Abstract
Significance: Hydrogen sulfide (H2S) plays roles in many physiological processes, including relaxation of vascular smooth muscles, mediation of neurotransmission, inhibition of insulin signaling, and regulation of inflammation. Also, hydropersulfide (R−S−SH) and polysulfide (−S−Sn−S−) have recently been identified as reactive sulfur species (RSS) that regulate the bioactivities of multiple proteins via S-sulfhydration of cysteine residues (protein Cys−SSH) and show cytoprotection. Chemical tools such as fluorescent probes and selective inhibitors are needed to establish in detail the physiological roles of H2S and polysulfide.
Recent Advances: Although many fluorescent probes for H2S are available, fluorescent probes for hydropersulfide and polysulfide have only recently been developed and used to detect these sulfur species in living cells.
Critical Issues: In this review, we summarize recent progress in developing chemical tools for the study of H2S, hydropersulfide, and polysulfide, covering fluorescent probes based on various design strategies and selective inhibitors of H2S- and polysulfide-producing enzymes (cystathionine γ-lyase, cystathionine β-synthase, and 3-mercaptopyruvate sulfurtransferase), and we summarize their applications in biological studies.
Future Directions: Despite recent progress, the precise biological functions of H2S, hydropersulfide, and polysulfide remain to be fully established. Fluorescent probes and selective inhibitors are effective chemical tools to study the physiological roles of these sulfur molecules in living cells and tissues. Therefore, further development of a broad range of practical fluorescent probes and selective inhibitors as tools for studies of RSS biology is currently attracting great interest. Antioxid. Redox Signal. 27, 669–683.
Keywords: : reactive sulfur species, fluorescence imaging, inhibitors
Introduction
Hydrogen sulfide (H2S) is a toxic gas smelling of rotten eggs, but it has been reported to function in many physiological processes, including relaxation of vascular smooth muscles (60, 85), mediation of neurotransmission (1, 40), inhibition of insulin signaling (38), and regulation of inflammation (19, 45). More recently, reactive sulfur species (RSS) such as hydropersulfide (R−S−SH) and polysulfide (−S−Sn−S−) were also found to have roles in regulating the activity of proteins via S-sulfhydration of cysteine (Cys) residues (SH→SSH) (55, 60) and the modification of synaptic transmission (41), in addition to a cytoprotective effect (42).
Since H2S, polysulfide and hydropersulfide are redox partners, they should coexist in biological systems. In contrast, hydropersulfide and polysulfide seem likely to be much more effective than H2S in S-sulfhydration from the view point of chemical reactivity (76), although the source of the persulfide donor whether its relationship with H2S is as a product or precursor is controversial (84).
There are several methods for selective detection of H2S, and the most commonly used are the methylene blue method (44), the sulfide-selective electrode method (77), the monobromobimane method (43, 57), and fluorescence detection (74). The monobromobimane method, that is, liquid chromatography mass spectrometry analysis of sulfur compounds labeled with monobromobimane, is also applicable to the detection of hydropersulfide and polysulfide (36). However, homogenization of biological samples is required in these methods, except for fluorescence detection, so they cannot be applied to living cells and tissues.
In contrast, fluorescent probes are easy to use, and enable real-time, nondestructive detection in living cells and tissues (25, 78); consequently they have been widely used in biological research to study the physiological functions of H2S, hydropersulfide, and polysulfide (71, 74). Many selective fluorescent probes for H2S have been reported, and more recently, fluorescent probes for hydropersulfide and polysulfide have also been developed (47, 71, 74, 87).
To study the role of H2S, hydropersulfide, and polysulfide in biological systems, several techniques to block the production of these sulfur molecules have been employed. For examples, mouse models genetically ablated of H2S- or polysulfide/hydropersulfide-producing enzymes (37, 56, 79, 85) and knockdown of these enzymes by siRNA are commonly used, but these techniques require additional expertise and access to specialized facilities (4, 59). Although selective inhibitors of H2S- or polysulfide/hydropersulfide-producing enzymes are also useful and indeed are frequently used in biological studies, their selectivity is generally not high, and there is still a need for highly selective inhibitors to investigate the precise functions of H2S, hydropersulfide, and polysulfide in living samples (4, 59, 81).
In this review, we summarize the current status of chemical tools for the study of H2S, polysulfide, and hydropersulfide signaling, focusing especially on fluorescent probes and inhibitors, and we also briefly introduce their applications to biological studies.
Development of Fluorescent Probes for H2S
Many fluorescent probes for H2S have been developed based on a variety of reactions, such as the reduction of an azide or nitro moiety to amine, the nucleophilicity of HS−, and the H2S-quenching effect of copper (II) ion (Cu2+), as summarized hereunder.
Fluorescent probes based on reduction of azide or nitro moiety to amine
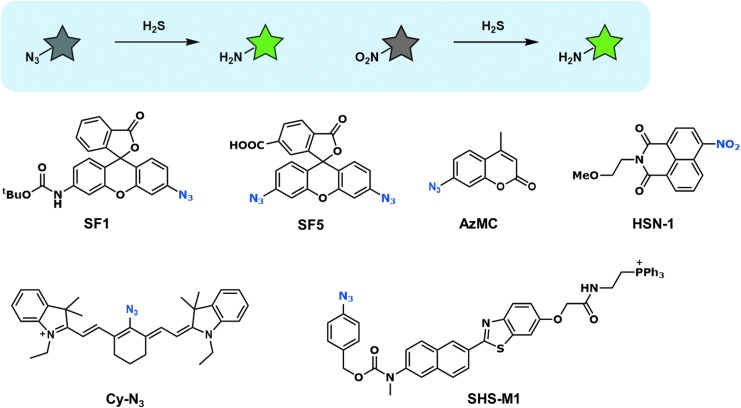
H2S is a reducing reagent and has been used to reduce azides in organic synthesis (69). The sulfide-mediated reduction of aryl azide was recently shown by Henthorn and Pluth to proceed through initial nucleophilic attack of sulfide anion on the electrophilic azide to form an azidethiol intermediate, followed by intramolecular attack of sulfide to generate the amine (31). Since the electron-withdrawing azide is converted to electron-donating amine in this reaction, the electron density of the molecule is greatly changed, and this change can be utilized to modulate the fluorescence of probes.
The first fluorescent probes utilizing azide reduction were SF1 (Fig. 1) and SF2, developed by Chang and colleagues (48). Reduction of the azide group of the xanthene moiety to amine leads to opening of the intramolecular spirocycle of the rhodamine scaffold: this restores the conjugated system of the xanthene moiety, resulting in strong fluorescence emission. The same group subsequently developed several improved probes (SF4–7) based on the same strategy (Fig. 1), using bis-azide rhodamines to achieve a lower background signal and an acetoxymethyl group to improve intracellular retention (46).
FIG. 1.
Fluorescent probes based on reduction of azide or nitro group. Gray or green star indicates nonfluorescent or strongly fluorescent molecule, respectively.
This azide reduction strategy is broadly applicable to various fluorophores, and various kinds of fluorescent probes for H2S have been developed with different colors or targeting specific organelles, as shown in Figure 1. For example, Barrios and colleagues reported a coumarin-based fluorescent probe, AzMC, which they used for cystathionine β-synthase (CBS) inhibitor screening (75). Han and colleagues reported a cyanine-based near-infrared (NIR) probe, Cy-N3 (88). This probe is a colorimetric and ratiometric probe, displaying an emission maximum shift of about 40 nm upon azide reduction.
H2S fluorescent probes targeting specific organelles have also been developed. For example, Kim and colleagues reported two-photon mitochondria-targeted fluorescent probes, SHS-M1 and SHS-M2, which incorporate a triphenylphosphonium group as a mitochondrial targeting moiety (5). These probes can ratiometrically detect different levels of mitochondrial H2S produced in live cells and living tissues expressing different levels of CBS.
Like the azide group, the nitro group can also be reduced by H2S, and Montoya and Pluth utilized this fact to design a fluorescent probe, HSN-1, that incorporates a nitro group into the 1,8-naphthalimide scaffold (54). Thus, several types of H2S fluorescent probes utilizing azide or nitro group reduction have been reported.
