Abstract
Background
Trueperella pyogenes is a worldwide known bacterium causing mastitis, abortion and various other pyogenic infections in domestic animals like ruminants and pigs. In this study we represent the first case report of three unusual fatal infections of Grey Slender Lorises caused by Trueperella pyogenes. Meanwhile, this study represents the first in-depth description of the multilocus sequence analysis (MLSA) on T. pyogenes species.
Case presentation
Three Trueperella pyogenes were isolated from three different Grey Slender Lorises, which died within a period of two years at Frankfurt Zoo (Frankfurt am Main - Germany). The three Grey Slender Loris cases were suffering from severe sepsis and died from its complication. During the bacteriological investigation of the three cases, the T. pyogenes were isolated from different organisms in each case. The epidemiological relationship between the three isolates could be shown by four genomic DNA fingerprint methods (ERIC-PCR, BOX-PCR, (GTG)5-PCR, and RAPD-PCR) and by multilocus sequence analysis (MLSA) investigating four different housekeeping genes (fusA-tuf-metG-gyrA).
Conclusion
In this study, we clearly showed by means of using three different rep-PCRs, by RAPD-PCR and by MLSA that the genomic fingerprinting of the investigated three T. pyogenes have the same clonal origin and are genetically identical. These results suggest that the same isolate contaminated the animal’s facility and subsequently caused cross infection between the three different Grey Slender Lorises. To the best of our knowledge, this is the first epidemiological approach concentrating on T. pyogenes using MLSA.
Keywords: Trueperella pyogenes, grey slender loris, Loris lydekkerianus nordicus, lorises, virulence genes, clonal relationship, DNA fingerprint, multilocus sequence analysis
Background
Trueperella pyogenes is a well-known pathogen of domestic ruminants and pigs causing mastitis, abortion and a variety of pyogenic infections [1]. As summarised by Jost and Billington [2] this bacterial pathogen is also able to cause diseases in a large number of different animal species including antelopes, bisons, camels, chickens, deer, elephants, gazelles, horses, macaws, reindeer, turkeys and wildebeest and also in companion animals such as dogs and cats. In 2010, Ülbegi-Mohyla et al. [3] characterised two T. pyogenes isolated from septicaemia of a gecko and a bearded dragon both phenotypically and genotypically. In 2012, Oikonomou et al. [4] reported that T. pyogenes is one of mainly frequently isolated pathogens from the investigated mastitis cases. The T. pyogenes acts as an opportunistic pathogen, causing endometritis in dairy cattle once the protective epithelium has been lost after parturition [5]. In later research, the complete genome sequencing of T. pyogenes was undertaken from a field isolate of a dairy cow suffering from metritis [6, 7] and from infected goats [8]. Grey Slender Lorises (Loris lydekkerianus nordicus) are a primate species from the family Lorisideae whose taxonomy is currently under revision. Their habitat is East and South India as well as Sri Lanka [9]. Grey Slender Lorises are primarily insectivorous nocturnal animals with loose social interactions. They forage on trees in dry zone forests where they also sleep in aggregations of several animals. In 2012, a T. pyogenes 11-07-D-03394 was isolated from a facial abscess of a six-year-old Grey Slender Loris kept in a terrarium at Frankfurt Zoo [10].
Case presentation
The Grey Slender Lorises in the present study originated from a European Association of Zoo and Aquaria (EAZA) breeding programme and were living at Frankfurt Zoo. The Lorises were kept in pairs in the nocturnal animal house. In October 2011, T. pyogenes 11-07-D-03394 was isolated, as previously described [10], from a facial abscess of a six-year-old male Grey Slender Loris. In May 2012, a second T. pyogenes 121,008,157 was isolated from a nasal swab of a ten-year-old male Grey Slender Loris which was living in the same terrarium and was suffering from purulent rhinitis. The T. pyogenes 121,008,157 was isolated together with Enterococcus spp., Pasteurella spp., Pseudomonas aeruginosa and coliform bacteria. After two weeks, this Grey Slender Loris died and T. pyogenes was isolated postmortem together with Enterococcus faecalis, Klebsiella pneumoniae and Escherichia coli from the skull of this animal. E. faecalis, K. pneumoniae and E. coli were additionally isolated from the liver, kidney, lung, spleen, tongue, brain and nasal mucosa. The post mortem analysis of the animal revealed a catarrhal purulent rhinitis, a catarrhal purulent sinusitis associated with an extended purulent catarrhal pneumonia, an acute fibrinous-purulent pericarditis as well as an interstitial nephritis. The animal had also lost two upper left molars and three top right molars. Later on, in December 2012, a third Grey Slender Loris (9.5 year old female) died in the same terrarium also from bacterial septicaemia following a dental abscess. In postmortem microbiological examinations, the T. pyogenes was isolated together with E. faecalis from the liver, kidney, lung, intestine, vagina and orbita and in high numbers from paranasal sinuses and dental cavities. The T. pyogenes isolate 121,018,522 used for further studies was isolated from the paranasal sinuses of this animal. Post mortem analysis of the animal also revealed a chronic nephritis of both kidneys. The animal had similar teeth problems as the two previously mentioned lorises. The bacteriological carcass inspection of the deceased lorises found T. pyogenes to be the predominating bacteria. In the present study the first isolate T. pyogenes 11-07-D-03394 obtained from the first case and the two T. pyogenes isolates (121,008,157 and 121,008,522) obtained from the second and third cases were identified both phenotypically and genotypically. Additionally, all three isolates were investigated for epidemiological relationships by means of various genotypical tests. For control purposes the strains T. pyogenes DSM 20630T, T. pyogenes DSM 20594 and Arcanobacterium haemolyticum DSM 20595T were included.
