Abstract
Polybrominated diphenyl ethers (PBDEs), produced as flame retardants worldwide, have been phased-out in many countries, and chlorinated and non-chlorinated organophosphates and non-PBDE brominated formulations (e.g., Firemaster 550 (FM550)) have entered the consumers’ market. Recent studies show that components of organophosphate esters and FM550 are frequently detected in many products common to human environments. Therefore, urinary metabolites of these compounds can be used as human exposure biomarkers. We developed a method to quantify nine compounds in 0.4 mL urine: diphenyl phosphate (DPhP), bis(1,3-dichloro-2-propyl) phosphate (BDCPP), bis-(1-chloro-2-propyl) phosphate, bis-2-chloroethyl phosphate, di-p-cresylphosphate, di-o-cresylphosphate (DoCP), di-n-butyl phosphate, dibenzyl phosphate (DBzP), and 2,3,4,5-tetrabromobenzoic acid. The method relies on an enzymatic hydrolysis of urinary conjugates of the target analytes, automated off-line solid phase extraction, reversed phase high performance liquid chromatography separation, and isotope dilution-electrospray ionization tandem mass spectrometry detection. The method is high-throughput (96 samples/day) with detection limits ranging from 0.05 to 0.16 ng mL−1. Spiked recoveries were 90–113 %, and interday imprecision was 2–8 %. We assessed the suitability of the method by analyzing urine samples collected from a convenience sample of adults (n = 76) and from a group of firefighters (n = 146). DPhP (median, 0.89; range, 0.26–5.6 ng mL−1) and BDCPP (median, 0.69; range, 0.31–6.8 ng mL−1) were detected in all of the non-occupationally exposed adult samples and all of the firefighter samples (DPhP [median, 2.9; range, 0.24–28 ng mL−1], BDCPP [median, 3.4; range, 0.30–44 ng mL−1]); DBzP and DoCP were not detected in any samples.
Keywords: Flame retardant, Metabolite, Urine, Liquid chromatography, Mass spectrometry
Introduction
Flame retardants (FRs) are either additive or reactive ingredients applied to household and consumer products to reduce the products flammability, and to meet state and federal fire safety standards and regulations. Until recently, the dominant class of FR additives used for household products was polybrominated diphenyl ethers (PBDEs) [1, 2]. Due to their persistence, bioaccumulation, and potential adverse health effects, PentaBDE and OctaBDE formulations were withdrawn from the consumer market in many regions of the world, including Europe and North America [3–5].
To continue to maintain the fire resistance requirements, alternative chemicals, such as chlorinated and non-chlorinated organophosphates and non-PBDE brominated formulations (e.g., Firemaster 550 (FM550)), have been introduced into the commercial flame retardant market [6]. Organophosphate flame retardants (OPFRs) include triphenyl phosphate (TPhP), tris(1,3-dichloro-2-propyl) phosphate (TDCPP), tris(1-chloro-2-propyl) phosphate, tris(2-chloroethyl) phosphate, tricresyl phosphates, tri-n-butyl phosphate (TBuP), and tribenzyl phosphate. Of interest, some organophosphates can also be used as plasticizers or lubricants [6–9]. For example, tricresyl phosphates, TPhP, and TBuP are commonly used as plasticizers and lubricants to regulate pore size [9] and as additives for hydraulic fluids [8]. FM550 contains, among other compounds, TPhP and 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB) [10, 11].
Some OPFRs and chemicals in non-PBDE brominated formulations are frequently detected in a variety of goods such as baby products, children’s hand wipes, furniture, as well as in office and house dust [2, 10, 12–16]. Moreover, reported OPFR levels in indoor environments are comparable or higher than those reported for PBDEs [17]. Several OPFRs are potential carcinogens, mutagens, and neurotoxicants [6, 11, 18, 19], with potential adverse health effects [20–22]. Laboratory animal studies show that OPFRs readily metabolize to their dialkyl or diaryl phosphates [23], and EH-TBB metabolizes to TBBA [24]. Therefore, these metabolites can be used as bio-markers of FR exposure. Monitoring urinary metabolites as biomarkers of exposure can be a valuable aid for understanding OPFRs and non-PBDE brominated formulations potential impact on human health.