Fluorescent probes based on the nucleophilicity of HS−
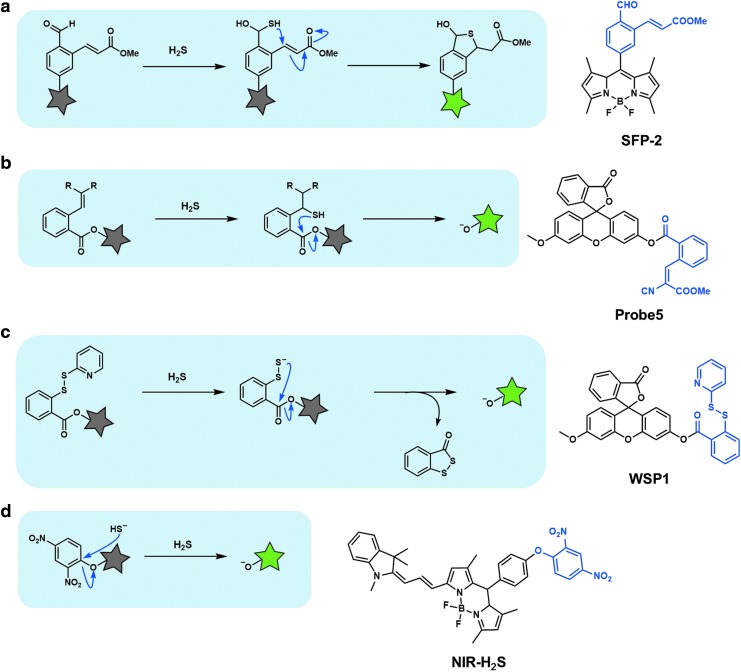
H2S is highly water soluble and shows pKa1 of 7.0 and pKa2 of ∼12 (66). So, about two-thirds of H2S is estimated to exist as strongly nucleophilic HS− at physiological pH (pH = 7.4). Qian et al. utilized this strong nucleophilicity to design fluorescent probes SFP-1 and SFP-2 (Fig. 2a) (64). In these probes, fluorescence off/on switching occurs via HS− addition to the aldehyde substituent, followed by Michael addition of the resulting intermediate to the unsaturated methyl acrylate moiety to form a thiohemiacetal under physiological conditions. The resulting stable tetrahydrothiophene shows strong fluorescence. Xian and colleagues also used a Michael addition strategy to achieve thioacetal cyclization, resulting in ester cleavage, to develop a fluorescent probe (Fig. 2b) (51).
FIG. 2.
Fluorescent probes based on the nucleophilicity of HS−. (a) Fluorescent probe based on HS− addition to the aldehyde substituent, followed by Michael addition. (b) Fluorescent probe based on a Michael addition strategy to achieve thioacetal cyclization. (c) Fluorescent probe based on disulfide exchange reaction. (d) Fluorescent probe based on nucleophilic addition to the electrophilic moiety of the probe. Gray or green star indicates nonfluorescent or strongly fluorescent molecule, respectively.
Disulfide exchange reaction by H2S has also been utilized for selective detection of H2S. Xian and colleagues reported fluorescein-based fluorescent probes, WSP1-5, in which the disulfide bond is cleaved by H2S, followed by intramolecular nucleophilic attack of the persulfide group on the ester moiety (Fig. 2c): this reaction releases the fluorophore, resulting in a large fluorescence increase (50, 62). Other similar fluorescent probes, such as a ratiometric probe (83) and a coumarin-based probe (26), have also been reported. Fluorescent probes based on these strategies (Fig. 2a–c) may react with other biothiols such as reduced glutathione (GSH) and Cys, but the resulting intermediates cannot continue to the intramolecular cyclization step, and consequently these probes show high selectivity for H2S over biothiols.
Nucleophilic addition to the electrophilic center of the fluorescent probe has been utilized for the development of ratiometric H2S probes. Guo et al. reported fluorescent probes, CouMC, based on the selective nucleophilic addition of sulfide anion to coumarin and merocyanine scaffolds (14). Moreover, 2,4-dinitrophenyl (DNP) derivatives were originally employed as masking groups for the phenol group of tyrosine during peptide synthesis, and this protecting group can be removed by thiolytic cleavage (20). Lin and colleagues applied this strategy to remove a highly electron-withdrawing group, DNP, from a probe by reaction with H2S, causing the probe to become strongly fluorescent (Fig. 2d) (8).
Fluorescent probes based on the quenching effect of Cu2+
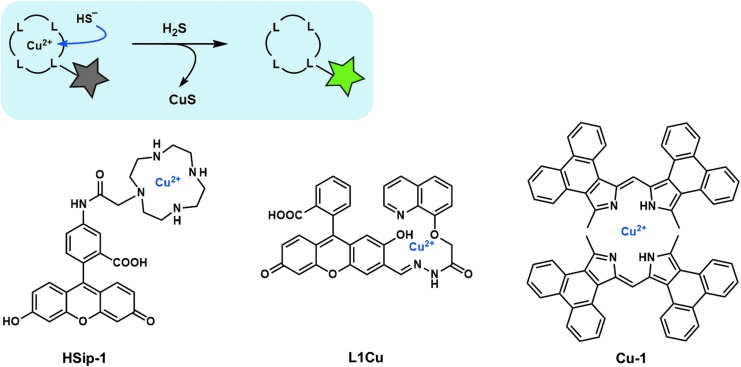
It is well known that heavy metal ions such as iron (III) ion (Fe3+) and Cu2+ quench the fluorescence of a nearby fluorophore. Moreover, based on the hard and soft acids and bases principle (61), sulfide anion has a strong affinity for Cu2+. On this basis, precipitation of CuS was adapted for H2S detection by incorporating a Cu2+ complex moiety into the fluorophore.
Chang and colleagues reported that a dipicolylamine–fluorescein complex with Cu2+ showed a turn-on fluorescence response to H2S, but the probe showed no selectivity over other biothiols (16). Our group designed and synthesized a H2S fluorescent probe, hydrogen sulfide imaging probe-1 (HSip-1) (Fig. 3), in which Cu2+ is complexed with an azamacrocyclic ring, cyclen (67). The high stability of this Cu2+-azamacrocyclic complex enabled us to achieve high selectivity for H2S: when H2S binds to the Cu2+ center, the probe shows a large fluorescence enhancement, whereas the azamacrocyclic Cu2+ complex is stable in the presence of other biothiols, such as 10 mM GSH.
FIG. 3.
Fluorescent probes based on precipitation of CuS. Gray or green star indicates nonfluorescent or strongly fluorescent molecule, respectively.
Other probes utilizing this approach, that is, with a Cu2+ complex as a sensing moiety for H2S, have been reported based on the combined scaffold of fluorescein and 8-hydroxyquinoline (33) and the phenanthrene-fused dipyrromethene scaffold (65) (Fig. 3).
HSip-1 is also useful to measure H2S production in the course of development of other chemical tools. For example, Nakagawa and colleagues reported photolysis-induced H2S donors utilizing ketoprofenate (22) and xanthone (21) as photolabile protecting groups. They used HSip-1 to evaluate photo-dependent H2S release from these H2S donors. Ichinose and colleagues developed H2S-releasing N-methyl-d-aspartate receptor (NMDAR) antagonists (combination of NMDAR antagonists with H2S donor) and evaluated changes of cell viability after incubation of cells with these agents (52). They compared the ability of various hybrid H2S donors to increase intracellular H2S by using HSip-1 for H2S detection.
Development of Fluorescent Probes for Hydropersulfide and Polysulfide
Increasing recognition of the importance of hydropersulfide and polysulfide in biological systems has led to the development of fluorescent probes for sulfane sulfur species; these species consist of sulfur atom(s) with six valence electrons but no charge bound to other sulfur atom(s), as in hydropersulfide and polysulfide. Hydropersulfide has significantly different chemical properties from structurally related thiols (R−SH). The pKa values of hydropersulfides are lower than that of H2S (32), so hydropersulfides should be stronger and more reactive nucleophiles than thiols. In addition, sulfane sulfur is electrophilic and can react with nucleophiles. By utilizing these chemical properties, several off/on type fluorescent probes for sulfane sulfur, that is, hydropersulfide and polysulfide, have been designed and developed.
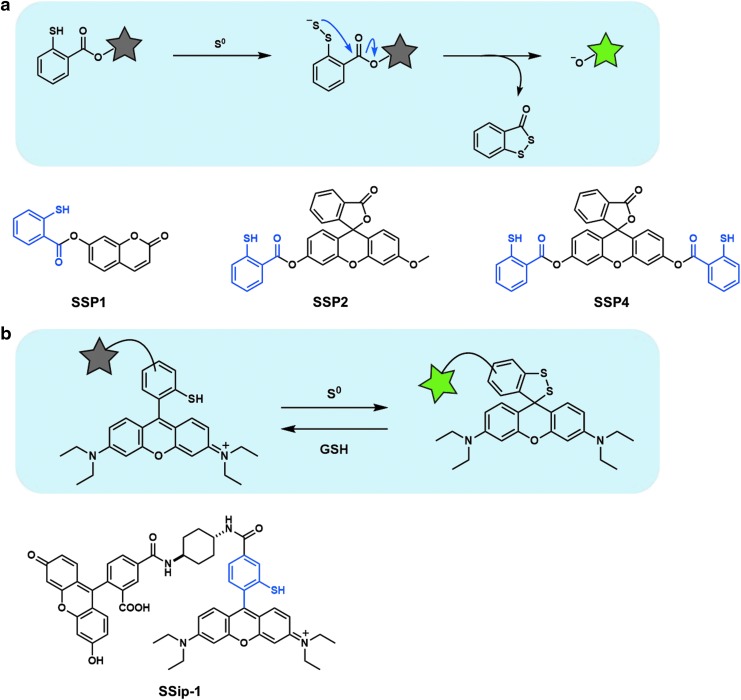
Fluorescent probes based on sulfane sulfur attachment to thiol
Sulfane sulfur functions as an electrophile and can react with nucleophiles such as thiol group (82). This property was utilized to develop the first fluorescent probes for sulfane sulfur, the SSP series, reported by Xian and colleagues (Fig. 4a) (9). The reaction mechanism for these probes is as follows. First, sulfane sulfur reacts with the sulfur atom of the thiol group of the probe, affording reactive persulfide (R−SS−). Then, intramolecular nucleophilic attack of persulfide (R−SS−) on the ester moiety occurs, resulting in strong fluorescence. Thus, the probes can detect sulfane sulfur, including polysulfide and elemental sulfur. Our group also very recently designed and synthesized a reversible off/on fluorescent probe for sulfane sulfur, SSip-1, by utilizing the ability of sulfane sulfur to bind reversibly to other sulfur atoms and the intramolecular spirocyclization reaction of xanthene dyes (Fig. 4b) (73). The fluorescent probe reversibly visualized sulfane sulfur in living A549 cells and primary-cultured hippocampal astrocytes.