Phenotypic and genotypic identification
Both newly isolated T. pyogenes were identified phenotypically as described by Eisenberg et al. [10]. A genotypic identification of all three T. pyogenes isolates was subsequently performed by sequencing the 16S rRNA gene [10–13] and the glyceraldehyde-3-phosphate dehydrogenase encoding gene gap [13]. Furthermore, the three T. pyogenes isolates were genotypically identified by amplifying the T. pyogenes species-specific region of the 16S-23S rRNA intergenic spacer region (ISR) and the T. pyogenes species-specific region of the superoxide dismutase A encoding gene sodA. The three T. pyogenes isolates were additionally characterised by amplifying of several known and putative virulence factor encoding genes. These virulence genes included gene plo encoding pyolysin, gene cbpA encoding a collagen-binding protein, gene nanH encoding neuraminidase H, gene nanP encoding neuraminidase P and the fimbriae encoding genes fimA, fimC and fimE [3, 11, 12]. In addition, tetracycline resistance encoding gene tet(W) was amplified as described by Zastempowska and Lassa [14].
Genomic fingerprinting
The genomic fingerprinting of the three T. pyogenes isolates originating from the lorises was performed using four genomic DNA fingerprinting methods. These included three repetitive element primed (rep)-PCRs [ERIC-PCR, BOX-PCR and (GTG)5-PCR] and random amplification polymorphic DNA (RAPD-PCR) analysis as described in detail by Glaeser et al. [15]. The sequences of the oligonucleotide primers and the thermocycler programs are summarised in Table 1. Cluster analysis of genomic fingerprinting patterns was performed in GelCompar II version 4.5 (Applied Maths) using the unweighted pair-group method using the arithmetic average (UPGMA) clustering method based on the Pearson correlation (0.5% optimisation; 1% position tolerance), which considers the presence/absence and the intensity of DNA bands. A consensus matrix was calculated and a composite clustering was performed.
Table 1.
The Oligonucleotide primer sequences and PCR conditions used in the present study
| Oligonucleotide primers | Sequence | Programa |
|---|---|---|
| 1. 16S rDNA UNI-L | 5′-AGA GTT TGA TCA TGG CTC AG-3′ | 1 |
| 2. 16S rDNA UNI-R (Amplification primer) | 5′-GTG TGA CGG GCG GTG TGT AC-3′ | |
| 3. 16S rDNA-533F | 5′-GTG CCA GCM GCC GCG GTA A-3′ | – |
| 4. 16S rDNA-907R (Sequencing primer) | 5′-CCG TCA ATT CMT TTG AGT TT-3′ | |
| 5. Gap-F | 5′-TCG AAG TTG TTG CAG TTA ACG A-3′ | 2 |
| 6. Gap-R | 5′-CCA TTC GTT GTC GTA CCA AG-3′ | |
| 7. ERIC1RF | 5′-ATG TAA GCT CCT GGG GAT TCA C-3′ | 3 |
| 8. ERIC2 | 5′-AAG TAA GTG ACT GGG GTG AGC-3′ | |
| 9. BOXA1R | 5′-CTA CGG CAA GGC GAC GCT GAC G-3′ | 3 |
| 10. (GTG)5 | 5′-GTG GTG GTG GTG GTG-3′ | 4 |
| 12. RAPD primer B | 5′- ATC TGG CAG C − 3′ | 5 |
| 13. fusA-F | 5′-GCT TCA TCA ACA AGA TGG AC-3′ | 6 |
| 14. fusA-R | 5′-CTC GAT TG CGA CGT GG AT-3′ | |
| 15. tuf-F | 5′-GGA CGG TGA TTG GAG AAG AAT GG-3′ | 7 |
| 16. tuf-R | 5′-CCA GGT TGA TTA CGC TCC AGA AGA-3′ | |
| 17. metG-F | 5′-GCC GGT TTT GGT GTT CC-3′ | 8 |
| 18. metG-R | 5′-GGC CAA ATC TGG GAA TGG-3′ | |
| 19. gyrA-F | 5′-CCA CCA GAT CGA GGT CAT C-3′ | 9 |
| 20. gyrA-R | 5′-TCG TCG GCA GTG AAA CGC A-3′ |
aPCR Program 1: ×1 (95 °C, 600 s), ×30 (95 °C, 30 s, 58 °C, 60 s, 72 °C, 60 s), ×1 (72 °C, 420 s). 2: ×1 (94 °C, 180 s), ×30 (94 °C, 30 s, 50 °C, 40 s, 72 °C, 60 s), ×1 (72 °C, 300 s). 3: ×1 (95 °C, 180 s), ×30 (94 °C, 30 s, 53 °C, 60 s, 70 °C, 480 s), ×1 (72 °C, 960 s). 4: ×1 (95 °C, 180 s), ×30 (94 °C, 30 s, 53 °C, 60 s, 70 °C, 180 s), ×1 (72 °C, 960 s). 5: ×1 (95 °C, 180 s), ×45 (94 °C, 15 s, 34 °C, 60 s, 70 °C, 120 s), ×1 (72 °C, 600 s). 6: ×1 (94 °C, 180 s), ×30 (94 °C, 45 s, 57 °C, 30 s, 72 °C, 90 s), ×1 (72 °C, 420 s). 7: ×1 (94 °C, 180 s), ×30 (94 °C, 45 s, 57 °C, 40 s, 72 °C, 60 s), ×1 (72 °C, 420 s). 8: ×1 (94 °C, 180 s), ×30 (94 °C, 45 s, 52 °C, 30 s, 72 °C, 90 s), ×1 (72 °C, 600 s). 9: ×1 (94 °C, 180 s), ×30 (94 °C, 45 s, 52 °C, 30 s, 72 °C, 90 s), ×1 (72 °C, 600 s)
Multilocus sequence analysis (MLSA)
