Abstract
Background
An increasing number of case reports suggest that acquired renal Fanconi syndrome may be associated with prolonged use of adefovir against hepatitis B virus. Renal Fanconi syndrome is an uncommon disease, and its complication with nephrolithiasis is quite rare. Herein, we report a rare coexistence of nephrolithiasis and acquired renal Fanconi syndrome in a chronic hepatitis B-positive patient with prolonged adefovir therapy.
Case presentation
The patient presented with osteomalacia and nephrolithiasis. Consequently, extracorporeal shock-wave lithotripsy and left double-J ureteral stent insertion were considered for obstructive nephropathy, which was caused by nephrolithiasis. However, osteomalacia had been misdiagnosed as osteoporosis before admission to our hospital. On admission, a complexity of multiple fractures, hypophosphataemia, glycosuria without hyperglycaemia and non–anion-gap metabolic acidosis indicated a diagnosis of acquired renal Fanconi syndrome induced by adefovir. After switching from adefovir to entecavir, the patient’s symptoms and laboratory findings improved significantly.
Conclusions
The mechanism responsible for nephrolithiasis in renal Fanconi syndrome is still unclear. We recommend regularly monitoring renal function and serum calcium and serum phosphate to prevent renal Fanconi syndrome during the prolonged use of adefovir for hepatitis B virus.
Keywords: Adefovir, Renal Fanconi syndrome, Osteomalacia, Osteoporosis, Nephrolithiasis, Obstructive nephropathy
Background
Adefovir dipivoxil-induced acute tubular necrosis and Fanconi syndrome have been well known to occur at a dose of 60 mg/d or 120 mg/d for human immunodeficiency virus (HIV) [1, 2]. The first case of acquired Fanconi syndrome associated with prolonged adefovir dipivoxil(10 mg/d) therapy for hepatitis B virus (HBV) had been published in 2008 [3]. Counted by ourselves, more than 40 cases of Fanconi syndrome caused by adefovir dipivoxil therapy for HBV have been reported in PubMed at present.
Fanconi syndrome is a generalized dysfunction of the proximal renal tubules leading to increased loss of amino acids, glucose, sodium, potassium, bicarbonate, calcium and phosphorus in the urine. Osteomalacia is a prevalent complication of Fanconi syndrome which contributes to bone demineralization through acidosis in the blood and urinary wasting of both phosphate and calcium. Hypercalciuria, another characteristic of renal Fanconi syndrome, is arguably the most common risk factor in calcium stone formation, but nephrolithiasis is rather uncommon in Fanconi syndrome [4]. Herein, we describe for the first time a case of nephrolithiasis and osteomalacia associated with adefovir-induced renal Fanconi syndrome.
Case presentation
A 60-year-old man was referred to our outpatient department for a 30-month history of generalized bone pain without antecedent trauma. He reported no fevers, chills, vomiting, diarrhoea, or other symptoms. The patient had a history of chronic hepatitis B treated with adefovir dipivoxil (10 mg/d) for 60 months and had no family history of nephrolithiasis or metabolic bone disease.
A whole-body bone scan was performed 18 months later in another hospital because of bone pain. It revealed multiple abnormal areas with increased tracer uptake in the bilateral ribs, bilateral sacroiliac joints, and bilateral tibias, which were consistent with multiple fractures. Serum intact parathyroid hormone (PTH) and tumour markers were normal. Osteoporosis was diagnosed and treated with alendronate, calcitriol, and calcium carbonate.
The patient’s bone pain was alleviated slightly after 6 months of treatment. Then, he was sent to the haematology department of the hospital mentioned above. Laboratory data are listed in Table 1. An ultrasound revealed stones in both kidneys and left ureteral stone combined with left hydronephrosis. A further CT scan of the urinary system without contrast revealed high-density images of both renal lower calyces, with left ureteral stone complicated by hydronephrosis on the left kidney, which suggested obstructive nephropathy caused by nephrolithiasis(Fig. 1). The total glomerular filtration rate was 41.89 ml/min evaluated by renal dynamic imaging with 99mTc-DTPA (techne-tium-99 m diethylene triamine penta-acetic acid) as exogenous markers. A DXA scan showed that the T-score of the left femoral neck was −2.60. A re-examination of whole-body 18-FDG-PET/CT showed similar findings as the whole body bone scan performed earlier at another institution. To eliminate bone pain and renal dysfunction caused by multiple myeloma, a bone marrow biopsy was performed, and the results were normal. The cause of renal impairment was presumably nephrolithiasis-induced obstructive nephropathy. Consequently, extracorporeal shock-wave lithotripsy and left double-J ureteral stent insertion were performed to alleviate the nephrolithiasis-induced obstructive nephropathy. Subsequently, alendronate, calcitriol, and calcium carbonate were suspended by the patient for personal reason.