FIG. 4.
Fluorescent probes based on sulfane sulfur attachment to thiol. (a) Fluorescent probes for sulfane sulfur, the SSP series. (b) Reversible off/on fluorescent probe for sulfane sulfur, SSip-1. Gray or green star indicates nonfluorescent or strongly fluorescent molecule, respectively.
Fluorescent probes based on the nucleophilicity of H2Sn
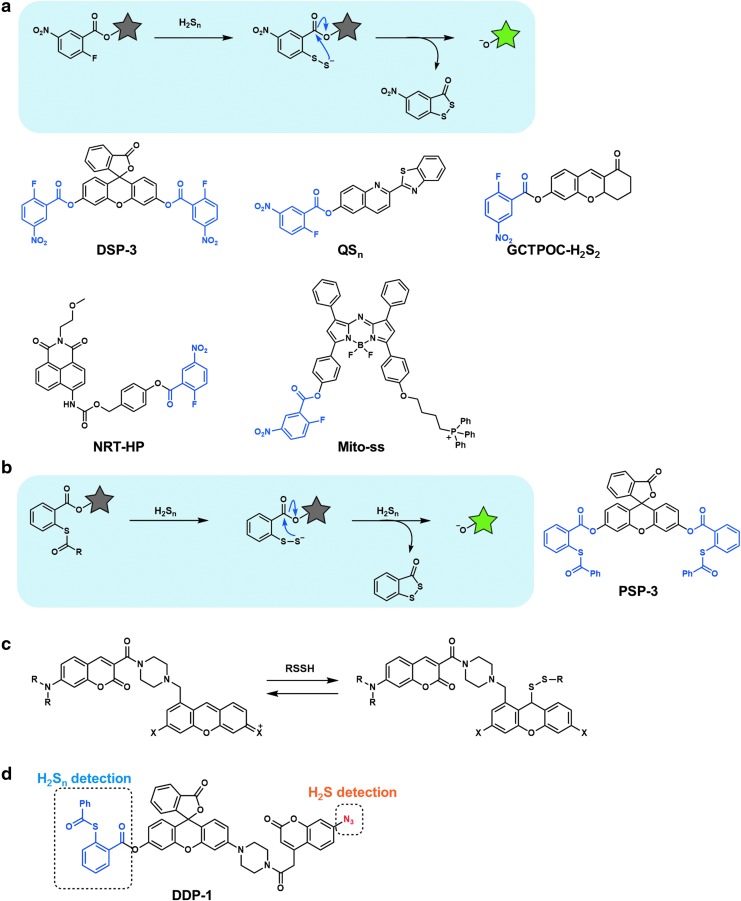
Xian and colleagues reported a specific fluorescent probe for hydrogen polysulfides (H2Sn, n > 1), the DSP series (Fig. 5a) (49). In this molecular design, the fluorophore is linked to 2-fluoro-5-nitrobenzoate via an ester bond. Nucleophilic aromatic substitution of hydrogen polysulfide with the fluorine group of the probe affords the persulfide intermediate, and then intramolecular nucleophilic attack of the persulfide (R−SS−) on the ester moiety in the probe structure releases the fluorophore. The electron-withdrawing nitro group on the 2-fluoro-5-nitrobenzoate moiety serves to enhance the reactivity of the probe. This approach can also be applied to other fluorophores (Fig. 5a) (23, 24, 28, 35, 70, 89).
FIG. 5.
Fluorescent probes based on the nucleophilicity of H2Sn. (a) Fluorescent probes based on nucleophilic aromatic substitution of hydrogen polysulfide with the fluorine group of the probe. (b) Highly specific fluorescent probe for hydrogen polysulfide, utilizing phenyl 2-(benzoylthio)benzoate as a recognition moiety. (c) Reversible fluorescent probe for hydropersulfide based on nucleophilic reaction of hydropersulfide with the pyronine unit of the probe. (d) Fluorescent probe for the detection of both H2S and H2Sn as different fluorescence signals. Gray or green star indicates nonfluorescent or strongly fluorescent molecule, respectively.
For example, Liu and colleagues reported a two-photon-excited fluorescent probe for hydrogen polysulfide, QSn, using 2-benzothiazol-2-yl-quinolin-6-ol as the two-photon fluorophore and 2-fluoro-5-nitrobenzoate as the H2Sn recognition moiety (89). They also reported a ratiometric two-photon fluorescent probe, NRT-HP, utilizing the same H2Sn recognition moiety and quinone methide chemistry (28). They successfully visualized both exogenous and endogenous H2S2/H2Sn in living cells and tissues.
Chen and colleagues developed NIR fluorescent probes, Mito-ss (24) and BD-ss (23), which have aza-BODIPY as the NIR fluorophore and 2-fluoro-5-nitrobenzoate as the polysulfide recognition moiety. Fluorescence of these probes is controlled via donor-excited photoinduced electron transfer because of the strong electron-withdrawing group, that is, the nitro group (78). These probes were utilized for visualization of exogenously added polysulfide, as well as polysulfide endogenously produced via enzymatic reaction in vivo.
Xian and colleagues also developed a H2Sn-specific chemosensor based on the nucleophilicity of H2Sn: they discovered a hydrogen polysulfide-mediated aziridine ring-opening reaction, and the developed chemosensor showed high sensitivity and selectivity for H2Sn (12).
Although 2-fluoro-5-nitrobenzoate is utilized as a recognition moiety for hydrogen polysulfide and hydropersulfide in many fluorescent probes, as already mentioned, it can also react with biothiols to form thioether products, leading to undesired consumption of the probes. So, Xian and colleagues developed an improved recognition moiety that reacts only with H2Sn (Fig. 5b) (11). Phenyl 2-(benzoylthio)benzoate showed appropriate selectivity, that is, its thioester bond is labile to polysulfide, but is stable even to biothiols. PSP-3 employed this reaction moiety on a fluorescein scaffold to detect intracellular polysulfide.
A reversible fluorescent probe for hydropersulfide (Fig. 5c) was recently reported by Ojida and colleagues (39). Its reaction mechanism involves nucleophilic reaction of hydropersulfide with the pyronine unit of the probe. The reversible character of this reaction enabled the probe to detect not only increase, but also decrease of hydropersulfide levels in real time. This probe also employed a fluorescence resonance energy transfer (FRET) mechanism as a fluorescence controlling mechanism, which modulates the intramolecular FRET efficiency to induce a dual emission change. Other strategies utilizing the high nucleophilicity of hydrogen polysulfide, such as selenium–sulfur exchange reaction (29) and Michael addition reaction to the cinnamate ester moiety (34), have also been reported.
Fluorescent probe for dual detection
To understand the mutual relationship and cellular cross-talk between H2S and H2Sn, Xian and colleagues developed a single fluorescent probe, DDP-1, that was able to visualize both H2S and H2Sn as different fluorescence signals (Fig. 5d) (10). DDP-1 employs an azide group for H2S detection and phenyl 2-(benzoylthio)benzoate for H2Sn detection.
Development of H2S-Producing Enzyme Inhibitors
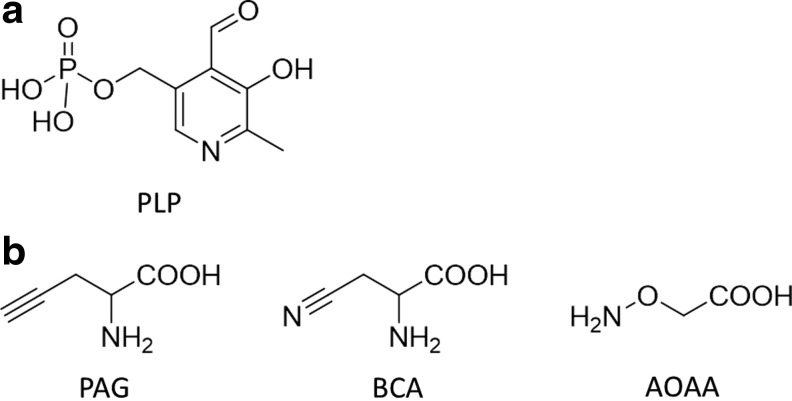
Physiological H2S is enzymatically synthesized by CBS, cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3MST) (59). Among them, CBS and CSE are pyridoxal 5′-phosphate (PLP)-dependent enzymes (Fig. 6a) (4, 63). Several inhibitors of these H2S-producing enzymes have been used in biological studies (Fig. 6b).
FIG. 6.
