Abstract
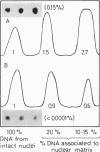
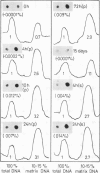
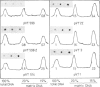
Supercoiled DNA loops linked to the nuclear matrix can be progressively cleaved with deoxyribonuclease I. The DNA which remains associated with the nuclear matrix can be purified and analysed for vitellogenin II sequence content by dot blot hybridization. Using this technique we show that vitellogenin II gene sequences are selectively associated with the nuclear matrix of liver but not with oviduct of laying hens. Following primary stimulation in immature chicks of vitellogenin synthesis with estradiol, the association of the gene with the nuclear matrix precedes vitellogenin mRNA synthesis. After 15 days when the level of vitellogenin mRNA has returned to zero, the gene is no longer preferentially associated with the nuclear matrix. At this time a second stimulation with estradiol results in a reassociation of the vitellogenin II gene with the nuclear matrix. In addition to the structural gene, both the 3' and 5' end flanking regions (1.5-2 kb) also bind to the nuclear matrix. However, beyond the limit of 1.5-2 kb upstream from the 5' end of the gene, there is no preferential binding of DNA to the nuclear matrix.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrack E. R., Coffey D. S. The specific binding of estrogens and androgens to the nuclear matrix of sex hormone responsive tissues. J Biol Chem. 1980 Aug 10;255(15):7265–7275. [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Identification of a nuclear protein matrix. Biochem Biophys Res Commun. 1974 Oct 23;60(4):1410–1417. doi: 10.1016/0006-291x(74)90355-6. [DOI] [PubMed] [Google Scholar]
- Berezney R., Coffey D. S. Nuclear matrix. Isolation and characterization of a framework structure from rat liver nuclei. J Cell Biol. 1977 Jun;73(3):616–637. doi: 10.1083/jcb.73.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciejek E. M., Nordstrom J. L., Tsai M. J., O'Malley B. W. Ribonucleic acid precursors are associated with the chick oviduct nuclear matrix. Biochemistry. 1982 Sep 28;21(20):4945–4953. doi: 10.1021/bi00263a018. [DOI] [PubMed] [Google Scholar]
- Ciejek E. M., Tsai M. J., O'Malley B. W. Actively transcribed genes are associated with the nuclear matrix. Nature. 1983 Dec 8;306(5943):607–609. doi: 10.1038/306607a0. [DOI] [PubMed] [Google Scholar]
- Cook P. R., Lang J., Hayday A., Lania L., Fried M., Chiswell D. J., Wyke J. A. Active viral genes in transformed cells lie close to the nuclear cage. EMBO J. 1982;1(4):447–452. doi: 10.1002/j.1460-2075.1982.tb01189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozens P. J., Cato A. C., Jost J. P. Characterization of cloned complementary DNA covering more than 6000 nucleotides (97%) of avian vitellogenin mRNA. Eur J Biochem. 1980 Dec;112(3):443–450. doi: 10.1111/j.1432-1033.1980.tb06106.x. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Gallinaro H., Puvion E., Kister L., Jacob M. Nuclear matrix and hnRNP share a common structural constituent associated with premessenger RNA. EMBO J. 1983;2(6):953–960. doi: 10.1002/j.1460-2075.1983.tb01527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg G. I., Collier I., Cassel A. Specific DNA sequences associated with the nuclear matrix in synchronized mouse 3T3 cells. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6887–6891. doi: 10.1073/pnas.80.22.6887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross-Bellard M., Oudet P., Chambon P. Isolation of high-molecular-weight DNA from mammalian cells. Eur J Biochem. 1973 Jul 2;36(1):32–38. doi: 10.1111/j.1432-1033.1973.tb02881.x. [DOI] [PubMed] [Google Scholar]
- Gschwendt M., Kittstein W. Specific binding of estradiol to the liver chromatin of estrogenized roosters. Biochim Biophys Acta. 1974 Aug 15;361(1):84–96. doi: 10.1016/0005-2787(74)90211-1. [DOI] [PubMed] [Google Scholar]
- Hamana K., Iwai K. Glucocorticoid-receptor complex binds to nonhistone protein and DNA in rat liver chromatin. J Biochem. 1978 Jan;83(1):279–286. doi: 10.1093/oxfordjournals.jbchem.a131902. [DOI] [PubMed] [Google Scholar]
