Abstract
Background
Guidelines recommend an initial intravenous (IV) fluid bolus of 30 ml/kg isotonic crystalloid for patients with sepsis and hypotension. However, there is a lack of evidence from clinical trials to support this. Accumulating observational data suggest harm associated with the injudicious use of fluids in sepsis. There is currently equipoise regarding liberal or restricted fluid-volume resuscitation as first-line treatment for sepsis-related hypotension. A randomised trial comparing these two approaches is, therefore, justified.
Methods/design
The REstricted Fluid REsuscitation in Sepsis-associated Hypotension trial (REFRESH) is a multicentre, open-label, randomised, phase II clinical feasibility trial. Participants will be patients presenting to the emergency departments of Australian metropolitan hospitals with suspected sepsis and a systolic blood pressure of < 100 mmHg, persisting after a 1000-ml fluid bolus with isotonic crystalloid. Participants will be randomised to either a second 1000-ml fluid bolus (standard care) or maintenance rate fluid only, with the early commencement of a vasopressor infusion to maintain a mean arterial pressure of > 65 mmHg, if required (restricted fluid). All will receive further protocolised fluid boluses (500 ml or 250 ml, respectively), if required during the 6-h study period. The primary outcome measure is total volume administered in the first 6 h. Secondary outcomes include fluid volume at 24 h, organ support ‘free days’ to day 28, 90-day mortality, and a range of feasibility and process-of-care measures. Participants will also undergo serial measurement, over the first 24 h, of biomarkers of inflammation, endothelial cell activation and glycocalyx degradation for comparison between the groups.
Discussion
This is the first randomised trial examining fluid volume for initial resuscitation in septic shock in an industrialised country. A pragmatic, open-label design will establish the feasibility of undertaking a large, international, multicentre trial with sufficient power to assess clinical outcomes. The embedded biomarker study aims to provide mechanistic plausibility for a larger trial by defining the effects of fluid volume on markers of systemic inflammation and the vascular endothelium.
Trial registration
Australia and New Zealand Clinical Trials Registry, ID: ACTRN12616000006448. Registered on 12 January 2016.
Electronic supplementary material
The online version of this article (doi:10.1186/s13063-017-2137-7) contains supplementary material, which is available to authorized users.
Keywords: Sepsis, Fluid therapy, Hypotension
Background
Sepsis is defined as life-threatening organ dysfunction caused by a dysregulated host response to infection [1]. A proportion of patients with sepsis develop hypotension, due to such factors as vasodilation and myocardial depression, that can lead to impaired tissue perfusion [2]. The term ‘septic shock’ has been used to describe a state of ‘acute circulatory failure’ in the setting of infection [3]. In the recently updated Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3), this term is reserved for the subset of patients in which the underlying circulatory and cellular metabolism abnormalities are such as to substantially increase mortality [1]. Recognising the importance of cellular and microcirculatory alterations in sepsis pathogenesis, a threshold blood pressure is no longer defined for septic shock. Notwithstanding these changes in nomenclature, the conventional initial clinical approach to the sepsis patient with evidence of hypotension/hypoperfusion, as recommended by the Surviving Sepsis Campaign (SSC) guidelines, remains rapid intravenous (IV) infusion of 30 ml/kg of isotonic crystalloid [4]. The rationale for this approach is to restore circulating volume, and to increase stroke volume by optimising cardiac preload. There is no clinical trial data to support this, however, and advances in our understanding of fluid physiology in critical illness have challenged the underlying assumptions [5].
Challenges to conventional approach
Historically, ‘septic shock’ was understood in simple terms to be synonymous with tissue hypoperfusion; however, the syndrome proves to be far more complex than this. Several lines of evidence challenge this over-simplification. For example:
Cardiac output may be preserved, increased or depressed in patients with sepsis, although there is a paucity of human studies undertaken in the ‘non-resuscitated’ state [6]
IV fluid loading has little, if any, effect on cardiac output in critical illness [7], and any increase in blood pressure is not sustained [8]
Cellular hypoxia is not observed in experimental models of sepsis or in clinical studies [9–11]
Elevated serum lactate in sepsis is produced aerobically, probably in response to adrenergic stimulation, as an adaptive response to increase bio-energetic efficiency, rather than because of tissue hypoxia [12]
Organ failure in sepsis involves cellular dysfunction unrelated to hypoxia/hypoperfusion, including structural mitochondrial changes and reduced oxygen consumption [13]
A key role is played by the microcirculation, including the vascular endothelium, in the pathogenesis of sepsis. These microcirculatory alterations are not related to macrocirculatory indices such as blood pressure [14]
It has been suggested that sepsis with hypotension and/or elevated serum lactate is not strictly a ‘shock’ state, but rather an adaptive alteration in haemodynamics and metabolism [5]. For these reasons we have utilised the term ‘sepsis-associated hypotension’ rather than ‘septic shock’ to denote the clinical phenotype of interest in this trial. Regardless of terminology, attempting to increase cardiac output by administering a fixed, large volume of fluid intravenously does not have a sound theoretical basis.
Is liberal intravenous fluid administration associated with harm?
Accumulating observational evidence links the injudicious use of intravenously administered fluids with adverse outcome in sepsis, including requirement for organ support and mortality [15–17]. The Fluid Expansion As Supportive Therapy (FEAST) trial randomised 3400 children (median age 24 months) with sepsis and evidence of hypoperfusion to receive bolus resuscitation with either crystalloid saline or albumin, and a control arm whose participants received only maintenance fluid without bolus [18]. Despite early improvement in indices of perfusion, the mortality rate at 48 h was 50% greater in both fluid-bolus groups compared to no bolus (10.6% versus 7.3%). Further analysis found that the excess deaths were due to cardiovascular collapse rather than to the respiratory or neurological complications of fluid administration [19]. However, the trial was undertaken in a resource-poor setting in sub-Saharan Africa. Consequently, there was no access to invasive organ support, over half the participants had malaria and one third were severely anaemic. These factors prevent translation of the results to adults in industrialised countries with ready availability of intensive care. Nonetheless, the FEAST trial is currently the only randomised clinical trial of fluid-bolus resuscitation in sepsis, and has prompted a critical re-evaluation of this approach.
Why might intravenously administered fluid be harmful?
There are a number of plausible mechanisms by which the inappropriate administration of fluids intravenously may cause harm:
Tissue oedema – leading to increased requirement for ventilatory support, increased translocation of gut organisms, and increased renal venous pressure compromising perfusion [5]
Opening of shut-down capillary beds (so called ‘hibernating circulation’) leading to ‘flooding’ of the systemic circulation with cytokine-rich blood, exacerbating systemic inflammation [5]
Degradation of the glycocalyx layer lining the luminal wall of the vascular endothelium [20]. The glycocalyx plays a critical role in vascular homeostasis by maintaining endothelial cells in a quiescent state. Loss of integrity of the glycocalyx is a critical step in endothelial cell activation and propagation of the systemic inflammatory state. Glycocalyceal damage occurs as a direct effect of fluid administration intravenously [21]
Alternative approach
Vasopressor medications, such as noradrenaline, have been used for decades to increase vascular tone and reverse the vasodilation and reduced afterload that characterises the ‘classic’ distributive picture of septic shock. Typically, a vasopressor infusion is utilised as a second-line approach to reverse critical hypotension that remains despite IV fluid loading of at least 30 ml/kg, as per SSC guidelines [4]. The optimal timing of vasopressor commencement remains a matter of clinical judgement; like fluids, this specific question has not been the subject of a randomised trial in sepsis. Concerns about the potential harmful effects of vasopressor use in patients who are ‘inadequately fluid-resuscitated’, coupled with the operational requirements that such drugs are administered via a central venous line, may lead to reluctance to utilise vasopressors in a timely fashion. Conversely, many patients with sepsis-associated hypotension ‘respond’ to fluid administration intravenously, at least initially, and the requirement to start vasopressors and admit to a scarce critical care bed may be avoided. These concerns are tempered by emerging evidence of the safety of short-term peripheral administration of vasopressors [22]. In addition, given in low doses, noradrenaline exerts effects on venous capacitance thereby increasing cardiac preload and acting as a physiological ‘volume challenge’ without the need for the administration of exogenous fluids [23].