Chemical structure of PLP and inhibitors of H2S-generating enzymes. (a) Chemical structure of PLP (63). (b) Chemical structures of inhibitors of H2S-generating enzymes. PAG and BCA are inhibitors of CSE, and AOAA is an inhibitor of CBS (4). AOAA, aminooxyacetic acid; BCA, β-cyano-l-alanine; CBS, cystathionine β-synthase; CSE, cystathionine γ-lyase; PAG, d,l-propargylglycine; PLP, pyridoxal 5′-phosphate.
d,l-Propargylglycine (PAG) and β-cyano-l-alanine (BCA) are commonly used as inhibitors of CSE: PAG is an irreversible inhibitor of CSE, while BCA is a reversible inhibitor. Both PAG and BCA inhibit CSE in a PLP-dependent manner, and they do not exhibit high selectivity for CSE (4, 59, 81), although their effects on about 140 other PLP-dependent enzymes have not been precisely established (59). Also, they have low cell permeability and need to be used at high concentrations (4). Aminooxyacetic acid (AOAA) is commonly used as an inhibitor of CBS (59), but it also blocks CSE. No selective inhibitor of 3MST, which is not a PLP-dependent enzyme, has yet been reported (81). Inhibitors of each enzyme are summarized hereunder.
Inhibitors of CSE
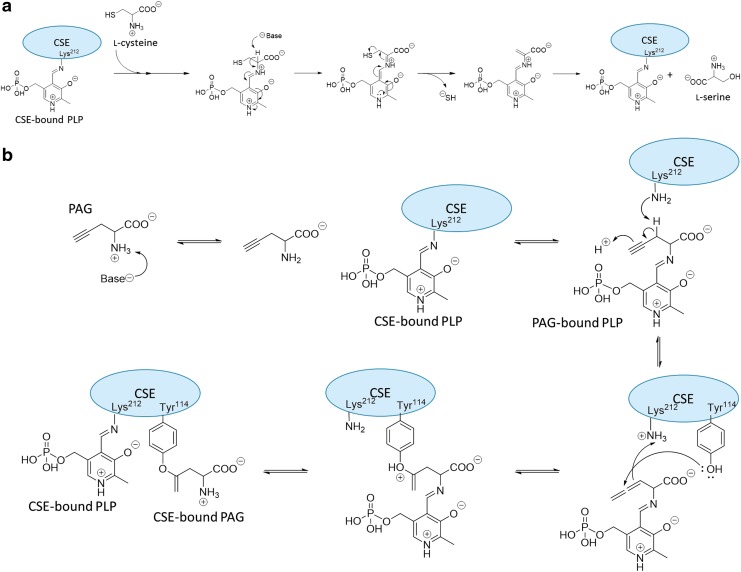
Various H2S-generating reactions are catalyzed by CSE, but the main pathway involves α,β-elimination reaction of l-Cys, as shown in Figure 7a. The first step of the reaction in CSE is the exchanging reaction from Schiff base between PLP and Lys212 of CSE to that between PLP and l-Cys. In this complex, l-Cys is deprotonated and the aromatic ring of PLP is reformed. After that, SH− is released from l-Cys (15).
FIG. 7.
H2S generation by CSE and mechanism of inhibition of CSE by PAG. (a) Mechanism of H2S generation by CSE: l-Cysteine acts as a substrate and undergoes α, β elimination to generate l-serine with release of SH− (15). (b) Mechanism of inhibition of CSE by PAG (72).
The reaction mechanism of CSE inhibition by PAG is shown in Figure 7b (72). The first reaction is deprotonation of the α-amino group of PAG, and this is followed by transaldimination. The bound PAG is deprotonated by Lys212 and an activated allene is formed. The phenoxy group of Tyr114 nucleophilically attacks the allene to deprotonate Lys212, affording a vinyl ether. Finally, transaldimination between Lys212 and PAG occurs, but PAG is covalently bound to Tyr114. The bound PAG occupies a space at the substrate-binding site of CSE, blocking access of the substrate to the active site so that the enzymatic activity is irreversibly inhibited (4, 81).
The inhibition mechanism of BCA appears to be different from that of PAG, because BCA inhibits CSE reversibly (4, 59, 81). BCA binds to the active site of CSE in a PLP-dependent manner, probably forming a Schiff base linkage, but this linkage can be hydrolyzed, which may account for the reversibility of this inhibition (2).
Inhibitors of CBS
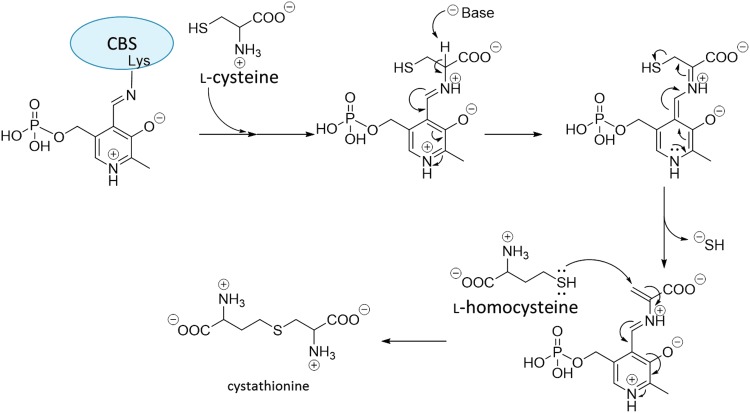
β-Replacement of l-Cys by l-homocysteine is the main route of H2S generation catalyzed by CBS (Fig. 8) (13). The first half of the reaction is similar to the reaction of CSE with l-Cys, that is, l-Cys and PLP form a Schiff base linkage and l-Cys is deprotonated. When PLP reforms its aromatic ring, HS− is released and the olefin in l-Cys is formed. Then, l-homocysteine attacks the olefin moiety, affording cystathionine (Fig. 8). AOAA has also been used as an inhibitor of CBS (4, 59, 81). Inhibition by AOAA is thought to be because of the formation of the Schiff base between AOAA and PLP at the active site of CBS, because it has been reported that AOAA forms a Schiff base linkage with PLP in CSE to block the generation of H2S (6). AOAA also inhibits other PLP-dependent enzymes, showing relatively low selectivity for CBS (4).
FIG. 8.
Inhibitors of 3MST
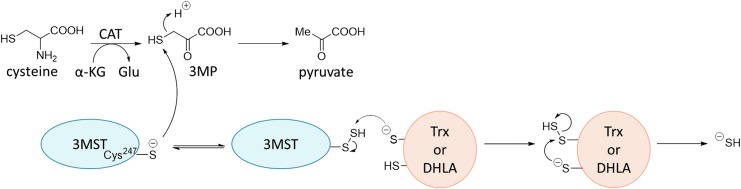
3MST generates H2S from 3-mercaptopyrvate (3MP) in the presence of dithiols, such as thioredoxin (Trx) or dihydrolipoic acid (DHLA) (Fig. 9) (53). 3MP is generated from l-Cys and α-oxoglutarate (α-KG), catalyzed by cysteine aminotransferase. The Cys residue at the active site of 3MST attacks the thiol group of 3MP, forming the persulfide at the active site Cys247. The persulfide is then attacked by one thiol of dithiol (Trx or DHLA), and the dithiol forms the persulfide group. The transferred persulfide is attacked by the other thiol of the dithiol, producing SH−. No selective inhibitor of 3MST has been reported so far, although pyruvate, menadione, 3-mercaptopropionic acid, and 3-chloropyruvate are nonselective inhibitors of 3MST (81).
FIG. 9.
Mechanism of H2S generation by 3MST (53). 3MST, 3-mercaptopyruvate sulfurtransferase.
Recent reports of selective inhibitors
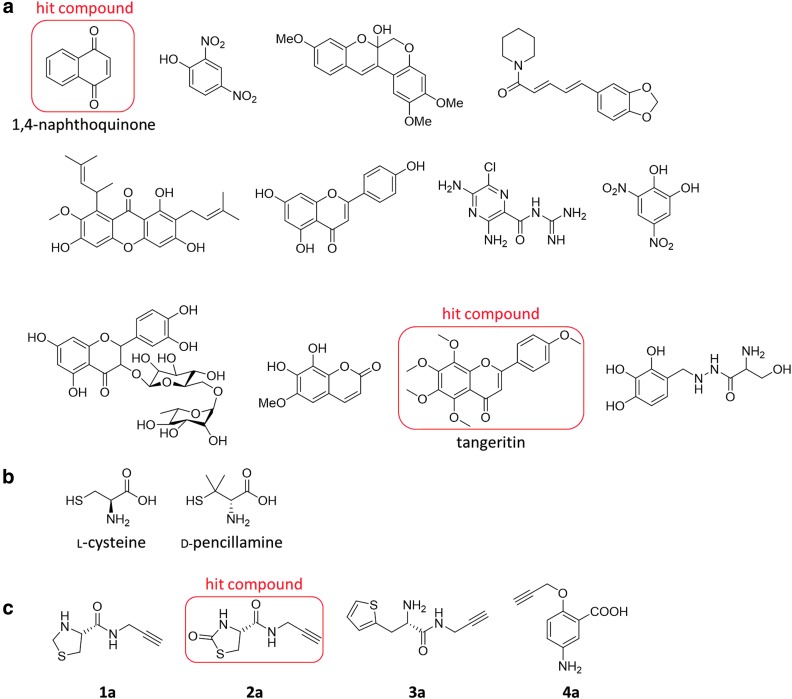
Thorson et al. (75) screened a structurally diverse library of 1900 chemical compounds with a wide range of biological activities for inhibitors of CBS by using AzMC as a fluorescence probe for H2S (Fig. 1), and found 12 hit compounds in the initial screening. After considering their selectivity for CBS and excluding compounds directly reacting with H2S, 1,4-naphthoquinone and tangeritin were concluded to be selective inhibitors of CBS; these compounds showed selectivity for CBS over CSE (Fig. 10a).