- Hentzen P. C., Rho J. H., Bekhor I. Nuclear matrix DNA from chicken erythrocytes contains beta-globin gene sequences. Proc Natl Acad Sci U S A. 1984 Jan;81(2):304–307. doi: 10.1073/pnas.81.2.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman R., Weymouth L., Penman S. Heterogeneous nuclear RNA-protein fibers in chromatin-depleted nuclei. J Cell Biol. 1978 Sep;78(3):663–674. doi: 10.1083/jcb.78.3.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D. A., McCready S. J., Cook P. R. RNA is synthesized at the nuclear cage. Nature. 1981 Aug 6;292(5823):552–555. doi: 10.1038/292552a0. [DOI] [PubMed] [Google Scholar]
- Jost J. P., Keller R., Dierks-Ventling C. Deoxyribonucleic acid and ribonucleic acid synthesis during phosvitin induction by 17beta-estradiol in immature chicks. J Biol Chem. 1973 Aug 10;248(15):5262–5266. [PubMed] [Google Scholar]
- Jost J. P., Ohno T., Panyim S., Schuerch A. R. Appearance of vitellogenin mRNA sequences and rate of vitellogenin synthesis in chicken liver following primary and secondary stimulation by 17 beta-estradiol. Eur J Biochem. 1978 Mar 15;84(2):355–361. doi: 10.1111/j.1432-1033.1978.tb12175.x. [DOI] [PubMed] [Google Scholar]
- Lebkowski J. S., Laemmli U. K. Evidence for two levels of DNA folding in histone-depleted HeLa interphase nuclei. J Mol Biol. 1982 Apr 5;156(2):309–324. doi: 10.1016/0022-2836(82)90331-x. [DOI] [PubMed] [Google Scholar]
- McCready S. J., Akrigg A., Cook P. R. Electron-microscopy of intact nuclear DNA from human cells. J Cell Sci. 1979 Oct;39:53–62. doi: 10.1242/jcs.39.1.53. [DOI] [PubMed] [Google Scholar]
- Miller T. E., Huang C. Y., Pogo A. O. Rat liver nuclear skeleton and ribonucleoprotein complexes containing HnRNA. J Cell Biol. 1978 Mar;76(3):675–691. doi: 10.1083/jcb.76.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelkin B. D., Pardoll D. M., Vogelstein B. Localization of SV40 genes within supercoiled loop domains. Nucleic Acids Res. 1980 Dec 11;8(23):5623–5633. doi: 10.1093/nar/8.23.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyim S., Ohno T., Jost J. P. In vitro RNA synthesis and expression of vitellogenin gene in isolated chicken liver nuclei. Nucleic Acids Res. 1978 Apr;5(4):1353–1370. doi: 10.1093/nar/5.4.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll D. M., Vogelstein B. Sequence analysis of nuclear matrix associated DNA from rat liver. Exp Cell Res. 1980 Aug;128(2):466–470. doi: 10.1016/0014-4827(80)90083-x. [DOI] [PubMed] [Google Scholar]
- Rennie P. S., Bruchovsky N., Cheng H. Isolation of 3 S androgen receptors from salt-resistant fractions and nuclear matrices of prostatic nuclei after mild trypsin digestion. J Biol Chem. 1983 Jun 25;258(12):7623–7630. [PubMed] [Google Scholar]
- Robinson S. I., Nelkin B. D., Vogelstein B. The ovalbumin gene is associated with the nuclear matrix of chicken oviduct cells. Cell. 1982 Jan;28(1):99–106. doi: 10.1016/0092-8674(82)90379-8. [DOI] [PubMed] [Google Scholar]
- Robinson S. I., Small D., Idzerda R., McKnight G. S., Vogelstein B. The association of transcriptionally active genes with the nuclear matrix of the chicken oviduct. Nucleic Acids Res. 1983 Aug 11;11(15):5113–5130. doi: 10.1093/nar/11.15.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. C., Berezney R. Nuclear matrix-bound deoxyribonucleic acid synthesis: an in vitro system. Biochemistry. 1982 Dec 21;21(26):6751–6761. doi: 10.1021/bi00269a021. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai S. Y., Roop D. R., Stumph W. E., Tsai M. J., O'Malley B. W. Evidence that deoxyribonucleic acid sequences flanking the ovalbumin gene are not transcribed. Biochemistry. 1980 Apr 29;19(9):1755–1761. doi: 10.1021/bi00550a005. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Pardoll D. M., Coffey D. S. Supercoiled loops and eucaryotic DNA replicaton. Cell. 1980 Nov;22(1 Pt 1):79–85. doi: 10.1016/0092-8674(80)90156-7. [DOI] [PubMed] [Google Scholar]
- Wilks A., Cato A. C., Cozens P. J., Mattaj I. W., Jost J. P. Isolation and fine structure organisation of an avian vitellogenin gene coding for the major estrogen-inducible mRNA. Gene. 1981 Dec;16(1-3):249–259. doi: 10.1016/0378-1119(81)90081-0. [DOI] [PubMed] [Google Scholar]