This represents the first MLSA-based study applied to field isolates of the species T. pyogenes. The MLSA was performed with the target genes translation elongation factor G encoding gene fusA, translation elongation factor Tu encoding gene tuf, methionyl-tRNA synthetase encoding gene metG and DNA gyrase, subunit A encoding gene gyrA. The sequences of the oligonucleotide primers for amplifying the four housekeeping genes were designed with the sequence data of the A. haemolyticum DSM 20595T genome project [16]. The target genes, the sequences of the oligonucleotide primers and the thermocycler programs are summarised in Table 1. Sequences of partial genes were concatenated in the following order: fusA (746 nt), tuf (795 nt), metG (836 nt) and gyrA (937 nt). Analyses were performed at the nucleotide and amino acid sequence level with T. pyogenes DSM 20630T, T. pyogenes DSM 20594 and A. haemolyticum DSM 20595T as controls. MLSA analysis was performed using the MEGA version 6 [17]. Full-length gene sequences from the genome of A. haemolyticum DSM 20595T (CP002045) were used as reference sequences to obtain the correct open reading frame (ORF) for translation into amino acid sequences. Alignments of nucleotide and the amino acid sequences were performed with MUSCLE implemented in MEGA version 6 [18]. The phylogenetic trees in single gene base analysis of the four target genes, respectively, were constructed with the neighbour-joining method [19] using the Kimura-2-parameter model for nucleotide sequences [20] or the JTT matrix-based method for amino acid sequences [21]. The phylogenetic trees of concatenated sequences were constructed using the maximum-likelihood method based on evolutionary distances calculated with the general time reversible model for nucleotide sequences [22] and again with the JTT matrix-based method for amino acid sequences. A discrete gamma distribution was used to model evolutionary rate differences among sites (5 categories; +G) and a rate variation model allowed for some sites to be evolutionarily invariable (+I). Nucleotide and amino acid sequence similarities of single and concatenated genes were determined based on p-distances calculated in MEGA version 6.
Results and discussion
Compared to previously identified T. pyogenes [23], the already described T. pyogenes 11-07-D-03394 [10] and the newly investigated T. pyogenes 121,008,157 and T. pyogenes 121,018,522 in the present study, could be identified by determining hemolysis and CAMP-like haemolytic reactions, by various other phenotypical and genotypical tests, together with T. pyogenes 11-07-D-03394, by sequencing 16S rRNA and gap genes (Fig. 1), and by amplifying of T. pyogenes species-specific regions of ISR and superoxide dismutase A encoding gene sodA as molecular targets, respectively (Table 2). The three loris isolates and the type strain T. pyogenes DSM 20630T shared 100% 16S rRNA gene sequence similarity and 95% to 99% 16S rRNA gene sequence similarity with type strains of other species of the genera Trueperella and Arcanobacterium (98.8% similarity with T. abortisuis DSM 19515T, 94.6% similarity with A. haemolyticum DSM 20595T). In addition, as shown in Fig. 1, the three loris isolates and T. pyogenes DSM 20594 shared identical gap gene sequences, with 99% sequence similarity to T. pyogenes DSM 20630T and 70% to 88% sequence similarity to the type strains of other species of the genera Trueperella and Arcanobacterium (88% similarity to T. abortisuis DSM 19515T, 73% similarity to A. haemolyticum DSM 20595T).
Fig. 1.

Phylogenetic trees based on sequences of 16S rRNA (a) and glyceraldehyde-3-phosphate dehydrogenase encoding gene gap (b) of the three T. pyogenes strains of the present study and reference strains of the genra Trueperella and Arcanobacterium obtained from GenBank (NCBI). Trees were constructed using the maximum-likelihood method based on evolutionary distances calculated with the general time reversible model. Numbers at branch nodes represent the percentage of replicate trees in which the associated taxa clustered together in bootstrap tests (1000 replicates). Only bootstrap values ≥70% are shown
Table 2.
