Abstract
Background
During the 2013–2016 West Africa Ebola virus disease (EVD) epidemic, some EVD patients, mostly health care workers, were evacuated to Europe and the USA.
Case presentation
In May 2015, a 37-year old male nurse contracted Ebola virus disease in Sierra Leone. After Ebola virus detection in plasma, he was medically-evacuated to Italy. At admission, rhabdomyolysis was clinically and laboratory-diagnosed and was treated with aggressive hydration, oral favipiravir and intravenous investigational monoclonal antibodies against Ebola virus. The recovery clinical phase was complicated by a febrile thrombocytopenic syndrome with pericardial effusion treated with corticosteroids for 10 days and indomethacin for 2 months. No evidence of recurrence is reported.
Conclusions
A febrile thrombocytopenic syndrome with pericardial effusion during the recovery phase of EVD appears to be uncommon. Clinical improvement with corticosteroid treatment suggests that an immune-mediated mechanism contributed to the pericardial effusion.
Keywords: Ebola Virus Disease, Rhabdomyolysis, Pericardial effusion
Background
The 2013–6 West Africa Ebola virus disease (EVD) epidemic resulted in 28,616 confirmed, probable and suspected cases reported in Guinea, Liberia and Sierra Leone, with 11,310 deaths [1]. A small number of EVD cases were medically-evacuated or imported to Europe and the U.S., with limited secondary transmission in Spain and USA, in health care workers [2]. Pericardial involvement has rarely been reported in EVD patients [3–5]. Here we describe a case of acute rhabdomyolysis with delayed pericardial effusion in a nurse with EVD.
Case presentation
In May 2015, a 37-year old male nurse who had been working in Sierra Leone was admitted to the Spallanzani Hospital, Rome, Italy for EVD clinical management. Medical, family and psychosocial history was non-contributory. Findings at admission, 3 days after symptom onset, included fever (39.0 °C), myalgia, conjunctivitis, diarrhoea, rhabdomyolysis [elevated serum creatine kinase (CK) level (785 IU/L, normal range 22–269)] with normal renal function, and Ebola virus (EBOV) load in plasma was 5 × 107 copies/ml.
Oral favipiravir (Toyama Chemical Co, Japan) was administered (6-g loading dose and 1200 mg twice daily for 10 days) [6, 7]. Two doses of investigational monoclonal antibodies against EBOV (MIL77, Mabworks Beijing China) were given (50 mg/kg IV) 3 days apart. Empiric antibiotic treatment with intravenous ceftriaxone (2 g daily) and oral levofloxacin (750 mg daily), and intravenous crystalloid solution, were administered daily with progressive clinical improvement. CK level peaked on illness day 5 (4400 IU/ml) and declined to normal on illness day 10 (Fig. 1a). Renal function remained normal. The plasma EBOV load was undetectable on day 11 (Fig. 1a).
Fig. 1.
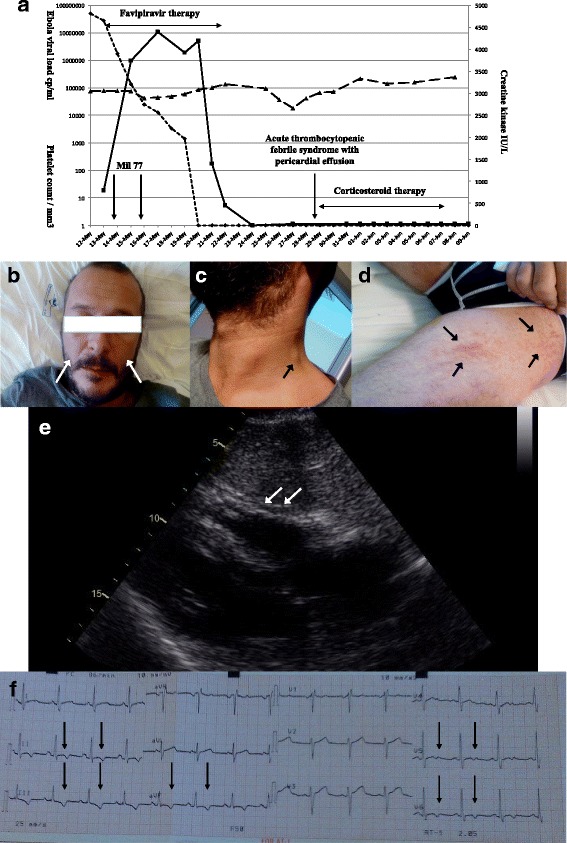
Ebola plasma viral load, creatine kinase levels, platelet count, timing of drug administration and of occurrence of the thrombocytopenic febrile syndrome (panel a); Skin lesions on the face and on the left thigh, and neck adenopathy (panels b-d); Echocardiographic evidence of MILD circumferential pericardial effusion at the time of the thrombocytopenic febrile syndrome and ECG showing ECG showed diffuse nonspecific abnormalities (panels e and f). Legend of panel A: The Y-axis indicates Ebola viral load (copies/ml) and platelet count (platelet/mm3). The Z-axis indicates creatine kinase levels (International Units/Liter). CK creatine kinase