FIG. 10.
Recently reported inhibitors of CSE and CBS. (a) Twelve hit compounds in the initial screening of CBS inhibitors by Thorson et al. 1,4-Naphthoquinone and tangeritin were reported to specifically inhibit H2S production by CBS (75). (b) Chemical structures of l-cysteine and d-pencillamine (7). (c) Chemical structures of compounds synthesized by Corvino et al. (17) as candidate inhibitors of CSE. All of them have a propargyl group. Compounds (1a) and (2a) are cysteine derivatives, and also have a thiazolidine moiety. Compounds (3a) and (4a) have an aromatic group that is expected to interact with CSE (17).
Cirino and colleagues (7) reported that d-penicillamine (d-pen), which has a structure similar to Cys and is used as an antirheumatic drug, acts as a selective inhibitor of CSE in a PLP-dependent manner (Fig. 10b). First, they showed that d-pen is not metabolized by CSE in vitro. Cell-free assay with recombinant CSE showed that d-pen reduced H2S synthesis, and was about 30 times more selective for CSE than CBS. Then, they showed that d-pen inhibits CSE by interacting with PLP, and they confirmed that it inhibits CSE in vivo. Thus, d-pen should be a useful scaffold for the design of novel selective inhibitors for CSE.
Structure-based and fragment-based approaches to find CSE inhibitors have been reported by Pastore and colleagues (17). They discovered that Cys derivatives with a propargyl group in the α-carboxyl position selectively inhibit CSE (Fig. 10c). They synthesized four new compounds, all having a propargyl group; this is an essential structural element for inhibition of CSE by PAG. Two of them have structure similar to Cys, whereas the other two contain an aromatic group, which is expected to interact with the amino acid residue of CSE at the active site. When pharmacological assays were performed, compound 2a (Fig. 10c) showed good water solubility, suggesting that it might be practically useful as an inhibitor. Compound 2a also showed high selectivity for CSE against CBS. They also reported that the inhibitory activity of compound 2a was greater than that of PAG.
Our group very recently reported a first 3MST-selective inhibitor (30). We performed high-throughput screening for 3MST inhibitors in a chemical library containing 174,118 compounds by using our selective fluorescent probe for H2S, HSip-1 (Fig. 3). In the assay, purified 3MST and the substrate 3-MP were used and HSip-1 reacted with H2S released by the enzymatic reaction. Compounds that suppressed the fluorescence increase of HSip-1 in response to H2S were selected, and we discovered several selective inhibitors of 3MST.
Cellular Applications of Fluorescent Probes and Inhibitors in Biological Studies
Although there is great interest in the biological roles of H2S, hydropersulfide, and polysulfide, many of the underlying molecular events remain poorly understood. Therefore, fluorescent probes for these sulfur molecules and inhibitors for related enzymes are needed to enable detailed studies of their molecular mechanisms. In some cases, both fluorescent probes and selective inhibitors have been used.
Investigation of intracellular endogenous H2S
Li and colleagues have developed a mitochondria-targeted fluorescent probe for H2S (58). When HeLa cells were preincubated with 1 mM PAG for 1 h and then incubated with their fluorescent probe (10 μM) for 30 min, the fluorescence intensity was clearly decreased, and this fluorescence decrease was considered to reflect the intracellular basal level of H2S.
Chang and colleagues have reported real-time visualization of endogenous H2S produced in live human umbilical vein endothelial cells (HUVECs) upon stimulation with vascular endothelial growth factor (VEGF), using a new fluorescent probe for H2S (46). CSE was identified as the key H2S-producing enzyme in the vasculature, and treatment of HUVECs with PAG attenuated the fluorescence signal of the probe. This indicates that CSE contributes to the observed H2S generation. They also reported that not only CSE but also CBS was expressed in HUVECs, so it is possible that CBS also has a role in this biological system.
Yi et al. reported a dual-response fluorescent probe for detecting H2O2 and H2S (86). When 200 μM GSH was added to HEK293 cells in the presence of their fluorescent probe, marked fluorescence enhancement was observed, representing endogenous H2S. In contrast, when HEK293 cells were coincubated with phorbol-12-myristate-13-acetate (PMA; an inducer of endogenous H2O2 production) and the probe, a marked fluorescence increase was observed, representing endogenous H2S production induced by endogenous H2O2 in living cells. The PMA-induced fluorescence increase was decreased by addition of PAG, implying that PAG may inhibit PMA-induced H2S biogenesis.
Dufton et al. investigated the regulation of leukocyte H2S synthesis by using a fluorescent probe for H2S, SF5 (Fig. 1) (18). To assess H2S synthesis in primary murine cells, they used P-Gel-derived macrophages, and they observed an increase of SF5 fluorescence in the living cells. Addition of l-Cys and PLP to the culture medium for 1 h had no effect on intracellular H2S generation, whereas the inhibition of CSE and CBS with l-PAG and AOAA significantly reduced H2S synthesis in a concentration-dependent manner.
Yi and colleagues have reported FRET-based ratiometric probes for H2S (80). Fluorescence imaging with these probes indicated that d-Cys induces greater H2S production than does l-Cys in mitochondria of HEK293 cells. PAG also inhibited Cys-dependent endogenous H2S production in a chiral-sensitive manner in living cells, that is, d-PAG inhibited d-Cys-dependent H2S production more efficiently than l-PAG, whereas l-PAG inhibited l-Cys-dependent H2S production more efficiently than d-PAG.
Pluth and colleagues have reported a fluorescent probe for detecting endogenously produced H2S in C6 rat glial cells, which express CBS (27). They measured the fluorescence response in cells that had been pretreated with AOAA (20 μM), and observed a significant reduction compared with untreated cells, presumably reflecting endogenous enzymatic production of H2S.
Investigation of intracellular endogenous hydropersulfide and polysulfide
Chen and colleagues have reported a fluorescent probe for mitochondrial hydrogen polysulfides, Mito-ss (Fig. 5a) (24). They tried to detect endogenously produced hydrogen polysulfides by perturbing the pool of hydrogen polysulfides in RAW264.7 cells. RAW264.7 cells were stimulated with lipopolysaccharide (LPS, 1 μg/mM) for 16 h and then incubated with Mito-ss for 15 min. LPS induces CSE mRNA overexpression, which resulted in an increase of hydrogen polysulfide production. The cells showed a dramatic increase in intracellular fluorescence intensity, suggesting that the probe could detect endogenously produced hydrogen polysulfides.
They also pretreated the cells with PAG (100 μM) for 10 min and then stimulated them with LPS for 16 h and incubated them with Mito-ss for another 15 min. In this case, the fluorescence response was attenuated, indicating that CSE contributes to the observed hydrogen polysulfide generation.
Yu and colleagues have reported a fluorescent probe for both superoxide anion and hydrogen polysulfides in mitochondria, Hcy-Mito (35). When HUVECs were incubated with 1 μM Hcy-Mito for 15 min, the cells did not show any significant fluorescence increase, and the apoptosis rate was almost 0%. But, when the cells were treated with VEGF 40 ng/ml for 15 min to trigger a superoxide anion burst and then incubated for 30 min, they showed strong fluorescence, indicating increased production of hydrogen polysulfides. Moreover, when the cells were preincubated with 100 μM PAG for 10 min to inhibit CSE, the rate of apoptosis increased to 20.9%. This result indicates that the main antioxidant activity in HUVECs was caused by hydrogen polysulfides.
The same group has also reported a ratiometric fluorescent probe for Cys hydropersulfide, Cy-Dise (29). When HL-7702 cells were pretreated with N-ethylmaleimide to deplete endogenous Cys hydropersulfides, almost no increase of the ratiometric fluorescence signal was detected with Cy-Dise. Pretreatment of the cells with PAG also gave a low ratiometric response, indicating a low level of Cys hydropersulfide. However, CSE-overexpressing cells showed a strong ratiometric increase, indicating a high level of Cys hydropersulfide in the cells. CSE-overexpressing cells were also treated with hydroxylamine to inhibit CSE activity, and in this case, the ratiometric signal showed a low level of Cys hydropersulfide.
In contrast, pretreatment with 100 μM cystine for 1 h increased the level of Cys hydropersulfide. When the cells were treated with 100 μM sulfasalazine for 3 h, they showed a low level of Cys hydropersulfide, presumably because sulfasalazine inhibits Cys/glutamine transporter, which is one of the transporters of cystine. Thus, the intracellular level of Cys hydropersulfide can be changed by exogenous stimuli.
Ojida and colleagues have reported a reversible ratiometric fluorescent probe for hydropersulfides (Fig. 5c) (39). When A549 cells were treated with the fluorescent probe for 20 min, bright fluorescence was observed in the cytosolic region. When A549 cells were preincubated with the probe, and then treated with cystine, a gradual increase of the hydropersulfide level was observed. This increase was suppressed by treatment with AOAA. Conversely, treatment of the cells with auranofin, an inhibitor of thioredoxin reductase (TrxR), induced an increase in the intracellular hydropersulfide level.