Phenotypical and genotypical properties of the investigated T. pyogenes
| T. pyogenes 121,018,522 | T. pyogenes 121,008,157 | T. pyogenes 11-07-D 03394 | T. pyogenes DSM 20630T* | T. pyogenes DSM 20594* | |
|---|---|---|---|---|---|
| Phenotypical properties | |||||
| Hemolysis on sheep blood agar | + | + | + | + | + |
| Hemolysis on rabbit blood agar | + | + | + | + | + |
| CAMP-like reaction with: | |||||
| Staphylococcus aureus β-hemolysin | + | + | + | + | + |
| Streptococcus agalactiae | − | − | − | − | − |
| Rhodococcus equi | + | + | + | + | + |
| Reverse CAMP reaction | − | − | − | − | − |
| Nitrate reduction1 | − | − | − | − | − |
| Pyrazinamidase1 | − | − | − | − | − |
| Pyrrolidonyl arylamidase1 | + | + | + | + | + |
| Alkaline phosphatase1 | + | + | + | − | − |
| β-Glucuronidase (β-GUR)1,2,3 | + | + | + | + | + |
| α-Galactosidase (α-GAL)2 | − | − | − | − | − |
| β-Galactosidase (β-GAL)1,3 | + | + | + | + | + |
| α-Glucosidase (α-GLU)1,2,3 | + | + | + | + | + |
| β-Glucosidase (β-GLU) | − | − | − | − | − |
| N-acetyl- β-Glucosaminidase (β-NAG)1,3 | + | + | + | + | + |
| Esculin (β-Glucosidase)1 | − | − | − | − | − |
| Urease1 | − | − | − | − | − |
| Gelatine1,4 | − | − | − | + | + |
| Fermentation of: | |||||
| D-Glucose1 | + | + | + | + | + |
| D-Ribose1 | + | + | + | + | + |
| D-Xylose1 | + | + | + | + | + |
| D-Mannitol1 | − | − | − | − | − |
| D-Maltose1 | + | + | + | + | + |
| D-Lactose1 | + | + | + | + | + |
| Glycogen1 | + | + | + | + | − |
| α-Mannosidase2 | − | − | − | − | − |
| Catalase | − | − | − | − | − |
| Serolysis on Loeffler agar | + | + | + | + | + |
| Caseinase | + | + | + | + | + |
| Starch hydrolysis (amylase) | + | + | + | + | − |
| Cross reaction with streptococcal serogroup G specific antiserum | + | + | + | + | + |
| Genotypical properties | |||||
| T. pyogenes 16S rRNA gene sequence | + | + | + | + | + |
| T. pyogenes gene gap sequence | + | + | + | + | + |
| T. pyogenes specific part of ISR | + | + | + | + | + |
| T. pyogenes specific part of gene sodA | + | + | + | + | + |
| Pyolysin encoding gene plo | + | + | + | + | + |
| Collagen-binding protein encoding gene cbpA | − | − | − | + | − |
| Neuraminidase H encoding gene nanH | + | + | + | + | + |
| Neuraminidase P encoding gene nanP | − | − | − | + | + |
| Fimbriae endoding gene fimA | + | + | + | − | + |
| Fimbriae endoding gene fimC | + | + | + | + | + |
| Fimbriae endoding gene fimE | + | + | + | + | + |
| Tetracycline resistance encoding gene tet(W) | + | + | + | +** | −** |
The reactions are shown as follows:* = synergistic CAMP-like reaction with staphylococcal β-hemolysin and Rhodococcus equi as indicator strains; ** = results mostly obtained from Eisenberg et al. [19]; +, positive reaction; −, negative reaction. 1 = Api-Coryne test system (Biomerieux, Nürtingen, Germany); 2 = tablets containing substrates (Rosco Diagnostica A/S, Taastrup, Denmark); 3 = 4-methylumbelliferyl conjugated substrates (Sigma, Steinheim, Germany)
To demonstrate the clonal lineage and the epidemiological relationship, the T. pyogenes isolates from this study were further found to possess known and putative virulence factor encoding genes plo, nanH, fimA, fimC, fimE and tet(W). The genes cbpA and nanP could not be detected by the amplification with the applied primer systems (Table 2). The possible importance of the known and putative virulence factors has been discussed previously [2, 10, 11].
The T. pyogenes were also investigated for an epidemiological relationship by four genomic fingerprinting methods, three (rep)-PCRs and one RAPD-PCR, respectively. (rep)-PCRs were introduced to differentiate microbes by combining the advantage of DNA amplification with the application of repetitive sequence based oligonucleotide primers. RAPD fingerprinting has also been described as a useful tool for analysing the genetic structure of closely related bacteria. Since RAPD fingerprints target the whole genome, this method is far more sensitive in terms of detecting genetic diversity than, for example, 16S rRNA gene sequencing [24]. The genomic fingerprint analysis undertaken in the present study yielded for all three T. pyogenes isolates of loris origin uniform patterns in ERIC-PCR, BOX-PCR, (GTG)5-PCR, and in RAPD-PCR analysis. The patterns differed from those of T. pyogenes DSM 20630T and T. pyogenes DSM 20594 (Fig. 2) indicating that the three isolates are genetically identical and therefore of same clonal origin, while the other strains are somehow genetically different. All four techniques had already been used successfully for molecular typing of various bacterial species [25–31]. BOX-PCR typing was used for DNA fingerprinting of a variety of bacteria including T. pyogenes from musk deer [32].