Rationale for a randomised trial and the need for pilot data
Excessive fluid administration leads to worse outcomes, and the only clinical trial data demonstrates increased mortality with fluid-bolus therapy [18]. However, for generations physicians have been conditioned to prescribe an IV fluid bolus as first-line treatment for shock. Substantial reductions in sepsis mortality seen in the past two decades have been associated with the introduction of protocolised resuscitation for sepsis, including liberal fluid administration. Waechter et al. examined the effect of the volume of intravenously administered fluid, and the timing of initiation of vasopressors on mortality in a retrospective analysis of over 2000 intensive care unit (ICU) patients [24]. They found that early, high-volume resuscitation in the first 6 h, moderate fluid administration over the subsequent 6–24 h and the timely initiation of vasopressors were associated with the lowest mortality. However, it is unknown whether reductions in mortality occurred as a result of, or in spite of, intravenously administered fluids. Thus, at the present time, there is equipoise regarding liberal or conservative fluid-volume resuscitation in patients with sepsis-associated hypotension [25, 26]. Given the frequency of sepsis among patients admitted to ICU, and the ubiquitous use of intravenously administered fluids in these patients, there is a need for high-quality clinical trial data to guide clinicians and optimise patient outcomes. The REFRESH trial will, therefore, compare a protocolised, restricted-volume resuscitation approach (including the early use of vasopressors, if required) with the standard-volume approach recommended by the SSC.
While a large, multicentre clinical trial is required to investigate the impact of this approach on clinically orientated outcomes such as mortality, pilot data is essential to demonstrate the clinical acceptability of the approach, adherence to the protocol, and whether clinically important differences in mean fluid volume can actually be achieved [27]. The REFRESH trial also aims to provide important mechanistic plausibility data via an embedded biomarker study. Collectively, the results will inform the design of a large trial with sufficient power to detect differences in clinical outcomes.
Methods/design
Aim, design and setting
This is an investigator-initiated, multicentre, prospective, randomised open-label pilot (phase II) clinical trial. It will be undertaken in the emergency departments (EDs) of a number of Australian hospitals. These include tertiary-referral and urban general hospitals; at the time of submission six sites are open to recruitment with a further two planned. A pragmatic trial design will test the clinical feasibility of recruitment and administration of the trial intervention, along with an embedded laboratory study that will explore mechanistic plausibility. The protocol adheres to the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) Statement (Table 1) (see Additional file 1).
Table 1.
REstricted Fluid REsuscitation in Sepsis-associated Hypotension (REFRESH) trial Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) protocol summary
| Scientific title | Fluid-restricted versus fluid-liberal resuscitation in sepsis; a randomised controlled pilot trial |
|---|---|
| Short title | REstricted Fluid REsucitation in Sepsis-associated Hypotension (REFRESH) trial |
| Health condition | Sepsis |
| Ethics | HREC/15/Austin/486 (Austin Health, Victoria) & HREC/15/114 (South Metropolitan Health Service, WA) |
| Protocol version | Version 3, October 2016 |
| Funding | Grants: |
| Emergency Medicine Foundation (EMSS-229R24-2015-KEIJZERS) | |
| University of WA/University of Queensland Bilateral Research Collaboration Award | |
| Royal Perth Hospital Medical Research Foundation | |
| Industry: | |
| Nil | |
| Primary sponsor | Investigator-initiated and -driven study. Centre for Clinical Research in Emergency Medicine, University of Western Australia. Chief investigator and study contact: Dr. Stephen Macdonald, Royal Perth Hospital, PO Box X2213, Perth, WA, Australia |
| Email: stephen.macdonald@uwa.edu.au | |
| Background | Sepsis is a common condition with high morbidity and mortality. Patients with sepsis can develop hypotension (low blood pressure) due to a combination of factors. The traditional first-line treatment of sepsis-associated hypotension is to give a rapid, large volume of intravenously administered fluid (fluid bolus). Emerging clinical data and advances in the understanding of fluid physiology suggest that fluid bolus may be associated with worse patient outcomes. This randomised pilot clinical trial will compare a restricted fluid-volume approach, including early use of vasopressor drugs if required, against standard (liberal fluid-volume) care to assess the feasibility and safety of this approach. An embedded laboratory study will measure the differences in a range of relevant blood markers (such as activation of the vascular endothelium) to provide mechanistic plausibility for a restricted-volume approach. Together these data will be used to inform the design of a large multicentre trial to assess clinical outcomes |
| Hypothesis | That a volume-restricted approach to sepsis-associated hypotension is clinically feasible; that this approach results in reduced systemic inflammation and associated biomarkers of endothelial cell activation |
| Study aims | 1. To investigate the feasibility of delivering volume-restricted resuscitation in sepsis-associated hypotension |
| 2. Compare the total volume of fluid administered at 6 h and 24 h | |
| 3. To assess effects on the biomarkers of the inflammatory response, endothelial activation and related pathways of interest | |
| 4. To record any differences in requirement for organ support and in clinical outcomes | |
| Study design | • Multi-centre (Armadale, Perth/Austin, Melbourne/Fiona Stanley, Perth/Gold Coast/Royal Hobart/Royal Perth/Sir Charles Gairdner, Perth/The Prince Charles, Brisbane) |
| • Randomised controlled/un-blinded/feasibility | |
| • Interventional | |
| Setting | 8 emergency departments (EDs) in Australia |
| Inclusion and exclusion criteria | Inclusion criteria: |
| 1. Suspected infection and | |
| 2. Systolic blood pressure (SBP) < 100 mmHg, despite 1000 ml intravenously administered isotonic crystalloid administered over not more than 60 minutes and | |
| 3. Study intervention can be administered within 2 hs of inclusion criteria being met | |
| Exclusion criteria: | |
| 1. Hypotension thought due to, or contributed to by, a non-sepsis cause (e.g. arrhythmia, haemorrhage) | |
| 2. Clinical requirement for fluid replacement (e.g. gastrointestinal losses) | |
| 3. Transfer from another hospital | |
| 4. More than 2000 ml intravenously administered fluid has been given (pre-hospital, in ED, or both) | |
| 5. Likely requirement for immediate surgery | |
| 6. Age < 18 years | |
| 7. Pregnancy (confirmed or suspected) | |
| 8. Patient in extremis or death deemed imminent and inevitable | |
| 9. Patient wishes or comorbidities such that either fluid loading or vasopressor support is not considered clinically appropriate | |
| Intervention | Intervention arm: |
| Commence vasopressor infusion (± maintenance IV fluid) if SBP < 90 mmHg or mean arterial pressure (MAP) < 65 mmHg). Reassess hourly next 6 h and administer further 250-ml IV fluid bolus if required | |
| Comparator arm: | |