Moreover, when fluorescence imaging of live A549 cells treated with l-Cys was performed using the fluorescent probe, the level of hydropersulfide increased in a time-dependent manner. Treatment of the cells with AOAA effectively suppressed the increase of hydropersulfide level induced by l-Cys. Unlike the case of the cystine experiment, inhibition of TrxR by auranofin did not cause an increase of the hydropersulfide level, implying that direct conversion of l-Cys to Cys hydropersulfide is not a major pathway of hydropersulfide formation in living cells.
Conclusions
Here, we have reviewed fluorescent probes for RSS (H2S, hydropersulfide, and polysulfide) based on a variety of design strategies, together with inhibitors of CSE, CBS, and 3MST, and we have introduced their biological applications. It is well established that H2S acts as a signaling and effector molecule, but so far its chemical mechanisms of action remain largely unknown.
In contrast, biological studies with recently developed fluorescent probes and detection methods for H2S, hydropersulfide, and polysulfide have suggested that hydropersulfide and polysulfide may be the actual signaling molecules mediating some of the physiological functions of H2S. Inhibitors of enzymes that synthesize these RSS are also powerful reagents for biological studies, but the selectivity and affinity of available small-molecular inhibitors are still insufficient and there are some conflicting results in the literature. Incorrect usage of inhibitors or failure to recognize pharmacologically relevant off targets would likely generate misleading results (3, 68), and detailed investigation of the specificity of various inhibitors seems to be needed for the high-quality biological research.
Further development and application of chemical tools for H2S, hydropersulfide, and polysulfide studies, especially tools able to detect and regulate the endogenous levels of these reactive sulfur molecules in cellulo, are expected to lead to an improved understanding of the role of these mediators in the control mechanisms of multiple physiological functions.
Abbreviations Used
- 3MP
3-mercaptopyrvate
- 3MST
3-mercaptopyruvate sulfurtransferase
- AOAA
aminooxyacetic acid
- BCA
β-cyano-l-alanine
- CBS
cystathionine β-synthase
- CSE
cystathionine γ-lyase
- Cys
cysteine
- DHLA
dihydrolipoic acid
- DNP
2,4-dinitrophenyl
- d-pen
d-penicillamine
- FRET
fluorescence resonance energy transfer
- GSH
glutathione
- H2S
hydrogen sulfide
- HSip-1
hydrogen sulfide imaging probe-1
- HUVEC
human umbilical vein endothelial cell
- LPS
lipopolysaccharide
- NIR
near-infrared
- NMDAR
N-methyl-d-aspartate receptor
- PAG
d,l-propargylglycine
- PLP
pyridoxal 5′-phosphate
- PMA
phorbol-12-myristate-13-acetate
- RSS
reactive sulfur species
- Trx
thioredoxin
- TrxR
thioredoxin reductase
- VEGF
vascular endothelial growth factor
Acknowledgments
This work was supported, in part, by grants by JSPS KAKENHI Grant Numbers 16H00823 and 16H05099 to K.H., and SENTAN, JST to K.H., who was also supported by Mochida Memorial Foundation for Medical and Pharmaceutical Research.
References
- 1.Abe K. and Kimura H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16: 1066–1071, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alston TA, Porter DJT, Mela L, and Bright HJ. Inactivation of alanine aminotransferase by the neurotoxin β-cyano-l-alanine. Biochem Biophys Res Commun 92: 299–304, 1980 [DOI] [PubMed] [Google Scholar]
- 3.Arrowsmith CH, Audia JE, Austin C, Baell J, Bennett J, Blagg J, Bountra C, Brennan PE, Brown PJ, Bunnage ME, Buser-Doepner C, Campbell RM, Carter AJ, Cohen P, Copeland RA, Cravatt B, Dahlin JL, Dhanak D, Edwards AM, Frederiksen M, Frye SV, Gray N, Grimshaw CE, Hepworth D, Howe T, Huber KVM, Jin J, Knapp S, Kotz JD, Kruger RG, Lowe D, Mader MM, Marsden B, Mueller-Fahrnow A, Müller S, O'Hagan RC, Overington JP, Owen DR, Rosenberg SH, Ross R, Roth B, Schapira M, Schreiber SL, Shoichet B, Sundström M, Superti-Furga G, Taunton J, Toledo-Sherman L, Walpole C, Walters MA, Willson TM, Workman P, Young RN, and Zuercher WJ. The promise and peril of chemical probes. Nat Chem Biol 11: 536–541, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Asimakopoulou A, Panopoulos P, Chasapis CT, Coletta C, Zhou Z, Cirino G, Giannis A, Szabo C, Spyroulias GA, and Papapetropoulos A. Selectivity of commonly used pharmacological inhibitors for cystathionine β synthase (CBS) and cystathionine γ lyase (CSE). Br J Pharmacol 169: 922–932, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bae SK, Heo CH, Choi DJ, Sen D, Joe EH, Cho BR, and Kim HM. A ratiometric two-photon fluorescent probe reveals reduction in mitochondrial H2S production in Parkinson's disease gene knockout astrocytes. J Am Chem Soc 135: 9915–9923, 2013 [DOI] [PubMed] [Google Scholar]
- 6.Beeler T. and Churchich JE. Reactivity of the phosphopyridoxal group of cystathionase. J Biol Chem 251: 5267–5271, 1976 [PubMed] [Google Scholar]
- 7.Brancaleone V, Esposito I, Gargiulo A, Vellecco V, Asimakopoulou A, Citi V, Calderone V, Gobbetti T, Perretti M, Papapetropoulos A, Bucci M, and Cirino G. d-Penicillamine modulates hydrogen sulfide (H2S) pathway through selective inhibition of cystathionine-γ-lyase. Br J Pharmacol 173: 1556–1565, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao XW, Lin WY, Zheng KB, and He LW. A near-infrared fluorescent turn-on probe for fluorescence imaging of hydrogen sulfide in living cells based on thiolysis of dinitrophenyl ether. Chem Commun 48: 10529–10531, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Chen W, Liu CR, Peng B, Zhao Y, Pacheco A, and Xian M. New fluorescent probes for sulfane sulfurs and the application in bioimaging. Chem Sci 4: 2892–2896, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen W, Pacheco A, Takano Y, Day JJ, Hanaoka K, and Xian M. A single fluorescent probe to visualize hydrogen sulfide and hydrogen polysulfides with different fluorescence signals. Angew Chem Int Ed 55: 9993–9996, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen W, Rosser EW, Matsunaga T, Pacheco A, Akaike T, and Xian M. The development of fluorescent probes for visualizing intracellular hydrogen polysulfides. Angew Chem Int Ed 54: 13961–13965, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W, Rosser EW, Zhang D, Shi W, Li Y, Dong WJ, Ma H, Hu D, and Xian M. A specific nucleophilic ring-opening reaction of aziridines as a unique platform for the construction of hydrogen polysulfides sensors. Org Lett 17: 2776–2779, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Jhee KH, and Kruger WD. Production of the neuromodulator H2S by cystathionine β-synthase via the condensation of cysteine and homocysteine. J Biol Chem 279: 52082–52086, 2004 [DOI] [PubMed] [Google Scholar]
- 14.Chen Y, Zhu C, Yang Z, Chen J, He Y, Jiao Y, He W, Qiu L, Cen J, and Guo Z. A ratiometric fluorescent probe for rapid detection of hydrogen sulfide in mitochondria. Angew Chem Int Ed 52: 1688–1691, 2013 [DOI] [PubMed] [Google Scholar]
- 15.Chiku T, Padovani D, Zhu W, Singh S, Vitvitsky V, and Banerjee R. H2S biogenesis by human cystathionine γ-lyase leads to novel sulfur metabolites lanthionine and homolanthionine and is responsive to the grade of hyperhomocysteinemia. J Biol Chem 284: 11601–11612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi MG, Cha S, Lee H, Jeon HL, and Chang SK. Sulfide-selective chemosignaling by a Cu2+ complex of dipicolylamine appended fluorescein. Chem Commun 739 0–7392, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Corvino A, Severino B, Fiorino F, Frecentese F, Magli E, Perissutti E, Santagada V, Vucci M, Cirino G, Kelly G, Servillo L, Popowicz G, Pastore A, and Caliendo G. Fragment-based de novo design of a cystathionine γ-lyase selective inhibitor blocking hydrogen sulfide production. Sci Rep 6: 34398, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dufton N, Natividad J, Verdu EF, and Wallace JL. Hydrogen sulfide and resolution of acute inflammation: a comparative study utilizing a novel fluorescent probe. Sci Rep 2: 499 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faro MLL, Fox B, Whatmore JL, Winyard PG, and Whiteman M. Hydrogen sulfide and nitric oxide interactions in inflammation. Nitric Oxide 41: 38–47, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Fridkin M, Hazum E, Tauber-Finkelstein M, and Shaltiel S. Thiolysis of O-2,4-dinitrophenyltyrosines: spectrophotomeric monitoring of reaction and its use in peptide synthesis. Arch Biochem Biophys 178: 517–526, 1977 [DOI] [PubMed] [Google Scholar]