Fig. 2.
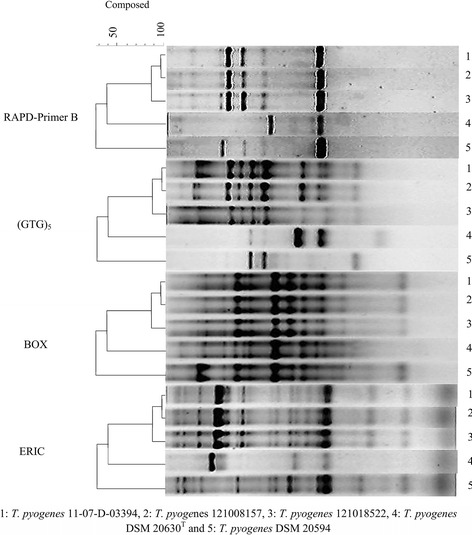
Genomic fingerprint pattern of the three Grey Slender Lorises strains in comparison to T. pyogenes reference strains with three different (REP)-PCRs (ERIC-PCR, BOX-PCR, and (GTG)5–PCR) and random amplification polymorphic DNA (RAPD-PCR)
The clonal relationship of the three T. pyogenes of loris origin could also be demonstrated by MLSA, investigating the target genes fusA, tuf, metG and gyrA. All four partially sequenced nucleotide sequences and amino acid sequences of the housekeeping genes were concatenated in the following order: fusA-tuf-metG-gyrA and FusA-Tuf-MetG-GyrA with a nucleotide sequence of 3314 bp and an amino acid sequence of 1103 aa. The targeted amplified DNA fragments were double sequenced on both strands and the sequences were deposited in the GenBank (National Center for Biotechnology Information) (Table 3). The phylogenetic analysis was based on the combined utilisation of the fusA-tuf-metG-gyrA partial gene sequences. The GenBank accession numbers of locus sequences obtained in this study are provided in Table 3. The cluster analyses of the phylogenetic trees of concatenated sequences succeeded in subdividing the three investigated T. pyogenes isolates originating from the three Grey Slender Lorises and the reference strains (T. pyogenes DSM 20594, T. pyogenes DSM 20630T and A. haemolyticum DSM 20595T). The concatenated sequence of the four loci on the nucleotide level among the 6 investigated isolates and strains with the corresponding 3314 bp resulted in 2468 conserved, 846 variable and 28 parsimony-informative sites being confirmed. The concatenated tree built with the nucleotide sequence clustered the investigated three isolates originating from the three Grey Slender Lorises and shared 100% sequence similarity (with 0.00 genetic distance) and bootstrap supports of 100%. However, the reference strains T. pyogenes DSM 20594 and T. pyogenes DSM 20630T shared with 98.9% and 99%, respectively the pairwise nucleotide sequence similarity (with 0.012 and 0.010 genetic distance) with the three isolates originating from the three Grey Slender Lorises (Fig. 3a – Table 4). The nucleotide composition (GC content) of the four investigated target genes (fusA-tuf-metG-gyrA) of the three T. pyogenes from the Grey Slender Lorises, T. pyogenes DSM 20594 and T. pyogenes DSM 20630T exhibited an identical GC content of 61.3 mol% and 54.7 mol% for the reference strain A. haemolyticum DSM 20595T. However, the percentage in the content of Guanine and Cytosine were identical for the three T. pyogenes isolates originating from the Grey Slender Lorises (29.7 mol%, 31.6 mol% respectively) and for the two reference strains T. pyogenes DSM 20594 and T. pyogenes DSM 20630T (29.8 mol% and 31.5 mol% respectively) (Table 5). The concatenated sequence of the four loci on the nucleotide level shows clearly on 15 different sites that the three T. pyogenes isolates originating from the Grey Slender Lorises were identical and differed from the other three reference strains (Table 6). On the other hand, the concatenated sequence of the four loci on the amino acid level among the 6 investigated isolates and strains revealed 1103 sites with 570 conserved, 217 variables sites and 1 parsimony-informative site. The concatenated tree built with the amino acid sequence succeeded in clustering the three investigated T. pyogenes isolates originating from the three Grey Slender Lorises and separating them from the other reference strains (T. pyogenes DSM 20594, T. pyogenes DSM 20630T and A. haemolyticum DSM 20595T). The three isolates originating from the three Grey Slender Lorises shared 100% amino acid sequence similarity and bootstrap supports of 87%, while the reference strains T. pyogenes DSM 20594 and T. pyogenes DSM 20630T shared 99.7% amino acid sequence similarity with the three isolates originating from the three Grey Slender Lorises (Fig. 3b). The percentage of amino acid similarities among T. pyogenes species and A. haemolyticum DSM 20595T ranged from 80.3% to 80.4%, while within the T. pyogenes species it ranged from 99.6% to 100%. Moreover, the concatenated sequence of the four loci on the amino acid level clearly showed on the site 248 that the three T. pyogenes isolates of Grey Slender Loris origin were identical (Aspartic acid) and differed from the other three reference strains (Glutamic acid).
Table 3.