Recent publications have demonstrated the presence of FR metabolites in human urine by liquid chromatography-mass spectrometry or gas chromatography-mass spectrometry after derivatization [25–29]. Even though these analytical methods are well developed, not all major metabolites are included in one single method and require relatively large sample volumes.
In this work, we present an high performance liquid chromatography (HPLC)-MS/MS method to concurrently quantify biomarkers of eight chlorinated and non-chlorinated organophosphates, and one non-PBDE brominated compound in human urine. We also assessed the suitability of the method by analyzing 76 randomly collected urine samples from the general population, and 146 urine samples collected from occupationally exposed persons.
Materials and methods
Reagents and standards
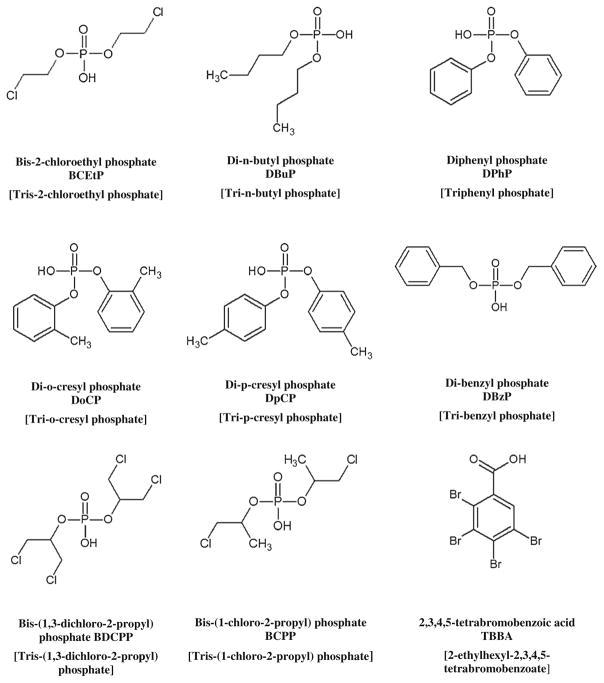
Methanol, acetonitrile, and ammonium hydroxide were purchased from Fisher Scientific (Pittsburgh, PA, USA), and formic acid, acetic acid, and ammonium acetate were purchased from Sigma-Aldrich (St. Louis, MO, USA). Methanol, acetonitrile, formic acid, and acetic acid were all HPLC-grade. Deionized water was organically and biologically purified using a NANOpure Infinity ultrapure water system (Barnstead/Thermolyne, IA). Diphenyl phosphate (DPhP), DPhP-d10, di-o-cresylphosphate (DoCP), DoCP-d14, di-p-cresylphosphate (DpCP), DpCP-d14, bis-(1-chloro-2-propyl) phosphate (BCPP), BCPP-d12, bis-2-chloroethyl phosphate (BCEtP), and BCEtP-d8 were purchased from Toronto Research Chemicals, TRC (Toronto, Canada). Bis-(1,3-dichloro-2-propyl) phosphate (BDCPP), BDCPP-d10, 2,3,4,5-tetrabromobenzoic acid (TBBA), and TBBA-13C6 were purchased from Wellington Laboratories (Guelph, Canada). Di-n-butyl phosphate (DBuP), DBuP-d18, di-benzyl-phosphate (DBzP), DBzP-d14, β-glucuronidase Type H-1 from Helix Pomatia, and 4-methylumbelliferyl β-D-glucuronidase hydrate (UMB) were purchased from Sigma-Aldrich. UMB-13C4 was purchased from Cambridge Isotope Laboratories (Andover, MA, USA). All chemicals and standard materials were used without further purification. The analytes chemical structures, abbreviations, and the names of parent compounds are shown in Fig. 1.
Fig. 1.