- 21.Fukushima N, Ieda N, Kawaguchi M, Sasakura K, Nagano T, Hanaoka K, Miyata N, and Nakagawa H. Development of photo-controllable hydrogen sulfide donor applicable in live cells. Bioorg Med Chem Lett 25: 175–178, 2015 [DOI] [PubMed] [Google Scholar]
- 22.Fukushima N, Ieda N, Sasakura K, Nagano T, Hanaoka K, Suzuki T, Naoki M, and Nakagawa H. Synthesis of a photocontrollable hydrogen sulfide donor using ketoprofenate photocages. Chem Commun 50: 587–589, 2014 [DOI] [PubMed] [Google Scholar]
- 23.Gao M, Wang R, Yu FB, You JM, and Chen LX. A near-infrared fluorescent probe for the detection of hydrogen polysulfides biosynthetic pathways in living cells and in vivo. Analyst 140: 3766–3772, 2015 [DOI] [PubMed] [Google Scholar]
- 24.Gao M, Yu FB, Chen H, and Chen LX. Near-infrared fluorescent probe for imaging mitochondrial hydrogen polysulfides in living cells and in vivo. Anal Chem 87: 3631–3638, 2015 [DOI] [PubMed] [Google Scholar]
- 25.Giepmans BN, Adams SR, Ellisman MH, and Tsien RY. The fluorescent toolbox for assessing protein location and function. Science 312: 217–224, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Gu X, Zhu H, Yang S, Zhu YC, and Zhu YZ. Development of a highly selective H2S fluorescent probe and its application to evaluate CSE inhibitors. RSC Adv 4: 50097–50101, 2014 [Google Scholar]
- 27.Hammers MD, Taormina MJ, Cerda MM, Montoya LA, Seidenkranz DT, Parthasarathy R, and Pluth MD. A bright fluorescent probe for H2S enables analyte-responsive, 3D imaging in live zebrafish using light sheet fluorescence microscopy. J Am Chem Soc 137: 10216–10223, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Han Q, Mou Z, Wang H, Tang X, Dong Z, Wang L, Dong X, and Liu W. Highly selective and sensitive one- and two-photon ratiometric fluorescent probe for intracellular hydrogen polysulfide sensing. Anal Chem 88: 7206–7212, 2016 [DOI] [PubMed] [Google Scholar]
- 29.Han X, Yu F, Song X, and Chen L. Quantification of cysteine hydropersulfide with a ratiometric near-infrared fluorescent probe based on selenium-sulfur exchange reaction. Chem Sci 7: 5098–5107, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hanaoka K, Sasakura K, Suwanai Y, Toma-Fukai S, Shimamoto K, Takano Y, Shibuya N, Terai T, Komatsu T, Ueno T, Ogasawara Y, Tsuchiya Y, Watanabe Y, Kimura H, Wang C, Uchiyama M, Kojima H, Okabe T, Urano Y, Shimizu T, and Nagano T. Discovery and mechanistic characterization of selective inhibitors of H2S-producing enzyme: 3-mercaptopyruvate sulfurtransferase (3MST) targeting active-site cysteine persulfide. Sci Rep 7: 40227, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Henthorn HA. and Pluth MD. Mechanistic insights into the H2S-mediated reduction of aryl azides commonly used in H2S detection. J Am Chem Soc 137: 15330–15336, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoffmann MR. Kinetics and mechanism of oxidation of hydrogen sulfide by hydrogen peroxide in acidic solution. Environ Sci Technol 11: 61–66, 1977 [Google Scholar]
- 33.Hou F, Huang L, Xi P, Cheng J, Zhao X, Xie G, Shi Y, Cheng F, Yao X, Bai D, and Zeng Z. A retrievable and highly selective fluorescent probe for monitoring sulfide and imaging in living cells. Inorg Chem 51: 2454–2460, 2012 [DOI] [PubMed] [Google Scholar]
- 34.Hou Y, Yang XF, Zhong YG, and Li Z. Development of fluorescent probes for hydrogen polysulfides by using cinnamate ester as the recognition unit. Sensor Actuat B Chem 232: 531–537, 2016 [Google Scholar]
- 35.Huang Y, Yu FB, Wang JC, and Chen LX. Near-infrared fluorescence probe for in situ detection of superoxide anion and hydrogen polysulfides in mitochondrial oxidative stress. Anal Chem 88: 4122–4129, 2016 [DOI] [PubMed] [Google Scholar]
- 36.Ida T, Sawa T, Ihara H, Tsuchiya Y, Watanabe Y, Kumagai Y, Suematsu M, Motohashi H, Fujii S, Matsunaga T, Yamamoto M, Ono K, Devarie-Baez NO, Xian M, Fukuto JM, and Akaike T. Reactive cysteine persulfides and S-polythiolation regulate oxidative stress and redox signaling. Proc Natl Acad Sci U S A 111: 7606–7611, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishii I, Akahoshi N, Yamada H, Nakano S, Izumi T, and Suematsu M. Cystathionine γ-lyase-deficient mice require dietary cysteine to protect against acute lethal myopathy and oxidative injury. J Biol Chem 285: 26358–26368, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaneko Y, Kimura Y, Kimura H, and Niki I. l-Cysteine inhibits insulin release from the pancreatic β-cell: possible involvement of metabolic production of hydrogen sulfide, a novel gasotransmitter. Diabetes 55: 1391–1397, 2006 [DOI] [PubMed] [Google Scholar]
- 39.Kawagoe R, Takashima I, Uchinomiya S, and Ojida A. Reversible ratiometric detection of highly reactive hydropersulfides using a FRET-based dual emission fluorescent probe. Chem Sci [Epub ahead of print]; DOI: 10.1039/C6SC03856E, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kimura H. Physiological role of hydrogen sulfide and polysulfide in the central nervous system. Neurochem Int 63: 492–497, 2013 [DOI] [PubMed] [Google Scholar]
- 41.Kimura H. Signaling molecules: hydrogen sulfide and polysulfide. Antioxid Redox Signal 22: 362–376, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kimura H, Shibuya N, and Kimura Y. Hydrogen sulfide is a signaling molecules and a cytoprotectant. Antioxid Redox Signal 17: 45–57, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klingerman CM, Trushin N, Prokopczyk B, and Haouzi P. H2S concentrations in the arterial blood during H2S administration in relation to its toxicity and effects on breathing. Am J Physiol Regul Integr Comp Physiol 305: R630–R638, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kubáň V, Dasgupta PK, and Marx JN. Nitroprusside and methylene blue methods for silicone membrane differentiated flow injection determination of sulfide in water and wastewater. Anal Chem 64: 36–43, 1992 [DOI] [PubMed] [Google Scholar]
- 45.Li L, Bhatia M, Zhu YZ, Zhu YC, Ramnath RD, Wang ZJ, Anuar FB, Whiteman M, Salto-Tellez M, and Moore PK. Hydrogen sulfide is a novel mediator of lipopolysaccharide-induced inflammation in the mouse. FASEB J 19: 1196–1198, 2005 [DOI] [PubMed] [Google Scholar]
- 46.Lin VS, Lippert AR, and Chang CJ. Cell-trappable fluorescent probes for endogenous hydrogen sulfide signaling and imaging H2O2-dependent H2S production. Proc Natl Acad Sci U S A 110: 7131–7135, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lippert AR. Designing reaction-based fluorescent probes for selective hydrogen sulfide detection. J Inorg Biochem 133: 136–142, 2014 [DOI] [PubMed] [Google Scholar]
- 48.Lippert AR, New EJ, and Chang CJ. Reaction-based fluorescent probes for selective imaging of hydrogen sulfide in living cells. J Am Chem Soc 133: 10078–10080, 2011 [DOI] [PubMed] [Google Scholar]