GenBank accession numbers of locus sequences of T. pyogenes strains obtained in this study
| Isolates and strains | fusA | Tuf | metG | gyrA | |
|---|---|---|---|---|---|
| 1 | T. pyogenes 121,018,522 | KJ605914 | HG941714 | HG941711 | HG941706 |
| 2 | T. pyogenes 121,008,157 | KJ605913 | HG941713 | HG941710 | HG530074 |
| 3 | T. pyogenes 11-07-D-03394 | KJ605912 | HG941712 | HG941709 | HG941702 |
| 4 | T. pyogenes DSM 20630T | KJ605911 | HG941716 | HG941708 | HG941704 |
| 5 | T. pyogenes DSM 20594 | KJ605910 | HG941715 | HG941707 | HG941703 |
Fig. 3.

Phylogenetic analysis based on concatenated partial fusA-tuf-metG-gyrA nucleotide sequences of a total of 3314 nucleotide positions (a) and FusA-Tuf-MetG-GyrA amino acid sequences of a total of 1103 amino acid positions (b) of the three investigated target genes of the three T. pyogenes of Grey Slender Loris origin, T. pyogenes DSM 20594, T. pyogenes DSM 20630T and A. haemolyticum DSM 20595T. Trees were constructed using the maximum-likelihood method based on evolutionary distances calculated with the general time reversible model (for nucleotide sequences) or the JTT matrix-based method (for amino acid sequences). Numbers at branch nodes represent the percentage of replicate trees in which the associated taxa clustered together in bootstrap tests (1000 replicates). Only bootstrap values ≥70% are shown
Table 4.
Average pairwise distances of the concatenated sequences of the investigated strains
| Isolates or strains | 1 | 2 | 3 | 4 | 5 | 6 | |
|---|---|---|---|---|---|---|---|
| 1 | T. pyogenes 121,018,522 | ||||||
| 2 | T. pyogenes 121,008,157 | 0 | |||||
| 3 | T. pyogenes 11-7-D-03394 | 0 | 0 | ||||
| 4 | T. pyogenes DSM 20630T | 0.010 | 0.010 | 0.010 | |||
| 5 | T. pyogenes DSM 20594 | 0.012 | 0.012 | 0.012 | 0.010 | ||
| 6 | A. haemolyticum DSM 20595T | 0.249 | 0.249 | 0.249 | 0.25 | 0.249 |
Table 5.
Nucleotide percentage of the concatenated sequences for the four loci sequences
| Isolates and strains | T | C | A | G | G + C | |
|---|---|---|---|---|---|---|
| 1 | T. pyogenes 121,018,522 | 18.3 | 31.6 | 20.5 | 29.7 | 61.3 |
| 2 | T. pyogenes 121,008,157 | 18.3 | 31.6 | 20.5 | 29.7 | 61.3 |
| 3 | T. pyogenes 11-7-D-03394 | 18.3 | 31.6 | 20.5 | 29.7 | 61.3 |
| 4 | T. pyogenes DSM 20630T | 18.3 | 31.5 | 20.5 | 29.8 | 61.3 |
| 5 | T. pyogenes DSM 20594 | 18.3 | 31.5 | 20.5 | 29.8 | 61.3 |
| 6 | A. haemolyticum DSM 20595T | 22.9 | 26.9 | 22.4 | 27.8 | 54.7 |
Table 6.
Matrix of the variable nucleotide positions of the concatenated sequences among the investigated strains
| Isolates and strains | Nucleotide position | |||||||||||||||||||||||||||
| 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 3 | 3 | 3 | 3 | ||||
| 1 a | 4 | 7 | 0 | 2 | 2 | 3 | 4 | 5 | 5 | 8 | 8 | 8 | 8 | 9 | 9 | 9 | 0 | 1 | 5 | 7 | 7 | 8 | 0 | 1 | 2 | 2 | 3 | |
| 6 | 3 | 4 | 4 | 4 | 6 | 9 | 8 | 5 | 8 | 1 | 1 | 3 | 5 | 3 | 4 | 5 | 3 | 9 | 5 | 3 | 9 | 9 | 7 | 3 | 4 | 5 | 1 | |
| 2 | 5 | 4 | 4 | 2 | 3 | 5 | 8 | 7 | 8 | 5 | 8 | 6 | 7 | 5 | 7 | 6 | 1 | 9 | 6 | 3 | 6 | 8 | 8 | 3 | 6 | 5 | 6 | |
| (1) T. pyogenes 121018522 | T | C | T | C | T | A | T | G | C | T | A | A | T | C | C | T | A | C | C | T | C | C | T | A | T | C | T | C |
| (2) T. pyogenes 121008157 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| (3) T. pyogenes 11-7-D-03394 | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . | . |
| (4) T. pyogenes DSM 20630T | C | T | G | . | . | G | C | C | T | C | G | G | C | T | T | A | G | . | . | C | . | T | A | G | C | T | C | . |
| (5) T. pyogenes DSM 20594 | C | T | G | T | C | G | C | C | T | C | G | G | C | T | T | A | G | T | T | C | T | . | A | G | C | . | C | T |
| (6) A. haemolyticum DSM 20595T | C | A | A | T | C | T | C | C | . | . | . | . | . | G | T | G | G | T | T | C | T | T | C | G | C | T | G | T |
aThe nucleotide positions in the three T. pyogenes from Grey Slender Lorises are identical and differ from other reference strains
Conclusion
The several genetic markers of the presented MLSA approach showed clearly that the three T. pyogenes originating from Grey Slender Lorises and the two T. pyogenes reference strains belong to three different clonal complexes, respectively. The results of the present investigation which represent the first detailed epidemiological study of T. pyogenes of this origin clearly indicated that all three T. pyogenes which contributed with other potentially pathogenic bacteria to the septicemia of the three lorises, respectively shared a clonal origin. However, whether the cross infection between the three animals with T. pyogenes isolates, which was present in the lorises’ terrarium over a certain period of time, occurred because of direct contact or a lack of disinfection of the animal facility after detecting the first or the second case remains unclear.