Table 1.
laboratory Data
| Variable | Reference range | Other hospital | On admission | After Treatment |
|---|---|---|---|---|
| Serum | ||||
| Phosphate | 0.8–1.6 mmol/l | 0.51 | 0.63 | 0.94 |
| Calcium | 2.17–2.75 mmol/l | 2.01 | 2.12 | 2.44 |
| Potassium | 3.50–5.30 mmol/l | 3.38 | 3.75 | 3.80 |
| Chloride | 96-108 mmol/l | 111 | 107.75 | 105 |
| Sodium | 135-145 mmol/l | 142 | 135 | 137 |
| Magnesium | 0.7–1.10 mmol/l | 0.90 | 0.97 | 1.01 |
| Urea nitrogen | 2.9–8.2 mmol/l | 6.04 | 4.95 | 5.06 |
| Creatinine | 44-133umol/l | 158 | 139.6 | 134 |
| Uric acid | 208-506umol/l | 136 | 133.1 | 230 |
| Anion-gap | 8.0–16.0 mmol/l | 9.0 | 10.7 | 12 |
| Albumin | 35-55 g/l | 47.10 | 45.0 | 50 |
| ALP | 10-150u/l | 109 | 304.9 | 147 |
| FBG | 3.9–6.1 mmol/l | 5.7 | 5.6 | 5.6 |
| 25(OH) D3 | 27.7–107.0 nmol/l | NA | 36.7 | NA |
| iPTH | 15-65 pg/ml | 22.40 | 92.29 | 72.7 |
| Lead(Pb) | 0-200μg/l | 12.5 | NA | NA |
| Fibroblast growth factor 23 | 0-180RU/ml | NA | NA | NA |
| Arterial blood gas | ||||
| pH | 7.35–7.45 | NA | 7.32 | 7.37 |
| AB | 21.4–27.3 mmol/l | NA | 18.20 | 22.0 |
| PaCO2 | 4.66–6.00 KPa | NA | 4.57 | 4.86 |
| Urine | ||||
| pH | 5.0–9.0 | 6.50 | 7.00 | 6.0 |
| Protein | Negative | 1+ | 2+ | Negative |
| Glucose | Negative | 2+ | 4+ | Negative |
| Albumin | 4-230 mg/24 h | NA | 300 | NA |
| Phosphate | 12.9–42.0 mmol/24 h | 11.0 | 56.5 | 84.9 |
| Calcium | 2.5–7.5 mmol/24 h | 5.60 | 0.4 | 1.8 |
| Uric acid | 1.48–4.43 mmol/24 h | NA | 3.64 | 3.77 |
| N-acetyl-glucosaminidase | 0.3–12.0u/l | NA | 21.0 | NA |
| β2-MG | 0–0.32 mg/l | NA | 82.5 | NA |
ALP-alkaline phosphatase, AB-actual bicarbonate, FBG-fasting blood-glucose, 25(OH)D3–25-hydroxyvitamin D3, β2-MG β2-microglobulin, NA-not available
Fig. 1.
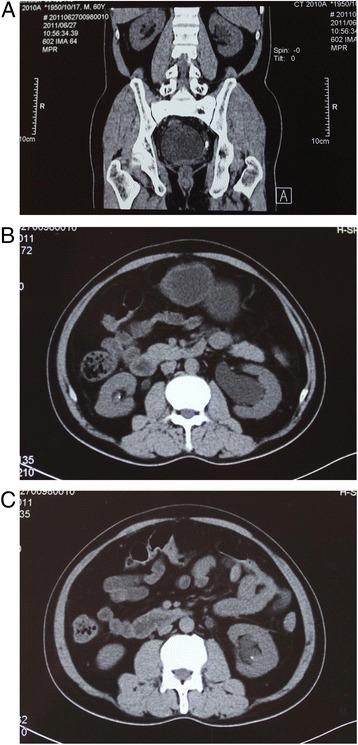
CT imagines. a: left ureteral stone combined with left hydronephrosis. b: a stone in the right kidney. c: a stone in the left kidney
The patient was then referred to our inpatient department because of bone pain for 30 months. At our first examination, the patient weighed 55 kg, and his height was 168 cm, giving a body mass index (BMI) of 19.5 kg/m2. The physical examination showed mild tenderness of the ribs. Laboratory investigations are presented in Table 1. A renal biopsy showed interstitial nephritis, and the immunohistochemistry test for HBV was negative. Mild-to-moderate interstitial nephritis and focal segmental glomerulosclerosis of the renal tissue were diagnosed by electron microscopy. The diagnosis of nephrolithiasis and osteomalacia due to renal Fanconi syndrome caused by adefovir is based on multiple fractures and an elevated level of alkaline phosphatase (ALP), hypophosphataemia due to hyperphosphaturia and non–anion-gap tubular metabolic acidosis accompanied with glycosuria without hyperglycaemia. Consequently, adefovir was substituted by entecavir (0.5 mg/d) against hepatitis B virus, along with the commencement of supplementation with neutral sodium phosphate (1.5 g/d), potassium citrate (4 g/d, divided into three equal parts), calcium carbonate (elemental calcium 0.6 g/d) and calcitriol (0.5 μg/d) orally for osteomalacia. The patient responded dramatically to the entecavir and supplementation therapy, which has now lasted five years.
