a001. [DOI] [PubMed] [Google Scholar]
- Woodland H. R., Adamson E. D. The synthesis and storage of histones during the oogenesis of Xenopus laevis. Dev Biol. 1977 May;57(1):118–135. doi: 10.1016/0012-1606(77)90359-1. [DOI] [PubMed] [Google Scholar]
- van Dongen W. M., Moorman A. F., Destrée O. H. The accumulation of the maternal pool of histone H1A during oogenesis in Xenopus laevis. Cell Differ. 1983 May;12(5):257–264. doi: 10.1016/0045-6039(83)90021-0. [DOI] [PubMed] [Google Scholar]