Acknowledgments
Funding
The authors received no financial support for this study.
Availability of data and materials
The manuscript includes all used materials and data that are of interest for potential readers.
Abbreviations
- (GTG)5
Repetitive bacterial DNA elements
- A
Arcanobacterium
- ERIC
Enterobacterial repetitive intergenic consensus
- MLSA
Multilocus sequence analysis
- nt
Nucleotide
- RAPD
Randomly amplified polymorphic DNA
- rep-PCRs
Repetitive sequence-based polymerase chain reaction
- T
Trueperella
Authors’ contributions
SN, AA, PK and CL were involved in the conception and design of the study. SN conceived the study, participated in its design and carried out the experimental work. PK, SPG conducted the genomic fingerprinting (rep-PCRs and RAPD-PCR). TE performed the initial examination of the isolates at the Veterinary Examination Office and helped in drafting the manuscript. OS and AB carried out the sequencing conducted the molecular investigations (DNA isolation, PCR assays and data interpretation). NS and CG provided valuable information on of the animals’ case history and the causes of death. UK performed the pathoanatomical investigations. EPB performed the primary microbiological test. CL, AA and AB performed the critical revision of the manuscript and gave important intellectual advice for approval. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lämmler C, Hartwigk H. In: Handbuch der bakteriellen Infektionen bei Tieren, Band II/3. Blobel H, Schliesser T, editors. Jena, Stuttgart: Gustav Fischer Verlag; 1995. pp. 196–240. [Google Scholar]
- 2.Jost BH, Billington SJ. Arcanobacterium pyogenes: molecular pathogenesis of an animal opportunist. Antonie Van Leeuwenhoek. 2005;88:87–102. doi: 10.1007/s10482-005-2316-5. [DOI] [PubMed] [Google Scholar]
- 3.Ülbegi-Mohyla H, Hijazin M, Alber J, Lämmler C, Hassan AA, Abdulmawjood A, et al. Identification of Arcanobacterium pyogenes isolated by post mortem examinations of a bearded dragon and a gecko by phenotypic and genotypic properties. J Vet Sci. 2010;11:265–267. doi: 10.4142/jvs.2010.11.3.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oikonomou G, Machado VS, Santisteban C, Schukken YH, Bicalho RC. Microbial diversity of bovine mastitic milk as described by pyrosequencing of metagenomic 16s rDNA. PLOS ONE. 2012. 10.1371/journal.pone.0047671. [DOI] [PMC free article] [PubMed]
- 5.Amos MR, Healey GD, Goldstone RJ, Mahan SM, Düvel A, Schuberth HJ, et al. Differential endometrial cell sensitivity to a cholesterol-dependent cytolysin links Trueperella pyogenes to uterine disease in cattle. Biol Reprod. 2014;90(3):1–13. doi: 10.1095/biolreprod.113.115972. [DOI] [PubMed] [Google Scholar]
- 6.Goldstone RJ, Amos M, Talbot R, Schuberth HJ, Sandra O, Sheldon IM, et al. Draft genome sequence of Trueperella pyogenes, isolated from the infected uterus of a postpartum cow with metritis. Genome Announc. 2014;2(2):e00194–e00114. doi: 10.1128/genomeA.00194-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Machado VS, Bicalho RC. Complete genome sequence of Trueperella pyogenes, an important opportunistic pathogen of livestock. Genome Announc. 2014;2(2):e00400–e00414. doi: 10.1128/genomeA.00400-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang S, Qiu J, Yang R, Shen K, Xu G, Fu L. Complete genome sequence of Trueperella pyogenes, isolated from infected farmland goats. Genome Announc. 2016;4(6):e01421–e01416. doi: 10.1128/genomeA.01421-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perera MSJ. A review of the distribution of grey slender loris (Loris lydekkerianus) in Sri Lanka. Primate Conserv. 2008;23:89–96. doi: 10.1896/052.023.0110. [DOI] [Google Scholar]
- 10.Eisenberg T, Nagib S, Hijazin M, Alber J, Lämmler C, Hassan AA, et al. Trueperella pyogenes as cause of a facial abscess in a grey slender loris (Loris lydekkerianus nordicus) a case report. Berl Münch Tierärztl Wochenschr. 2012;125:407–410. [PubMed] [Google Scholar]
- 11.Hijazin M, Ülbegi-Mohyla H, Alber J, Lämmler C, Hassan AA, Abdulmawjood A, et al. Molecular identification and further characterization of Arcanobacterium pyogenes isolated from bovine mastitis and from various other origins. J Dairy Sci. 2011;94:1813–1819. doi: 10.3168/jds.2010-3678. [DOI] [PubMed] [Google Scholar]