On illness day 19, a febrile syndrome with diffuse adenopathy, confluent skin rash and marked thrombocytopenia (18,000/mm3) occurred (Fig. 1b-d). ECG showed diffuse nonspecific abnormalities in repolarisation, and an echocardiogram showed a mild circumferential pericardial effusion (largest echo-free space in tele-diastole <10 mm) (Fig. 1e-f). Chest pain and pericardial rub were absent. High-dose corticosteroid therapy was initiated with immediate clinical improvement; methylprednisolone, 1 g IV daily for 2 days, reduced to 500 mg on day 21 and 250 mg on day 22, and then switched to oral prednisone on day 23, with normalization of platelet count. Serum tested positive for rheumatoid factor, Waaler Rose, and circulating immune complexes. At discharge on illness day 29, a minimal pericardial effusion was present. Corticosteroid treatment was stopped and oral indomethacin 25 mg twice daily was prescribed. Echocardiographic examination performed 60 days after discharge showed complete resolution of the pericardial effusion and indomethacin therapy was stopped. There was no evidence of pericardial effusion at 18 month follow-up visit.
Discussion and conclusions
A febrile thrombocytopenic syndrome with pericardial effusion during the recovery phase of EVD appears to be uncommon. Pericarditis was suggested as a cause of retrosternal pain in some patients and pericardial effusion was confirmed in one fatal EVD case during the 1995 Kikwit outbreak [3]. Pericardial effusion was reported in a critically ill EVD patient in Germany [4], and in two EVD patients in Guinea in 2014 [5].
Immune activation has been described in a small number of EVD patients [8]. In this case, EBOV infection may have triggered inflammation resulting in rhabdomyolysis, and after viremia resolved, prolonged immune activation may have caused pericardial tissue injury [9]. A serum-sickness disease induced by the monoclonal antibody against EBOV that was administered is another possible explanation [8]. Clinical improvement with corticosteroid treatment suggests that an immune-mediated mechanism likely contributed to the development of the pericardial effusion.
Acknowledgements
*Members of the INMI’s Ebola Team (to be listed in PubMed as collaborators if possible).
IDs specialists: Nicola Petrosillo, Emanuele Nicastri, Nazario Bevilacqua, Evangelo Boumis, Pierangelo Chinello, Stefania Cicalini, Angela Corpolongo, Vincenzo Galati, Andrea Mariano, Silvia Rosati, Fabrizio Taglietti, Laura Vincenzi; Intensive care Physicians: Mario Antonini, Ilaria Caravella, Gabriele Garotto, Luisa Marchioni, Micaela Maritti; Psicologist: Pietro Balestra and Martina Ricottini, Radiologist: Elisa Busi Rizzi, Cardiologist: Gianluigi Biava;
Virology and Microbiology Laboratorians: Maria Rosaria Capobianchi, Antonino di Caro, Concetta Castilletti, Licia Bordi, Eleonora Lalle, Mirella Biava, Silvia Meschi, Daniele Lapa, Patrizia Marsella, Francesca Colavita, Roberta Chiappini, Antonio Mazzarelli, Serena Quartu, Chiara Agrati, Fabrizio Carletti, Federica Forbici, Maria Beatrice Valli, Isabella Abbate, Alessandra Amendola, Anna Rosa Garbuglia, Maria Grazia Paglia, Eugenio Bordi, Damiano Travaglini, Antonietta Toffoletti; Nurses: Gianni Battisti, Alessanda Coppola, Loredana De Marchis, Nicola De Marco, Paolo Giacomini, Fabio Di Gianbattista, Mario Guiducci, Antonio Marasco, Antonella Marzolini, Alessandro Mercuri, Paola Nieddu, Silvia Ondedei, Maurizio Vescovo, Laura Vitolo; Radiology Technician: Maurizio Morea; Drivers biocontainment ambulance: Gaetano Battisti, Marco Liguori;
Members of the INMI Crisis unit: Nicola Petrosillo, Emanuele Nicastri, Francesco Nicola Lauria, Vincenzo Puro, Mario Antonini, Antonio Russo, Maria Rosaria Capobianchi, Antonino Di Caro, Paolo D’Aprile, Antonella Petrecchia, Evangelo Boumis, Marco Gentile, Damiano Travaglini, Silvia Pittalis, Lorena Martini, Concetta Castilletti, Francesco Maria Fusco, Simone Lanini, Andrea Antinori, Marina Cerimele, Giuseppe Ippolito, and Marta Branca.