| Give a second 1000-ml fluid bolus plus further 500-ml boluses as clinically indicated until judged to be euvolaemic. Commence vasopressor infusion (± maintenance IV fluid) if SBP < 90 mmHg or MAP < 65 mmHg). Reassess hourly next 6 h and administer further 500-ml IV fluid bolus if required | |
| Primary outcome measure | Total volume of intravenously administered fluid (including pre-randomisation) at 6 h |
| Secondary outcome measures | • Mortality (all-cause) at 90 days post enrolment |
| • ICU length of stay | |
| • Hospital length of stay | |
| • Hospital-free days to day 90 | |
| • Organ failure | |
| o Cardiovascular | |
| ▪ Requirement for vasopressors | |
| ▪ Duration of vasopressor requirement (h) | |
| ▪ Vasopressor-free days to day 28 | |
| o Respiratory | |
| ▪ Requirement for ventilation (NIV/IPPV) | |
| ▪ Duration of ventilator support (h) | |
| ▪ Ventilator-free days to day 28 | |
| o Renal | |
| ▪ Requirement for renal replacement therapy (RRT) | |
| ▪ Duration of RRT | |
| ▪ RRT-free days to day 28 | |
| ▪ AKIN score to day 7 | |
| Laboratory/mechanistic outcome measures | Peak values, as well as patterns of expression over time (T0–T24), will be analysed for a range of biomarkers and compared between the groups including: |
| • Atrial natriuretic peptide | |
| • Troponin | |
| • Inflammatory cytokines | |
| o IL-6, IL-10, resistin | |
| • NGAL (a biomarker of renal injury) | |
| • Endothelial cell activation biomarkers | |
| o sVCAM, sICAM, sE-Selectin, sFlt-1 | |
| • Soluble markers of glycocalyx degradation | |
| o Heparan sulphate, syndecan-1, hyaluronan | |
| Feasibility outcome measures | • Proportion of eligible patients enrolled |
| • Randomisation errors | |
| • Compliance – protocol violations | |
| • Proportion with completely recorded data | |
| • Proportion with complete study blood sampling | |
| Sample size | 50 per arm/100 total |
| Randomisation | Eligible participants in whom a consent process has been commenced will be randomised to either the fluid-volume restricted or the fluid-liberal (standard-care arm) |
| Randomisation procedure | • Eligibility assessment and randomisation conducted by clinical staff with support by research personnel (in person or by telephone) |
| • Computer-generated randomisation sequence | |
| • Real-time web-based randomisation | |
| • 1:1 randomisation in blocks of 2 or 4 | |
| Blinding | • Un-blinded to patients and to treating team |
| • Blinding of laboratory staff and data analysts | |
| Data collection methods | • Paper CRF completed for each participant |
| • CRF populated by research staff in real time or from clinical record | |
| • Subsequent data entry into secure database by trained research assistant | |
| • Audit of data entry against source documentation | |
| Data collected | • All fluids administered pre-randomisation |
| • All fluids administered post randomisation – 6 h (recorded hourly) | |
| • All fluids administered over 6–24 h | |
| • Urine output to 24 h | |
| • Vital signs at enrolment and hourly until 6 h | |
| • Antimicrobials – agent, dose and time administered | |
| • Corticosteroid administration | |
| • Source of sepsis (suspected/proven) | |
| • Charlson Comorbidity Score | |
| • Microbiological/serological results from samples obtained in first 24 h | |
| • Sequential Organ Failure Assessment (SOFA) score at admission and at 24 h | |
| • Acute Physiology and Chronic Health Evaluation (APACHE) II score (peak first 24 h) | |
| • Acute Kidney INjury (AKIN) score up to 7 days | |
| • Study blood sampling at enrolment (T0), 3 h (T3), 6 h (T6) and 12–24 h (T24) | |
| Statistical analysis | • Intention-to-treat analysis |
| • Statistician blinded to treatment allocation | |
| Data and safety monitoring | • DSMC – emergency physician, intensive care physician, statistician |
| • SAE reporting to study coordination centre within 24 h | |
| • DSMC review all SAE | |
| • Periodic data review by DSMC and recommendations to PI in event of study conduct of SAE issues | |
| • Trial coordinator will monitor trial conduct at each site at regular intervals | |
| Ethics and governance | • HREC approvals obtained for all sites |
| • Clinical governance (site-specific authority) procedures in place for all sites | |
| • Any subsequent protocol amendments to be submitted to lead ethics site with appropriate dissemination to participating study sites | |
| • The study will be conducted in accordance with GCP | |
| Consent | • Where possible, prospective informed consent will be obtained |
| • Due to the time-critical nature of the condition, approval in place for verbal consent to randomise and initiate care followed by formal written consent process | |
| • Some participants will lack capacity so consent will be sought from next of kin | |
| • Where next of kin not immediately available, provision in place for enrolment under initial waiver of consent (or procedural authorisation) followed by delayed consent to continue in the trial | |
| Confidentiality | • All data will be de-identified |
| • Data (paper and electronic) will be stored securely | |
| Dissemination | • Trial results to be presented at relevant scientific meetings and published in peer-reviewed journal |
| Study status | • Opened to recruitment July 2016 (Protocol V2) |
| • Protocol amended October 2016 (Protocol V3) after 6 participants enrolled | |
| • As at 22 June 2017, 42 participants enrolled at 6 of 7 sites active | |
| • Anticipated to complete enrolment of 100 participants within 12 months |
AKIN Acute Kidney Injury Network, CRF Case Report Form, DSMC Data Safety Monitoring Committee, GCP Good Clinical Practice, ICAM intercellular adhesion molecule, IL interleukin, IPPV intermittent positive-pressure ventilation; IV intravenous, NGA neutrophil gelatinase-associated lipocalin, NIV non-invasive ventilation, PI principal investigator, SAE serious adverse event, VCAM vascular cell adhesion molecule
Participant characteristics
The trial will enrol 100 adult patients (aged 18 years or older) with sepsis, according to the recently revised standard consensus definition (Sepsis3) [1], presenting to the ED of a participating hospital, and who have a systolic blood pressure (SBP) < 100 mmHg, persisting despite intravenous administration of a fluid bolus.
Inclusion criteria
Suspected infection and
Systolic blood pressure (SBP) < 100 mmHg*, despite 1000 ml isotonic crystalloid administered intravenously over not more than 60 mins and
Study intervention can be administered within 2 h of inclusion criteria being met
Exclusion criteria
Hypotension thought due to, or contributed to by, a non-sepsis cause (e.g. arrhythmia, haemorrhage)
Clinical requirement for fluid replacement (e.g. gastrointestinal losses)
Transfer from another hospital
More than 2000 ml* of intravenously administered fluid has been given (pre-hospital, in ED, or both)
Likely requirement for immediate surgery
Age below 18 years
Pregnancy (confirmed or suspected)
Patient in extremis or death deemed imminent and inevitable
Patient wishes or comorbidities such that either fluid loading or vasopressor support is not considered clinically appropriate
*The previous version of the protocol (V2, dated April 2016) required a systolic blood pressure < 90 mmHg for inclusion, and excluded patients who had received > 1000 ml of fluid. These criteria were amended because of a slow recruitment rate. A total of six participants were enrolled under this protocol, all of which meet eligibility for the trial in the current version.