- 12.Al-Tarazi Y, Hijazin M, Alber J, Lämmler C, Hassan AA, Timke M, et al. Phenotypic and genotypic characteristics of Trueperella (Arcanobacterium) pyogenes isolated from lung abscesses of one-humped camels (Camelus dromedarius) in Jordan. J Camelid Sci. 2012;5:99–104. [Google Scholar]
- 13.Sammra O, Balbutskaya A, Nagib S, Alber J, Lämmler C, Abdulmawjood A, et al. Properties of an Arcanobacterium haemolyticum strain isolated from a donkey. Berl Münch Tierärztl Wochenschr. 2014;127:10–14. [PubMed] [Google Scholar]
- 14.Zastempowska E, Lassa H. Genotypic characterization and evaluation of an antibiotic resistance of Trueperella pyogenes (Arcanobacterium pyogenes) isolated from milk of dairy cows with clinical mastitis. Vet Microbiol. 2012;161:153–158. doi: 10.1016/j.vetmic.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 15.Glaeser S, Galatis H, Martin K, Kämpfer P. Niabella hirudinis and Niabella drilacis sp. nov., isolated from the medicinal leech Hirudo verbana. Int J Syst Evol Microbiol. 2013;63:3487–3493. doi: 10.1099/ijs.0.050823-0. [DOI] [PubMed] [Google Scholar]
- 16.Yasawong M, Teshima H, Lapidus A, Nolan M, Lucas S, Glavina Del Rio T, et al. Complete genome sequence of Arcanobacterium haemolyticum type strain (11018) Stand Genomic Sci. 2010;3:126–135. doi: 10.4056/sigs.1123072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 20.Kimura M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J Mol Evol. 1980;16:111–120. doi: 10.1007/BF01731581. [DOI] [PubMed] [Google Scholar]
- 21.Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Comput Appl Biosci. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- 22.Nei M, Kumar S. Molecular evolution and phylogenetics. New York: Oxford: Oxford University Press; 2000. [Google Scholar]
- 23.Nagib S, Rau J, Sammra O, Lämmler C, Schlez K, Zschöck M, et al. Identification of Trueperella pyogenes isolated from bovine mastitis by Fourier transform infrared spectroscopy. PLOS ONE. 2014. 10.1371/journal.pone.0104654. [DOI] [PMC free article] [PubMed]
- 24.Ziemke F, Brettar I, Höfle M. Stability and diversity of the genetic structure of a Shewanella putrefaciens population in the water column of the central Baltic. Aquat Microb Ecol. 1997;13:63–74. doi: 10.3354/ame013063. [DOI] [Google Scholar]
- 25.Martin B, Humbert O, Camara M, Guenzi E, Walker J, Mitchell T, et al. A highly conserved repeated DNA element located in the chromosome of Streptococcus pneumoniae. Nucleic Acids Res. 1992;20:3479–3483. doi: 10.1093/nar/20.13.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Belkum A, Hermans PW. BOX PCR fingerprinting for molecular typing of Streptococcus pneumoniae. Methods Mol Med. 2001;48:159–168. doi: 10.1385/1-59259-077-2:159. [DOI] [PubMed] [Google Scholar]
- 27.Hijazin M, Sammra O, Ülbegi-Mohyla H, Nagib S, Alber J, Lämmler C, et al. Arcanobacterium phocisimile sp. nov., isolated from harbour seals. Int J Syst Evol Microbiol. 2013;63:2019–2024. doi: 10.1099/ijs.0.045591-0. [DOI] [PubMed] [Google Scholar]
- 28.Da Silva RB, Valicente FH. Molecular characterization of Bacillus thuringiensis using rep-PCR. London: Springerplus; 2013. doi:10.1186/2193-1801-2-641. [DOI] [PMC free article] [PubMed]
- 29.Kämpfer P, Glaeser SP, Raza MW, Abbasi SA, Perry JD. Pseudocitrobacter gen. nov., a novel genus of the Enterobacteriaceae with two new species Pseudocitrobacter faecalis sp. nov., and Pseudocitrobacter anthropi sp. nov, isolated from fecal samples from hospitalized patients in Pakistan. Syst Appl Microbiol. 2013;37:17–22. doi: 10.1016/j.syapm.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Versalovic J, Koeuth T, Lupski JR. Distribution of repetitive DNA sequences in eubacteria and application to fingerpriting of bacterial genomes. Nucleic Acids Res. 1991;19:6823–6831. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Versalovic J, Schneider M, De Bruijn F, Lupski JR. Genomic fingerprinting of bacteria using repetitive sequence-based polymerase chain reaction. Meth Mol Cell Biol. 1994;5:25–40. [Google Scholar]
- 32.Zhao K, Tian Y, Yue B, Wang H, Zhang X. Virulence determinants and biofilm production among Trueperella pyogenes recovered from abscesses of captive forest musk deer. Arch Microbiol. 2013;195:203–209. doi: 10.1007/s00203-013-0869-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The manuscript includes all used materials and data that are of interest for potential readers.