Discussion
Osteomalacia is a metabolic bone disorder in adults caused by general defective mineralization of the organic bone matrix. Bone biopsy with double tetracycline labeling is the best procedure to differentiate osteomalacia from osteoporosis [5]. However, it is not easily executed unless the differential diagnosis cannot be made from the medical history. In osteoporosis, normal levels of serum calcium, phosphate, alkaline phosphatase, and PTH are present; by contrast, abnormalities in at least one of these measurements are common in osteomalacia [6]. The bone disorders point to the diagnosis of osteomalacia instead of osteoporosis in the case.
Nephrolithiasis is infrequently encountered in renal Fanconi syndrome [4]. Type 3 renal tubular acidosis is a mixture of distal renal tubular acidosis and renal Fanconi syndrome. It seems more reasonable to explain the coexistence of osteomalacia and nephrolithiasis in this case. During admission to our hospital, secondary hyperparathyroidism appears to counterpoise calcium wasting in the proximal renal tubules by increasing the reabsorption of calcium in the distal tubules. Therefore, the possibility of distal renal tubular acidosis occurring is inadequate.
In theory, the insolubility of adefovir in urinary environment may cause chemical stones. Nephrolithiasis has been demonstrated to be an adverse effect of indinavir when used as an antiretroviral agent for HIV [7]. However, the association between prolonged use of adefovir and nephrolithiasis formation has not been found regardless in basic research or clinical observation [8, 9]. Because extracorporeal shock-wave lithotripsy had been performed for obstructive nephropathy before the patient’s admission to our hospital, we could not precisely identify the character of nephrolithiasis in the patient. Therefore, whether the formation kidney stones directly related to insolubility of adefovir is uncertain in this case.
In the case, calcium stones seem most likely caused by hypercalciuria according to the radio opaque stones in both kidneys. Hypercalciuria was defined in adults as 24-h urine calcium excretion >4 mg/kg of body weight/day [10]. In this case, after adjusting for body weight the value of 24-h urine calcium excretion was 4.07 mg/kg which fulfilled the criteria of hypercalciuria. Hypercalciuria seems to be the major factor contributing to nephrolithiasis in the case. The causes of hypercalciuria in this case were complex. Because calcium reabsorption is defective in Fanconi syndrome, calcium supplementation would aggravate the hypercalciuria [10]. Hypophosphataemia may exacerbate hypercalciuria by increasing the intestinal calcium absorption [10].
Interestingly, mitochondria dysfunction may underline the association between adefovir nephrotoxicity and Fanconi syndrome [11, 12]. Furthermore, a clinical study demonstrated that disruption of the Sodium-inorganic phosphate cotransporter NaPi-IIa (NaPi-IIa) may play a crucial role in the pathogenesis of nephrolithiasis and metabolic bone disease associated with Fanconi syndrome [13, 14]. Therefore, the NaPi-IIa should be a focus of future research regarding adefovir induced Fanconi syndrome.
Conclusions
It remains a challenge for scientists and clinicians to understand the relationships among adefovir, Fanconi syndrome and nephrolithiasis. Until the mechanisms responsible for adefovir nephrotoxicity are clarified, we recommend regularly monitoring renal function, serum calcium and serum phosphate to prevent renal Fanconi syndrome during the prolonged use of adefovir for HBV particularly for patients with high-risk factors for renal impairment, such as low BMI, old age and decreased GFR.
Acknowledgments
We would like to acknowledge the helpful comments on this paper received from our reviewers.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Funding
Not applicable.
Abbreviations
- 18-FDG-PET/CT
18F–fluorodeoxyglucose positron emission tomography computed tomography
- 25(OH)D3
25-hydroxyvitamin D3
- 99mTc-DTPA
technetium-99 m diethylene triamine penta-acetic acid
- AB
actual bicarbonate
- ALP
alkaline phosphatase
- BMI
body mass index
- DXA
dual energy X-ray absorptiometry
- FBG
fasting blood-glucose
- HBV
hepatitis B virus
- HIV
human immunodeficiency virus
- mg/d
milligram/day
- NA
not available
- NaPi-IIa
Renal sodium-inorganic phosphate cotransporter NaPi-IIa
- PTH
parathyroid hormone
- β2-MG
β2-microglobulin
Authors’ contributions
JL performed the clinical assessments and drafted the manuscript and Table. DZ performed the clinical assessments and assisted in drafting the manuscript. YZ was responsible for clinical care of the patient. DZ and YZ revised the manuscript critically for intellectual content. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that there are no conflicts of interest.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jueying Lin, Email: linjueying566@163.com.