Screening and randomisation
Patients with clinical features of infection will be screened for sepsis using standard clinical procedures including recording of vital signs, and collection of blood samples including full blood count, urea and electrolytes and a venous blood gas analysis including serum lactate. Those who have a systolic blood pressure < 100 mmHg on arrival, or who develop this during their ED stay, will receive a 1000-ml IV fluid challenge with isotonic crystalloid over a maximum of 60 min. Patients whose blood pressure remains < 100 mmHg, or whose blood pressure falls below this threshold within 60 min of completing the fluid challenge, and who do not have any exclusion criteria will be invited to participate in the trial. Participants who fulfil the eligibility criteria and for whom consent is obtained (see ‘Ethics and consent’ below) will be randomised (1:1) to one of the study arms. The treating clinician will perform randomisation in real time using a dedicated, secure, password-protected web-based interface. Allocation concealment will be maintained until the conclusion of the online randomisation process. Randomisation, using a computer-generated random sequence, will be stratified by site in pre-allocated blocks of 2 and 4, thus making it impossible to predict the last allocation in a block. For practical and safety purposes randomised trial participants and the treating team will not be blind to the trial intervention. The staff conducting the laboratory analyses and the trial statistician will be blinded to the treatment allocation. The screening and enrolment procedure is summarised in (see Additional file 2: Figure S1).
Study interventions
General management – all participants
Supplemental oxygen to maintain SpO2 > 92%
Antibiotics within 60 min of enrolment
Ventilation (non-invasive) or intubation and mechanical ventilation, if clinically indicated, using a lung preventive strategy as recommended by SSC guidelines [4]; consider ketamine for induction to minimise risk of hypotension
All fluids administered (bolus or maintenance) subsequent to enrolment to be balanced isotonic crystalloid during the first 6 h. Hypotonic fluids and synthetic colloids and 0.9% saline are to be avoided. Blood products and albumin may be given at treating clinician discretion. Actual (unadjusted) volumes of all non-crystalloid fluids will be recorded
Fluid management will be as per study protocol for the first 6 h post randomisation regardless of whether the patient remains in the ED or is transferred to ICU or to a ward. For patients with an unanticipated transfer to theatre for surgery during this time the trial protocol will be suspended
Restricted-volume (±early vasopressor) arm (see Additional file 3: Figure S2)
Management following randomisation after the initial 1000-ml fluid bolus for the next 6 h:
Continue fluid infusion intravenously at a maintenance rate of 1–2 ml/kg/h (max 150 ml/h) via infusion pump, as clinically indicated
- If SBP < 90 mmHg or mean arterial pressure (MAP) < 65 mmHg:
- Start noradrenaline* infusion by peripheral IV route (large vein) and titrate to maintain MAP at 65–70 mmHg
- Insertion of urinary catheter and hourly urine output measures
- Insertion of central venous access (central venous catheter (CVC)/ peripheral intravenous central catheter (PICC)) and commencement of central noradrenaline infusion once correct position confirmed by chest X-ray. Alternatively, at physician discretion and according to local protocol, if the anticipated duration of vasopressor requirement is less than 6 h the infusion can continue via a dedicated secure peripheral large venous cannula if the patient is monitored in a critical care environment and there is no other indication for central access
- Arterial line at the discretion of the treating clinician
- Titration and weaning of vasopressors as per treating clinician and local protocols
- Additional therapies, e.g. vasopressin, corticosteroids at the treating clinician’s discretion
- If, in the judgement of the treating clinician (based upon clinical assessment, central venous pressure (CVP), ultrasound, etc.), the patient remains hypovolaemic, a further 1000-ml isotonic crystalloid bolus is permitted
- Reassess each hour for the first 6 h. At each reassessment consider need for further fluid bolus based upon clinical assessment, including:
- Mental status, skin perfusion
- Urine output
- Repeat lactate (if initially elevated)
- Ultrasound/echocardiography or CVP if available
If the treating clinician judges additional fluid to be required, administer an additional 250-ml isotonic crystalloid IV bolus.
Standard treatment arm (see Additional file 4: Figure S3)
Management following randomisation after the initial 1000-ml fluid bolus for the next 6 h:
Give a second fluid bolus of 1000 ml (balanced isotonic crystalloid)
- Reassess
- If SBP ≥ 90 mmHg and MAP ≥ 65 mmHg give maintenance rate of 1–2 ml/kg/h (max 150 ml/h) via infusion pump, as clinically indicated
- If SBP < 90 mmHg or MAP < 65 mmHg:
-
i.Give further 500-ml boluses, at least every 30 min, until the treating clinician judges euvolaemia to be achieved
-
ii.Once euvolaemia is deemed present (e.g. inferior vena cava (IVC) non-collapsing on ultrasound, CVP > 8 mmHg, distended neck veins, etc.) and if the patient remains hypotensive:
- Start noradrenaline* infusion by peripheral IV route (large vein) and titrate to maintain MAP at 65–70 mmHg
- Insertion of urinary catheter and hourly urine output measures
- Insertion of a central venous access (CVC/PICC) and commencement of central noradrenaline infusion once correct position confirmed by chest X-ray. Alternatively, at physician discretion and according to local protocol, if the anticipated duration of vasopressor requirement is less than 6 h the infusion can continue via a dedicated secure peripheral large venous cannula if the patient is monitored in a critical care environment and there is no other indication for central access
- Arterial line at the discretion of the treating clinician
- Titration and weaning of vasopressors as per treating clinician and local protocols
- Additional therapies, e.g. vasopressin, corticosteroids at the treating clinician’s discretion
-
i.
- Reassess each hour for the first 6 h. At each reassessment consider the need for further fluid boluses based upon clinical assessment, including:
- Mental status, skin perfusion
- Urine output
- Repeat lactate (if initially elevated)
- Ultrasound/echocardiography or CVP if available
If the treating clinician judges additional fluid to be required, administer an additional 500-ml isotonic crystalloid IV bolus.
*An infusion of metaraminol via a peripheral IV cannula may be considered as an alternative to noradrenaline in accordance with local hospital policy.
Study measurements
Clinical
All fluids administered pre-randomisation
All fluids administered post-randomisation – 6 h (recorded hourly)
All fluids administered over 6–24 h
Urine output to 24 h
Vital signs at enrolment and hourly until 6 h
Antimicrobials – agent, dose and time administered
Corticosteroid administration
Source of sepsis (suspected/proven)
Charlson Comorbidity Score [28]
Microbiological/serological results from samples obtained in first 24 h
Sequential Organ Failure Assessment (SOFA) score [29] at admission and at 24 h
Acute Physiology and Chronic Health Evaluation (APACHE) II score [30] (peak first 24 h)
Laboratory
Study blood sampling:
Enrolment (T0), 3 h (T3), 6 h (T6) and 12–24 h (T24)
Plasma and serum samples (total 5 ml) at each sampling time point
Blood gas (arterial or venous) including lactate and haemoglobin at each sampling time point
Blood samples should not to be taken during fluid-bolus administration and should be taken from the opposite arm from any maintenance fluid being administered
Samples will be centrifuged and the extracted sera/plasma divided into 500-μl aliquots and stored at −80 °C for subsequent batch analysis.