- 49.Liu C, Chen W, Shi W, Peng B, Zhao Y, Ma H, and Xian M. Rational design and bioimaging applications of highly selective fluorescence probes for hydrogen polysulfides. J Am Chem Soc 136: 7257–7260, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu C, Pan J, Li S, Zhao Y, Wu LY, Berkman CE, Whorton AR, and Xian M. Capture and visualization of hydrogen sulfide by a fluorescent probe. Angew Chem Int Ed 50: 10327–10329, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu CR, Peng B, Li S, Park CM, Whorton AR, and Xian M. Reaction based fluorescent probes for hydrogen sulfide. Org Lett 14: 2184–2187, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Marutani E, Sakaguchi M, Chen W, Sasakura K, Liu J, Xian M, Hanaoka K, Nagano T, and Ichinose F. Cytoprotective effects of hydrogen sulfide-releasing N-methyl-d-aspartate receptor antagonists mediated by intracellular sulfane sulfur. Med Chem Comm 5: 1577–1583, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mikami Y, Shibuya N, Kimura Y, Nagahara N, Ogasawara Y, and Kimura H. Thioredoxin and dihydrolipoic acid are required for 3-mercaptopyruvate sufurtransferase to produce hydrogen sulfide. Biochem J 439: 479–485, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Montoya LA. and Pluth MD. Selective turn-on fluorescent probes for imaging hydrogen sulfide in living cells. Chem Commun 48: 4767–4769, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mustafa AK, Gadalla MM, Sen N, Kim S, Mu W, Gazi SK, Barrow RK, Yang G, Wang R, and Snyder SH. H2S signals through protein S-sulfhydration. Sci Signal 2: ra72, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nagahara N, Nagano M, Ito T, Shimamura K, Akimoto T, and Suzuki H. Antioxidant enzyme, 3-mercaptopyruvate sulfurtransferase-knockout mice exhibit increased anxiety-like behaviors: a model for human mercaptolactate-cysteine disulfiduria. Sci Rep 3: 1986, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishida M, Sawa T, Kitajima N, Ono K, Inoue H, Ihara H, Motohashi H, Yamamoto M, Suematsu M, Kurose H, van der Vliet A, Freeman BA, Shibata T, Uchida K, Kumagai Y, and Akaike T. Hydrogen sulfide anion regulates redox signaling via electrophile sulfhydration. Nat Chem Biol 8: 714–724, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pak YL, Li J, Ko KC, Kim G, Lee JY, and Yoon J. Mitochondria-targeted reaction-based fluorescent probe for hydrogen sulfide. Anal Chem 88: 5476–5481, 2016 [DOI] [PubMed] [Google Scholar]
- 59.Papapetropoulos A, Whiteman M, and Cirino G. Pharmacological tools for hydrogen sulphide research: a brief, introductory guide for beginners. Br J Pharmacol 172: 1633–1637, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Paul BD. and Snyder SH. H2S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Biol 13: 499–507, 2012 [DOI] [PubMed] [Google Scholar]
- 61.Pearson RG. Hard and soft acids and bases. J Am Chem Soc 85: 3533–3539, 1963 [Google Scholar]
- 62.Peng B, Chen W, Liu C, Rosser EW, Pacheco A, Zhao Y, Aguilar HC, and Xian M. Fluorescent probes based on nucleophilic substitution-cyclization for hydrogen sulfide detection and bioimaging. Chem Eur J 20: 1010–1016, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Percudani R. and Peracchi A. A genomic overview of pyridoxal-phosphate-dependent enzymes. EMBO Rep 4: 850–854, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Qian Y, Karpus J, Kabil O, Zhang SY, Zhu HL, Banerjee R, Zhao J, and He C. Selective fluorescent probes for live-cell monitoring of sulphide. Nat Commun 2: 495, 2011 [DOI] [PubMed] [Google Scholar]
- 65.Qu XY, Li CJ, Chen HC, Mack J, Guo ZJ, and Shen Z. A red fluorescent turn-on probe for hydrogen sulfide and its application in living cells. Chem Commun 49: 7510–7512, 2013 [DOI] [PubMed] [Google Scholar]
- 66.Reiffenstein RJ, Hulbert WC, and Roth SH. Toxicology of hydrogen sulfide. Annu Rev Pharmacol Toxicol 32: 109–134, 1992 [DOI] [PubMed] [Google Scholar]
- 67.Sasakura K, Hanaoka K, Shibuya N, Mikami Y, Kimura Y, Komatsu T, Ueno T, Terai T, Kimura H, and Nagano T. Development of a highly selective fluorescence probe for hydrogen sulfide. J Am Chem Soc 133: 18003–18005, 2011 [DOI] [PubMed] [Google Scholar]
- 68.Schreiber SL, Kotz JD, Li M, Aubé J, Austin CP, Reed JC, Rosen H, White EL, Sklar LA, Lindsley CW, Alexander BR, Bittker JA, Clemons PA, de Souza A, Foley MA, Palmer M, Shamji AF, Wawer MJ, McManus O, Wu M, Zou B, Yu H, Golden JE, Schoenen FJ, Simeonov A, Jadhav A, Jackson MR, Pinkerton AB, Chung TDY, Griffin PR, Cravatt BF, Hodder PS, Roush WR, Roberts E, Chung DH, Jonsson CB, Noah JW, Severson WE, Ananthan S, Edwards B, Oprea TI, Conn PJ, Hopkins CR, Wood MR, Stauffer SR, Emmitte KA, and NIH Molecular Libraries Project Team. Advancing biological understanding and therapeutics discovery with small-molecule probes. Cell 161: 1252–1265, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Scriven EFV. and Turnbull K. Azides—their preparation and synthetic uses. Chem Rev 88: 297–368, 1988 [Google Scholar]
- 70.Shang HM, Chen H, Tang YH, Guo R, and Lin WY. Construction of a two-photon fluorescent turn-on probe for hydrogen persulfide and polysulfide and its bioimaging application in living mice. Sensor Actuat B Chem 230: 773–778, 2016 [Google Scholar]
- 71.Shimamoto K. and Hanaoka K. Fluorescent probes for hydrogen sulfide (H2S) and sulfane sulfur and their applications to biological studies. Nitric Oxide 46: 72–79, 2015 [DOI] [PubMed] [Google Scholar]
- 72.Sun Q, Collins R, Huang S, Holmverg-Schiavone L, Anand GS, Tan CH, van-den-Berg S, Deng LW, Moore PK, Karlberg T, and Sivaraman J. Structural basis for the inhibition mechanism of human cystathionine γ-lyase, an enzyme responsible for the production of H2S. J Biol Chem 284: 3076–3085, 2009 [DOI] [PubMed] [Google Scholar]
- 73.Takano Y, Hanaoka K, Shimamoto K, Miyamoto R, Komatsu T, Ueno T, Terai T, Kimura H, Nagano T, and Urano Y. Development of a reversible fluorescent probe for reactive sulfur species, sulfane sulfur, and its biological application. Chem Commun 53: 1064–1067, 2017 [DOI] [PubMed] [Google Scholar]
- 74.Takano Y, Shimamoto K, and Hanaoka K. Chemical tools for the study of hydrogen sulfide (H2S) and sulfane sulfur and their applications to biological studies. J Clin Biochem Nutr 58: 7–15, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thorson MK, Majtan T, Kraus JP, and Barrios AM. Identification of cystathionine β-synthase inhibitors using a hydrogen sulfide selective probe. Angew Chem Int Ed 52: 4641–4644, 2013 [DOI] [PubMed] [Google Scholar]
- 76.Toohey JI. Sulfur signaling: is the agent sulfide or sulfane? Anal Biochem 413: 1–7, 2011 [DOI] [PubMed] [Google Scholar]
- 77.Tsai DM, Kumar AS, and Zen JM. A highly stable and sensitive chemically modified screen-printed electrode for sulfide analysis. Anal Chim Acta 556: 145–150, 2006 [DOI] [PubMed] [Google Scholar]
- 78.Ueno T. and Nagano T. Fluorescent probes for sensing and imaging. Nat Method 8: 642–645, 2011 [DOI] [PubMed] [Google Scholar]
- 79.Watanabe M, Osada J, Aratani Y, Kluckman K, Reddick R, Malinow MR, and Maeda N. Mice deficient in cystathionine β-synthase: animal models for mild and severe homocyst(e)inemia. Proc Natl Acad Sci U S A 92: 1585–1589, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wei L, Yi L, Song F, Wei C, Wang B, and Xi Z. FRET ratiometric probes reveal the chiral-sensitive cysteine-dependent H2S production and regulation in living cells. Sci Rep 4: 4521, 2014 [Google Scholar]
- 81.Whiteman M, Trionnaire SL, Chopra M, Fox B, and Whatmore J. Emerging role of hydrogen sulfide in health and disease: critical appraisal of biomarkers and pharmacological tools. Clin Sci 121: 459–488, 2011 [DOI] [PubMed] [Google Scholar]
- 82.Wood JL. Sulfane sulfur. Methods Enzymol 143: 25–29, 1987 [DOI] [PubMed] [Google Scholar]
- 83.Xu Z, Xu L, Zhou J, Xu YF, Zhu WP, and Qian XH. A highly selective fluorescent probe for fast detection of hydrogen sulfide in aqueous solution and living cells. Chem Commun 48: 10871–10873, 2012 [DOI] [PubMed] [Google Scholar]
- 84.Yadav PK, Martinov M, Vitvitsky V, Seravalli J, Wedmann R, Filipovic MR, and Banerjee R. Biosynthesis and reactivity of cysteine persulfides in signaling. J Am Chem Soc 138: 289–299, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang G, Wu L, Jiang B, Yang W, Qi J, Cao K, Meng Q, Mustafa AK, Mu W, Zhang S, Snyder SH, and Wang R. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine γ-lyase. Science 322: 587–590, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yi L, Wei L, Wang R, Zhang C, Zhang J, Tan T, and Xi Z. A dual-response fluorescent probe reveals the H2O2-induced H2S biogenesis through a cystathionine β-synthase pathway. Chem Eur J 21: 15167–15172, 2015 [DOI] [PubMed] [Google Scholar]
- 87.Yu F, Han X, and Chen L. Fluorescent probes for hydrogen sulfide detection and bioimaging. Chem Commun 50: 12234–12249, 2014 [DOI] [PubMed] [Google Scholar]
- 88.Yu F, Li P, Song P, Wang B, Zhao J, and Han K. An ICT-based strategy to a colorimetric and ratiometric fluorescence probe for hydrogen sulfide in living cells. Chem Commun 48: 2852–2854, 2012 [DOI] [PubMed] [Google Scholar]
- 89.Zeng LY, Chen SY, Xia T, Hu W, Li CY, and Liu ZH. Two-photon fluorescent probe for detection of exogenous and endogenous hydrogen persulfide and polysulfide in living organisms. Anal Chem 87: 3004–3010, 2015 [DOI] [PubMed] [Google Scholar]