Yufeng Zhuo, Email: xmzyf3@163.com.
Dongdong Zhang, Phone: 86-592-2292015, Email: zd860320@hotmail.com.
References
- 1.Izzedine H, Kheder-Elfekih R, Housset P, Sarkozy C, Brocheriou I, Deray G. Adefovir dipivoxil-induced acute tubular necrosis and Fanconi syndrome in a renal transplant patient. AIDS (London, England) 2009;23(4):544–545. doi: 10.1097/QAD.0b013e32832407f7. [DOI] [PubMed] [Google Scholar]
- 2.Earle KE, Seneviratne T, Shaker J, Shoback D. Fanconi's syndrome in HIV+ adults: report of three cases and literature review. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2004;19(5):714–721. doi: 10.1359/jbmr.2004.19.5.714. [DOI] [PubMed] [Google Scholar]
- 3.Lee HJ, Choi JW, Kim TN, Eun JR. a case of severe hypophosphatemia related to adefovir dipivoxil treatment in a patient with liver cirrhosis related to hepatitis B virus. Korean J Hepatol. 2008;14(3):381–386. doi: 10.3350/kjhep.2008.14.3.381. [DOI] [PubMed] [Google Scholar]
- 4.Laing CM, Toye AM, Capasso G, Unwin RJ. Renal tubular acidosis: developments in our understanding of the molecular basis. International Journal of Biochemistry & Cell Biology. 2005;37(6):1151–1161. doi: 10.1016/j.biocel.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Bingham CT, Fitzpatrick LA. Noninvasive testing in the diagnosis of osteomalacia. Am J Med. 1993;95(5):519–523. doi: 10.1016/0002-9343(93)90335-M. [DOI] [PubMed] [Google Scholar]
- 6.Cukierman T, Gatt ME, Hiller N, Chajek-Shaul T. Clinical problem-solving. A fractured diagnosis. N Engl J Med. 2005;353(5):509–514. doi: 10.1056/NEJMcps040047. [DOI] [PubMed] [Google Scholar]
- 7.Rho M, Perazella MA. Nephrotoxicity associated with antiretroviral therapy in HIV-infected patients. Curr Drug Saf. 2007;2(2):147–154. doi: 10.2174/157488607780598269. [DOI] [PubMed] [Google Scholar]
- 8.Hartono JL, Aung MO, Dan YY, Gowans M, Lim K, Lee YM, Lee GH, Low HC, Tan PS, Thwin MA, et al. Resolution of adefovir-related nephrotoxicity by adefovir dose-reduction in patients with chronic hepatitis B. Aliment Pharmacol Ther. 2013;37(7):710–719. doi: 10.1111/apt.12251. [DOI] [PubMed] [Google Scholar]
- 9.Vigano M, Lampertico P, Colombo M. Drug safety evaluation of adefovir in HBV infection. Expert Opin Drug Saf. 2011;10(5):809–818. doi: 10.1517/14740338.2011.593507. [DOI] [PubMed] [Google Scholar]
- 10.Tasca A, Dalle Carbonare L, Nigro F, Giannini S. Bone disease in patients with primary hypercalciuria and calcium nephrolithiasis. Urology. 2009;74(1):22–27. doi: 10.1016/j.urology.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 11.Tanji N, Tanji K, Kambham N, Markowitz GS, Bell A, D'Agati VD. Adefovir nephrotoxicity: possible role of mitochondrial DNA depletion. Hum Pathol. 2001;32(7):734–740. doi: 10.1053/hupa.2001.25586. [DOI] [PubMed] [Google Scholar]
- 12.Carney EF. Tubular disease: mistargeted protein disrupts mitochondrial metabolism in inherited Fanconi syndrome. Nat Rev Nephrol. 2014;10(3):125. doi: 10.1038/nrneph.2014.6. [DOI] [PubMed] [Google Scholar]
- 13.Prie D, Huart V, Bakouh N, Planelles G, Dellis O, Gerard B, Hulin P, Benque-Blanchet F, Silve C, Grandchamp B, et al. Nephrolithiasis and osteoporosis associated with hypophosphatemia caused by mutations in the type 2a sodium-phosphate cotransporter. N Engl J Med. 2002;347(13):983–991. doi: 10.1056/NEJMoa020028. [DOI] [PubMed] [Google Scholar]
- 14.Levi M, Breusegem S. Renal phosphate-transporter regulatory proteins and nephrolithiasis. N Engl J Med. 2008;359(11):1171–1173. doi: 10.1056/NEJMe0805943. [DOI] [PMC free article] [PubMed] [Google Scholar]