Strategies to ensure protocol compliance and data integrity
Digital copies of de-identified Case Report Forms will be submitted electronically to the trial coordination centre at the following time points; enrolment, completion of hospital stay, 28 days and 90 days. This will allow for checking and data queries to be undertaken in a timely manner. This will also identify any protocol deviations and provide opportunity for feedback to the site investigator. A trained research assistant at the trial coordination centre will upload data into a secure trial database. There will be periodic internal audits of data accuracy and compliance.
Outcome measures
Consistent with the exploratory pilot nature of this study, multiple feasibility, process-of-care, clinical and mechanistic outcomes will be evaluated. The primary outcome relates to achieving a difference in the volume of intravenously administered fluid to the groups. All analyses will be by intention-to-treat.
Primary outcome
A clinically and statistically significant difference in mean total fluid volume administered at 6 h post randomisation (including pre-randomisation). In addition pre- and post-randomisation volumes will be reported separately. See ‘Statistical methods and sample size calculation’ below.
Secondary outcomes: feasibility
Proportion of screened patients eligible
Proportion of eligible patients enrolled
Enrolment rate (i.e. number of enrolments per month per site)
Protocol compliance
Secondary outcomes: process-of-care
Admission rate to ICU versus general ward
Rates and timing of CVC insertion
Peripheral versus central administration of vasopressors
Secondary outcomes: clinical
Mortality (all-cause) at 90 days post enrolment
ICU length of stay
Hospital length of stay (acute)
Hospital-free days to day 90 (acute)
Organ failure
- o Cardiovascular
- ▪ Administration of vasopressors
- ▪ Duration of vasopressor requirement (h)
- ▪ Vasopressor-free days to day 28
- o Respiratory
- ▪ Requirement for ventilation (non-invasive ventilation (NIV)/intermittent positive-pressure ventilation (IPPV))
- ▪ Duration of ventilator support (h)
- ▪ Ventilator-free days to day 28
- o Renal
- ▪ Requirement for renal replacement therapy (RRT)
- ▪ Duration of RRT
- ▪ RRT-free days to day 28
- ▪ Acute Kidney Injury Network (AKIN) score to day 7 [31]
Laboratory/mechanistic outcomes
Peak values, as well as patterns of expression over time (T0–T24), will be analysed for a range of biomarkers and compared between the groups including:
Atrial natriuretic peptide
Troponin
- Inflammatory cytokines
- o Interleukin (IL)-6, IL-10, resistin
Neutrophil gelatinase-associated lipocalin (NGAL; a biomarker of renal injury)
- Endothelial cell activation biomarkers
- o sVCAM, sICAM, sE-Selectin, sFlt-1
- Soluble markers of glycocalyx degradation
- o Heparan sulphate, syndecan-1, hyaluronan
All laboratory analyses will be performed and the results collated by laboratory staff blinded to the study group allocation. As a pragmatic, open-label trial, treating clinicians and participants will be aware of the treatment allocation.
The schedule of enrolment, interventions and assessments is shown in Fig. 1.
Fig. 1.
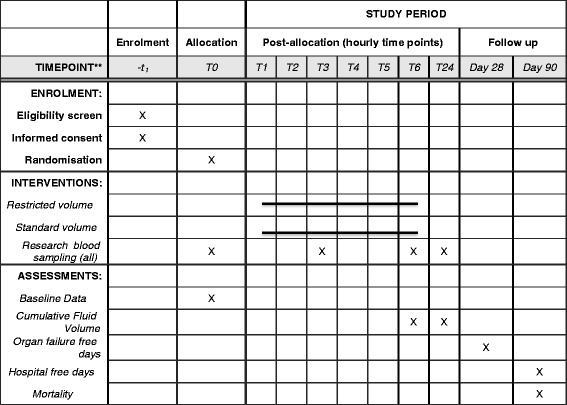
Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) Figure
Statistical methods and sample size calculation
The primary outcome is the achievement of a minimum clinically significant relative difference of 33% in the total cumulative fluid volume administered until T6 (including pre-randomisation fluids). In the control arm of an Australasian, multicentre randomised controlled trial of early goal-directed therapy in septic shock, the mean total volume administered within the first 6 h was 4200 ± 2650 ml [32]. We aim to achieve a reduction of 33%, to a mean of 2800 ± 1800 ml over 6 h in the restricted-volume arm compared to the standard-care arm. This will require 43 participants in each group to achieve 80% power with α = 0.05. This pilot trial is not designed to have sufficient power to detect differences in secondary clinical outcomes.
The trial will also measure a range of biochemical markers. There is limited relevant data to inform sample size calculations for these analyses. Based upon feasible recruitment within a 12-month time period, a sample size of 100 is attainable. This will allow a margin of safety for achieving differences in the primary outcome. It will also enable the detection of differences between means of the two treatment groups of 55.6% of the standard deviation (or larger) for the measured biomarker, with 80% power and α = 0.05. This calculation is based upon a cross-sectional t test, not a repeated measures test (which affords additional power). As such, these calculations are conservative.
Baseline clinical and demographic data will be analysed using chi-square or Fisher’s exact test for categorical data, and the Wilcoxon rank-sum test for continuous data.
Where biomarkers are normally or log-normally distributed, linear mixed models with maximum likelihood estimation will be employed to analyse the pattern over time. Differences in the pattern of expression of each biomarker between the groups will be tested by the interaction of time and group in the model. Additional covariates will be added to adjust for treating centre, illness severity and comorbidities. For biomarkers with skewed distributions resistant to successful transformation, quantile regression with per-person cluster adjustment will be used.
A biostatistician will perform the statistical analysis.
Trial administration and Data Safety Monitoring Committee
Trial coordination will be by the Centre for Clinical Research in Emergency Medicine (CCREM) at the University of Western Australia, Perth, Australia. Case Report Forms will be completed by site investigators and sent electronically (after removal of individual patient identifiers) to the trial coordination centre for checking and data entry into a purpose-designed secure Research Electronic Data Capture (REDCap – Vanderbilt University, Nashville, TN, USA) database hosted by the University of Western Australia. Study blood samples will be processed at local hospital laboratories and stored for subsequent bulk shipping to the coordinating centre. All biomarker analyses will be performed in house at the CCREM laboratory facility in Perth.
The trial will be overseen by a Steering Committee, comprising site investigators from each participating site. The Steering Committee will meet monthly by teleconference. An independent Data Safety Monitoring Committee (DSMC) comprising an emergency physician, intensive care physician and biostatistician has been appointed to oversee the trial and will undertake periodic reviews at the discretion of the chair. The DSMC will review all deaths and adverse events (see below) and have the authority to suspend or halt recruitment if necessary. The DMSC will also assess relevant emerging published academic literature favouring one of the arms of the trial. Due to the small numbers precluding determination of any statistically significant difference in clinical outcomes between the arms, no formal interim analysis will be undertaken and no stopping rules are specified.
Adverse events
Adverse events (AEs) are defined as any untoward medical occurrence in a patient administered an investigational intervention which does not necessarily require to have a causal relationship with this intervention.