We are particularly grateful to the colleagues and friends who helped us in obtaining Mill77: Mike Jacobs from Royal Free Hospital in London, UK; Gary P. Kobinger from National Laboratory for Zoonotic Diseases and Special Pathogens, Public Health Agency of Canada, Winnipeg, Canada and Nakono Shindo from World Health Organization. We are also grateful to pharmaceutical companies who provide faviravir for free.
Funding
This paper was supported by grant from the “Ricerca Corrente” of Italian Ministry of Health.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to privacy reasons but are available from the corresponding author on reasonable request.
Abbreviations
- CK
Creatine kinase
- EBOV
Ebola virus
- EVD
Ebola virus disease
- PCR
Polymerase chain reaction
Authors’ contributions
EN, NP and GI designed the study. EN and GI drafted the manuscript. NP, EN, GB contributed to the diagnosis and treatment. AB and TMU reviewed and helped to revise the manuscript. All the authors approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
The INMI’s Institutional Ethical Board assessed the criteria for access to experimental drugs and invasive procedures, approved informed consent form and analyzed ethical issues and possible solutions to minimize the physical and psychological harm for the patient. The patient signed an informed consent for any single procedure or treatment performed, after thoroughly explanation of reasonably anticipated benefits and potential hazards of intervention.
Emergency Use Authorization for investigational new drugs was issued by the Italian Drug Agency (AIFA), the authority entitled to approve medical agents to be used for therapy of disease when they are not the standard of care or supported by research that proves their safety.
Competing interests
The authors declare that they have no competing interest.
Disclaimer: The views expressed are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Emanuele Nicastri, Phone: +390655170393, Email: emanuele.nicastri@inmi.it.
Antonio Brucato, Email: albrucato@asst-pg23.it.
Nicola Petrosillo, Email: nicola.petrosillo@inmi.it.
Gianluigi Biava, Email: gianluigi.biava@inmi.it.
Timothy M. Uyeki, Email: tmu0@cdc.gov
Giuseppe Ippolito, Email: giuseppe.ippolito@inmi.it.