Patients with severe sepsis in the ED and ICU commonly have aberrations in laboratory values, signs and symptoms due to the severity of their underlying disease and the impact of standard therapies [33]. These will not necessarily constitute an AE unless they require significant amelioration or are considered to be of concern in the investigator’s clinical judgment. AEs include all unexpected and/or untoward medical events experienced by the patient which are not part of the expected course of sepsis.
The following will be considered AEs, regardless of study treatment allocation and subsequent intervention required:
Extravasation of peripherally administered noradrenaline infusion (whether or not any tissue injury occurred)
- CVC-related complications
- Bleeding
- Inadvertent arterial puncture
- Pneumothorax
- Thrombosis
- Infection
- Arterial catheter-related complications, including thrombosis
- Any other event that is considered to be of concern by the site investigator
The AEs listed above will be recorded from the time of commencement of study treatment to 72 h post intervention. CVC-related complications, including infection, will be considered an AE for as long as the CVC remains in situ.
All AEs will be reported, irrespective of treatment allocation to the chief investigator and/or the REFRESH Management Committee and then forwarded to the chair of the DSMC.
Serious adverse events
A serious adverse event (SAE) is defined as any untoward medical occurrence that:
Results in death
Is life-threatening
Requires inpatient hospitalisation or prolongation of existing hospitalisation
Results in persistent or significant disability/incapacity
Is an important medical event, which may require intervention to prevent one of the previously listed outcomes
In this study, all SAEs will be reported regardless of suspected causality.
Adverse event and serious adverse event reporting
Separate Case Report Forms will be developed to record AEs and SAEs. SAEs occurring from the time of commencement of study treatment to 72 h post intervention will be reported to the coordinating centre by faxing the supplied SAE Form within 24 h of site study staff becoming aware of the event. In this pilot trial, all deaths up to day 30 will be reported as a SAE. Coordinating centre staff will be responsible for following up SAEs to ensure that all details are available. It is the responsibility of each site to inform their Human Research Ethics Committee (HREC) of all SAEs which occur at their site, in accordance with local requirements.
All SAEs will be forwarded to the chair of the DSMC within 24 h of receipt at the REFRESH coordinating centre. As detailed in the Patient Information and Consent Form, any injury or complication occurring as a result of trial participation is to be reported to the study team who will arrange all necessary medical treatment. In Australia, Medicare will provide any required medical treatment, free of charge, in a public hospital.
Strategies to achieve adequate enrolment
An education package has been developed for staff at enrolling sites. In addition, individual site visits (physical or virtual) will be undertaken at trial commencement. The Trial Steering Committee will meet by teleconference at least monthly and review enrolment rates and discuss strategies to maximise these. Several sites have dedicated research nurses in the ED who will actively screen for eligibility. Finally, the trial will be publicised by presentations at academic meetings and via social media platforms – e.g. Twitter @REFRESH_Trial.
Discussion
Significance
This will be the first randomised trial examining the question of fluid volume in the early resuscitation of adult patients with sepsis-associated hypotension in a mature health care system with access to advanced critical care. This is an important clinical question given the findings of observational studies and the FEAST trial [18]. Given the universal use of fluids to manage this condition, superiority of a restricted approach is likely to have significant clinical and economic impact. A recent trial demonstrated the feasibility of a restricted-volume approach to sepsis in ICU [34]. However, the median volume of fluid administered prior to randomisation was over 4 l. By recruiting patients in the ED, at the earliest point in the course of their care, we aim to investigate very early resuscitation. This essentially replicates the approach of the FEAST trial [18], but in an industrialised country with access to advanced critical care.
Undertaking such a trial in the ED setting presents considerable challenges. Unlike the ICU, patients present to the ED with undifferentiated illness. Sepsis is a highly heterogeneous condition. Often the onset of illness is insidious, and the clinical presentations highly variable. In addition, sepsis is a clinical syndrome and there is a lack of a standard diagnostic test. Nevertheless, the ARISE trial [32] demonstrated that the identification and enrolment of patients with sepsis into a clinical trial in Australian EDs is possible.
The REFRESH trial will provide essential feasibility and mechanistic plausibility data to support a large-scale clinical trial. A multicentre pilot will allow assessment of the feasibility of delivering the protocol in a range of settings. For example, participating sites are inclusive of both tertiary referral centres and urban general hospitals. A number of sites have substantial research infrastructure, including research nurses within the ED, whereas at other sites the trial will rely on enrolments by clinical staff. Metrics, such as rates of recruitment of eligible patients and adherence to the trial protocol, will be assessed as part of the feasibility objectives. All this information will be utilised in designing the optimal protocol for a trial that is clinically acceptable and operationally achievable.
Limitations
An open-label design confers challenges but is unavoidable given the nature of the trial intervention. Potential bias arises due to the treating clinician being aware of the treatment allocation. The pragmatic nature of the trial allows for some latitude for the clinician to determine fluid volumes within set parameters; however, it is difficult to control for individual behaviours and treating physician preference for one approach over another. An additional potential challenge may be the reluctance to give fluid in the standard-care arm on the basis of perceived potential harms (the rationale for the trial). To counter this, regular feedback and educational sessions with clinicians will occur throughout the trial. For this pilot trial, the primary feasibility outcomes are objective measures (e.g. fluid volumes, processes of care). Clinical outcome measures are also objective and pre-specified, e.g. mortality, organ failure. However to minimise any potential risk of bias, a physician-investigator will adjudicate clinical outcomes blinded to group allocation. Similarly, laboratory analyses will be conducted in blinded fashion.
Stakeholder engagement and dissemination of findings
The investigator team comprise clinicians in emergency medicine and intensive care who have substantial experience in conducting research among critically ill patients. The REFRESH protocol has been developed over an 18-month period during which extensive consultations took place. The protocol has been informed by practical feedback including from clinician groups locally at participating sites, and following presentation at the Australian and New Zealand Intensive Care Society Clinical Trials Group (CTG), and the Annual Scientific Meeting of the Australasian College for Emergency Medicine (ACEM). The REFRESH trial has received formal endorsement from the ACEM CTG.
The trial results will be of wide interest to the global emergency and critical care communities. It is anticipated that the results will be used to inform the design of, and secure funding for, a large-scale clinical trial to evaluate patient-centred outcomes. The results of the trial will be submitted for publication in a suitable international journal. A writing committee will be convened from among the Trial Steering Committee. Authorship criteria will be according to the International Committee of Medical Journal Editor definition.
Trial status
At the time of submission the REFRESH trial has enrolled 42 of its planned 100 participants.
Additional files
SPIRIT 2013 Checklist: recommended items to address in a clinical trial protocol and related documents*. (DOC 120 kb)
Figure. Screening and enrolment flowchart. (PDF 422 kb)
Figure. Restricted fluid volume arm. (PDF 497 kb)
Figure. Standard fluid volume arm. (PDF 419 kb)
Appendix. Verbal consent script. (DOCX 131 kb)
Figure. REFRESH trial consent procedure. (PDF 400 kb)
Acknowledgements
Trial coordination and management: Ellen Macdonald and Sophie Damianopoulos, Centre for Clinical Research in Emergency Medicine, Harry Perkins Institute of Medical Research, University of Western Australia, Perth, WA, Australia.
Laboratory analyses: Erika Bosio, Centre for Clinical Research in Emergency Medicine, Harry Perkins Institute of Medical Research, University of Western Australia, Perth, WA, Australia.