INMI’s Ebola Team:
Nicola Petrosillo, Emanuele Nicastri, Nazario Bevilacqua, Evangelo Boumis, Pierangelo Chinello, Stefania Cicalini, Angela Corpolongo, Vincenzo Galati, Andrea Mariano, Silvia Rosati, Fabrizio Taglietti, Laura Vincenzi, Mario Antonini, Ilaria Caravella, Gabriele Garotto, Luisa Marchioni, Micaela Maritti, Pietro Balestra, Martina Ricottini, Elisa Busi Rizzi, Gianluigi Biava, Maria Rosaria Capobianchi, Antonino di Caro, Concetta Castilletti, Licia Bordi, Eleonora Lalle, Mirella Biava, Silvia Meschi, Daniele Lapa, Patrizia Marsella, Francesca Colavita, Roberta Chiappini, Antonio Mazzarelli, Serena Quartu, Chiara Agrati, Fabrizio Carletti, Federica Forbici, Maria Beatrice Valli, Isabella Abbate, Alessandra Amendola, Anna Rosa Garbuglia, Maria Grazia Paglia, Eugenio Bordi, Damiano Travaglini, Antonietta Toffoletti, Gianni Battisti, Alessanda Coppola, Loredana De Marchis, Nicola De Marco, Paolo Giacomini, Fabio Di Gianbattista, Mario Guiducci, Antonio Marasco, Antonella Marzolini, Alessandro Mercuri, Paola Nieddu, Silvia Ondedei, Maurizio Vescovo, Laura Vitolo, Maurizio Morea, Gaetano Battisti, Marco Liguori, Francesco Nicola Lauria, Vincenzo Puro, Antonio Russo, Paolo D’Aprile, Antonella Petrecchia, Marco Gentile, Silvia Pittalis, Lorena Martini, Francesco Maria Fusco, Simone Lanini, Andrea Antinori, Marina Cerimele, Giuseppe Ippolito, and Marta Branca
References
- 1.WHO Ebola Response Team. Agua-Agum J, Allegranzi B, Ariyarajah A, Aylward R, Blake IM, Barboza P, et al. After Ebola in West Africa--Unpredictable Risks, Preventable Epidemics. N Engl J Med. 2016;375:587–596. doi: 10.1056/NEJMsr1513109. [DOI] [PubMed] [Google Scholar]
- 2.Uyeki TM, Mehta AK, Davey RT, Jr, Liddell AM, Wolf T, Vetter P, et al. Clinical Management of Ebola Virus Disease in the United States and Europe. N Engl J Med. 2016;374:636–646. doi: 10.1056/NEJMoa1504874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bwaka MA, Bonnet MJ, Calain P, Colebunders R, De Roo A, Guimard Y, et al. Ebola hemorrhagic fever in Kikwit, Democratic Republic of the Congo: clinical observations in 103 patients. J Infect Dis. 1999;179(Suppl 1):S1–S7. doi: 10.1086/514308. [DOI] [PubMed] [Google Scholar]
- 4.Kreuels B, Wichmann D, Emmerich P, Schmidt-Chanasit J, de Heer G, Kluge S, Sow A, Renné T, Günther S, Lohse AW, Addo MM, Schmiedel S. A case of severe Ebola virus infection complicated by gram-negative septicemia. N Engl J Med. 2014;371:2394–2401. doi: 10.1056/NEJMoa1411677. [DOI] [PubMed] [Google Scholar]
- 5.Cellarier GR, Bordes J, Karkowski L, Gagnon N, Billhot M, Cournac JM, et al. Safety, feasibility, and interest of transthoracic echocardiography in a deployed French military Ebola virus disease treatment center in Guinea. Intensive Care Med. 2015;41:1491–1492. doi: 10.1007/s00134-015-3821-2. [DOI] [PubMed] [Google Scholar]
- 6.Sissoko D, Laouenan C, Folkesson E, M'Lebing AB, Beavogui AH, Baize S, et al. Experimental Treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A Historically Controlled. Single-Arm Proof-of-Concept Trial in Guinea PLoS Med. 2016;13:e1001967. doi: 10.1371/journal.pmed.1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mora-Rillo M, Arsuaga M, Ramírez-Olivencia G, de la Calle F, Borobia AM, Sánchez-Seco P, et al. Acute respiratory distress syndrome after convalescent plasma use: treatment of apatient with Ebola virus disease contracted in Madrid. Spain Lancet Respir Med. 2015;3:554–562. doi: 10.1016/S2213-2600(15)00180-0. [DOI] [PubMed] [Google Scholar]
- 8.McElroy AK, Akondy RS, Davis CW, Ellebedy AH, Mehta AK, Kraft CS, Lyon GM, Ribner BS, Varkey J, Sidney J, Sette A, Campbell S, Ströher U, Damon I, Nichol ST, Spiropoulou CF, Ahmed R. Human Ebola virus infection results in substantial immune activation. Proc Natl Acad Sci U S A. 2015;112:4719–4724. doi: 10.1073/pnas.1502619112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bogdanos DP, Smyk DS, Invernizzi P, Rigopoulou EI, Blank M, Pouria S, et al. Infectome: a platform to trace infectious triggers of autoimmunity. Autoimmun Rev. 2013;12:726–740. doi: 10.1016/j.autrev.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to privacy reasons but are available from the corresponding author on reasonable request.