Data Safety Monitoring Committee: Anthony FT Brown, Royal Brisbane and Women’s Hospital, Brisbane, QLD, Australia; Robert Boots, Royal Brisbane and Women’s Hospital, Brisbane, QLD, Australia; Michael Phillips, Harry Perkins Institute of Medical Research, University of Western Australia, Perth, WA, Australia.
We also acknowledge the contribution of the following: Kane Guthrie (Sir Charles Gairdner Hospital); Anton Leonard (Armadale Health Service and Royal Perth Hospitals); Victoria Trubody, David Cooper, (Royal Hobart Hospital); the trial research nurses, and clinical staff of the participating departments.
Funding
The REFRESH trial is an investigator-initiated and publically funded ‘common good’ research trial. As such, there is no commercial sponsorship. In addition to substantial institutional support from the participating hospitals, grant funding has been obtained from the Emergency Medicine Foundation (formerly the Queensland Emergency Medicine Research Foundation) to support laboratory processing costs and research nurse hours (EMSS-229R24-2015-KEIJZERS). A separate funding application to the Australian National Health and Medical Research Council will be submitted in 2017 in order to complete the laboratory work that comprises the mechanistic arm of the trial.
Availability of data and materials
The datasets used and/or analysed during this study will be available from the corresponding author upon reasonable request.
Abbreviations
- ACEM
Australasian College for Emergency Medicine
- AE
Adverse event
- AKIN
Acute Kidney Injury Network
- APACHE
Acute Physiology And Chronic Health Evaluation
- ARISE
Australasian Resuscitation in Sepsis Evaluation
- CVC
Central venous catheter
- DSMC
Data Safety Monitoring Committee
- ED
Emergency department
- FEAST
Fluid expansion as supportive therapy
- HREC
Human Research Ethics Committee
- ICAM
Intercellular adhesion molecule
- ICU
Intensive care unit
- IL-6/10
Interleukin-6/10
- IPPV
Intermittent positive-pressure ventilation
- IV
Intravenous
- MAP
Mean arterial pressure
- NGAL
Neutrophil gelatinase-associated lipocalin
- NIV
Non-invasive ventilation
- PICC
Peripheral intravenous central catheter
- SAE
Serious adverse event
- SBP
Systolic blood pressure
- SOFA
Sequential Organ Failure Assessment
- SpO2
Peripheral transcutaneous oxygen saturation
- SSC
Surviving Sepsis Campaign
- VCAM
Vascular cell adhesion molecule
Authors’ contributions
SPJM drafted the study protocol based upon an original proposal by SGAB. Steering Committee members DMT, GK, GA, DMF, FBK, RB, JFF, EL, MA, DM, LS, IV, JW, BW and JA-L contributed important intellectual content. GK, SPJM and FK sourced funding from the EMF. SB advised on the randomisation schedule, wrote the statistical plan and will be responsible for the analysis. All authors approved the final version of the manuscript.
Authors’ information
Not applicable.
Ethics approval and consent to participate
The trial protocol has been endorsed by the Human Research Ethics Committees:
South Metropolitan Health Service, Western Australia (Reference 15-114, 13 October 2015) for Western Australian Sites
Austin Health, Victoria (HREC/15/Austin/486, 20 April 2016) for all other sites
The Australian National Health and Medical Research Council (NHMRC) National Statement on the Ethical Conduct of Research in Humans, Chapter 4.4 addresses the conduct of research among people highly dependent upon medical care and who may be unable to give consent [35]. This includes emergency and intensive care settings, such as applies in the present study, where research is necessary to improve the safety and efficiency of interventions used in their treatment.
Participants in this trial will have low blood pressure in the setting of sepsis and there is a short time window in which this must be treated by the administration of one of the two trial interventions. Several approaches will be adopted. When a conscious and comprehending patient is eligible, a brief verbal explanation of the study (following a standard script) will be given and a provisional verbal consent obtained to allow randomisation and initiation of timely intervention. This script is attached as an Appendix (see Additional file 5). This will be followed by provision of a written Participant Information Sheet. If the patient chooses not to continue they will be withdrawn from the trial and their care will revert to standard management according to the treating physician. This will be recorded in the trial screening logs. Where a patient’s condition precludes giving prospective informed consent, they may be enrolled under an initial waiver of consent (or procedural authorisation in Victoria). Following this, formal consent will be obtained from the ‘person responsible’ for the patient, generally a close family member. This may be in person, or by telephone. If telephone consent is obtained this will be clearly documented in the clinical notes, and followed up by a formal written consent, as soon as practicable.
When the participant regains capacity, consent will be sought to continue in the study. Participants may choose to withdraw from the study (or be withdrawn by the ‘person responsible’ if enrolled under waiver of consent) at any stage. Under these circumstances, permission will be sought to use data and samples collected to that time point. This withdrawal will be recorded in the study logs and care will revert to standard management according to the treating team. The consent process is summarised in (see Additional file 6: Figure S4).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Footnotes
Electronic supplementary material
The online version of this article (doi:10.1186/s13063-017-2137-7) contains supplementary material, which is available to authorized users.
Contributor Information
Stephen P. J. Macdonald, Phone: +61 8 9224 8458, Email: stephen.macdonald@uwa.edu.au
David McD Taylor, Email: david.taylor@austin.org.au.
Gerben Keijzers, Email: gerben.keijzers@health.qld.gov.au.
Glenn Arendts, Email: glenn.arendts@uwa.edu.au.
Daniel M. Fatovich, Email: Daniel.fatovich@health.wa.gov.au
Frances B. Kinnear, Email: frances.kinnear@health.qld.gov.au
Simon G. A. Brown, Email: simon.brown@uwa.edu.au
Rinaldo Bellomo, Email: rinaldo.bellomo@austin.org.au.
Sally Burrows, Email: sally.burrows@uwa.edu.au.
John F. Fraser, Email: john.fraser@health.qld.gov.au
Edward Litton, Email: edward.litton@health.wa.gov.au.
Juan Carlos Ascencio-Lane, Email: juan.ascencio-lane@ths.tas.gov.au.
Matthew Anstey, Email: matthew.anstey@health.wa.gov.au.
David McCutcheon, Email: david.mccutcheon@health.wa.gov.au.
Lisa Smart, Email: lisa.smart@research.uwa.edu.au.
Ioana Vlad, Email: ioana.vlad@health.wa.gov.au.
James Winearls, Email: james.winearls@health.qld.gov.au.
Bradley Wibrow, Email: Bradley.wibrow@health.wa.gov.au.
References
- 1.Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche J-D, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent J-L, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016;315:801–10. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–51. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 3.Levy MM, Fink MP, Marshall JC, et al. International Sepsis Definitions Conference 2001; SCCM/ESICM/ACCP/ATS/SIS. Intensive Care Med. 2003;29:530–8. doi: 10.1007/s00134-003-1662-x. [DOI] [PubMed] [Google Scholar]
- 4.Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, et al. Surviving Sepsis Campaign: international guidelines for the management of severe sepsis and septic shock 2016. Crit Care Med. 2017. doi:10.1097/CCM.0000000000002255. [DOI] [PubMed]
- 5.Hilton AK, Bellomo R. A critique of fluid bolus resuscitation in severe sepsis. Crit Care. 2012;16:302. doi: 10.1186/cc11154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cioccari L, Luethi N, Weber U, Hilton A, Takala J, Bellomo R. The native cardiac output in human sepsis: a systematic review. Crit Care Resusc. 2016;18:148–56. [PubMed] [Google Scholar]
- 7.Magder S. Volume and its relationship to cardiac output and venous return. Crit Care. 2016;20:271. doi: 10.1186/s13054-016-1438-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bihari S, Teubner DJ, Prakash S, Beatty T, Morphett M, Bellomo R, Bernstein A. Fluid bolus therapy in emergency department patients: indications and physiological changes. Emerg Med Australas. 2016;28:531–7. doi: 10.1111/1742-6723.12621. [DOI] [PubMed] [Google Scholar]
- 9.May C, Wan L, Williams J, Wellard MR, Pell G, Langenberg C, et al. A technique for the simultaneous measurement of renal ATP, blood flow and pH in a large animal model of septic shock. Crit Care Resusc. 2007;9:30–3. [PubMed] [Google Scholar]
- 10.Levy B, Gibot S, Franck P, Cravoisy A, Bollaert P-E. Relation between muscle Na + K ATPase activity and raised lactate concentrations in septic shock: a prospective study. Lancet. 2005;365:871–5. doi: 10.1016/S0140-6736(05)71045-X. [DOI] [PubMed] [Google Scholar]
- 11.Boekstegers P, Weidenhöfer S, Kapsner T, Werdan K. Skeletal muscle partial pressure of oxygen in patients with sepsis. Crit Care Med. 1994;22:640–50. doi: 10.1097/00003246-199404000-00021. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Alvarez M, Marik P, Bellomo R. Stress hyperlactataemia: present understanding and controversy. Lancet Diabetes Endocrinol. 2014;2:339–47. doi: 10.1016/S2213-8587(13)70154-2. [DOI] [PubMed] [Google Scholar]
- 13.Singer M. The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence. 2014;5:66–72. doi: 10.4161/viru.26907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ince C. The microcirculation is the motor of sepsis. Crit Care. 2005;9(Suppl 4):S13. doi: 10.1186/cc3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelm DJ, Perrin JT, Cartin-Ceba R, Gajic O, Schenck L, Kennedy CC. Fluid overload in patients with severe sepsis and septic shock treated with early goal-directed therapy is associated with increased acute need for fluid-related medical interventions and hospital death. Shock. 2015;43:68–73. doi: 10.1097/SHK.0000000000000268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Acheampong A, Vincent J-L. A positive fluid balance is an independent prognostic factor in patients with sepsis. Crit Care. 2015;19:251. doi: 10.1186/s13054-015-0970-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sirvent J-M, Ferri C, Baro A, Murcia C, Lorencio C. Fluid balance in sepsis and septic shock as a determining factor for mortality. Am J Emerg Med. 2015;33:186–9. doi: 10.1016/j.ajem.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 18.Maitland K, Kiguli S, Opoka RO, Engoru C, Olupot-Olupot P, et al. Mortality after fluid bolus in African children with severe infection. N Engl J Med. 2011;364:2483–95. doi: 10.1056/NEJMoa1101549. [DOI] [PubMed] [Google Scholar]
- 19.Maitland K, George EC, Evans JA, Kiguli S, Olupot-Olupot P, et al. Exploring mechanisms of excess mortality with early fluid resuscitation: insights from the FEAST trial. BMC Med. 2013;11:68. doi: 10.1186/1741-7015-11-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Burke-Gaffney A, Evans TW. Lest we forget the endothelial glycocalyx in sepsis. Crit Care. 2012;16:121. doi: 10.1186/cc11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Powell M, Mathru M, Brandon A, Patel R, Frölich M. Assessment of endothelial glycocalyx disruption in term parturients receiving a fluid bolus before spinal anesthesia: a prospective observational study. Int J Obstet Anesth. 2014;23:330–4. doi: 10.1016/j.ijoa.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Ricard J-D, Salomon L, Boyer A, Thiery G, Meybeck A, Roy C, et al. Central or peripheral catheters for initial venous access of ICU patients: a randomized controlled trial. Crit Care Med. 2013;41:2108–15. doi: 10.1097/CCM.0b013e31828a42c5. [DOI] [PubMed] [Google Scholar]
- 23.Hamzaoui O, Georger J-F, Monnet X, Ksouri H, Maizel J, Richard C, Teboul J-L. Early administration of norepinephrine increases cardiac preload and cardiac output in septic patients with life-threatening hypotension. Crit Care. 2010;14:R142. doi: 10.1186/cc9207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Waechter J, Kumar A, Lapinsky SE, Marshall J, Dodek P, Arabi Y, et al. Interaction between fluids and vasoactive agents on mortality in septic shock: a multicenter, observational study. Crit Care Med. 2014;42:2158–68. doi: 10.1097/CCM.0000000000000520. [DOI] [PubMed] [Google Scholar]
- 25.Perner A, Singer M. Fixed minimum fluid volume for resuscitation: con. Intensive Care Med. 2016. doi:10.1007/s00134-016-4581-3. [DOI] [PubMed]
- 26.Machado FR, Levy MM, Rhodes A. Fixed minimum volume resuscitation: pro. Intensive Care Med. 2016. doi:10.1007/s00134-016-4590-2. [DOI] [PubMed]
- 27.Thabane L, Ma J, Chu R, Cheng J, Ismalia A, et al. A tutorial on pilot studies: the what, why and how. BMC Med Res Methodol. 2010;10:1. doi: 10.1186/1471-2288-10-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 29.Vincent J-L, Moreno R, Takala J, Willats S, De Mendonca A, Bruining H, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Int Care Med. 1996;22:707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 30.Knaus WA, Draper EA, Wagner DP. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–29. doi: 10.1097/00003246-198510000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, et al. Acute Kidney Injury Network. Report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11:R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.The ARISE Investigators Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014;371:1496–506. doi: 10.1056/NEJMoa1404380. [DOI] [PubMed] [Google Scholar]
- 33.Cook D, Lauzier F, Rocha MG, Sayles MJ, Finfer S. Serious adverse events in academic critical care research. CMAJ. 2008;179:1181–4. doi: 10.1503/cmaj.080758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hjortrup PB, Haase N, Bundgaard H, Thomsen SL, Winding R, et al. Restricting volumes of resuscitation fluid in adults with septic shock after initial management: the CLASSIC randomised, parallel-group, multicentre feasibility trial. Intensive Care Med. 2016;42:1695–705. doi: 10.1007/s00134-016-4500-7. [DOI] [PubMed] [Google Scholar]
- 35.National Health and Medical Research Council (NHMRC). National Statement on the Ethical Conduct of Research in Humans (2007, updated May 2015). Available at URL: https://www.nhmrc.gov.au/guidelines-publications/e72.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SPIRIT 2013 Checklist: recommended items to address in a clinical trial protocol and related documents*. (DOC 120 kb)
Figure. Screening and enrolment flowchart. (PDF 422 kb)
Figure. Restricted fluid volume arm. (PDF 497 kb)
Figure. Standard fluid volume arm. (PDF 419 kb)
Appendix. Verbal consent script. (DOCX 131 kb)
Figure. REFRESH trial consent procedure. (PDF 400 kb)
Data Availability Statement
The datasets used and/or analysed during this study will be available from the corresponding author upon reasonable request.