Chemical structures, abbreviations, and [parent compound] for target flame retardant metabolites
Individual stock solutions of standards and labeled internal standards were prepared by dissolving measured amounts of the solid compound or by diluting in appropriate solvent. Using these individual stock solutions, two intermediate stock solutions with all target analytes were prepared in 1:1 (v/v) methanol/water giving a concentration of individual compounds of 1000 and 500 ng mL−1. Ten calibration standard solutions containing all target analytes were prepared by diluting appropriate amounts from intermediate stock solutions in 1:1 (v/v) methanol/water. A 100-μL spike from these calibration standards to 400 μL of urine covers a final concentration range of 0.05 to 40 ng mL−1. 4-Methylumbelliferyl glucuronide and 13C4-4-methylumbelliferone were used as deconjugation standards to monitor the extent of the enzymatic reaction. The individual stock solutions were prepared by dissolving measured amounts of 4-methylumbelliferyl glucuronide and 13C4-4-methylumbelliferone in methanol. By mixing appropriate amounts from isotope-labeled standards and deconjugation standards in 1:1 (v/v) methanol/water, the spiking solution of isotope-labeled standards and deconjugation standards mixture was prepared, so that a 100-μL spike to 400 μL of urine would result in 10 ng mL−1 concentration of the individual labeled compounds, 750 ng mL−1 of 4-methylumbelliferyl glucuronide, and 150 ng mL−1 of 13C4-4-methylumbelliferone. All stock solutions and standards were stored at or below −10 °C in amber glass vials to prevent photo degradation.
Human urine collection for method development and validation
Urine samples were collected anonymously in Atlanta, GA, in 2015 from a diverse group of adult volunteers with no documented occupational exposure to the target flame retardants. The Centers for Disease Control and Prevention Human Subjects Institutional Review Board (IRB) reviewed and approved the study protocol. A waiver of informed consent was requested under 45 CFR 46.116(d). We did not have access to any personal or demographic data.
The individual urine samples with the overall lowest concentrations (N = 52) of endogenous target analytes were combined to form a blank pool. The blank pool was stored at or below −20 °C in glass vials. Quality control (QC) materials were prepared by spiking portions of blank urine with native target compounds. The approximate concentrations of the target analytes were 4 ng mL−1 (low concentration QC (QCL)) and 15 ng mL−1 (high concentration QC (QCH)). The spiked QC materials were refrigerated, mixed for over 24 h, then dispensed in 1 mL aliquots into polypropylene vials, and stored at or below −20 °C until use.
We also analyzed urine samples from firefighters collected in 2010–2011 for a US National Institute for Occupational Safety and Health (NIOSH) study to evaluate firefighters’ exposures to potential toxic chemicals during structural firefighting while wearing fireproof clothing and self-contained breathing apparatus (SCBA) [30, 31]. Samples were collected ~20 min after or 3 h after structural firefighting performed while wearing full protective clothing and SCBA respirators. All participants gave consent to have their residual urine stored without identifiers for future research purposes, and the study protocol was approved by the NIOSH IRB. The analysis of these de-identified specimens for urinary flame retardants biomarkers was determined not to constitute engagement in human subjects research.
Sample preparation and automated off-line solid phase extraction (SPE)
Each analytical run, prepared in a 96-well plate (2 mL square well, Varian, Lake Forest, CA, USA), included a solvent blank, ten calibration standards, two QCL, two QCH, and the study samples. A 96-well format spreadsheet with sample locations was used to guide the spiking. One hundred microliters from the labeled/deconjugation standard spiking mixture was aliquoted to each well. Then 100 μL of calibration standard solutions were spiked to the wells assigned for each calibration level. Subsequently, 400 μL of deionized water was added to solvent blank and calibration standards, and 400 μL of QCs or study urines was added to the corresponding wells. After that, 400 μL of enzyme solution was dispensed to each well. The enzyme solution (pH ~5) was prepared immediately before every analytical run by adding β-glucuronidase/sulfatase with a specific activity of about 500 units mg−1 to 0.2 M sodium acetate buffer to produce a solution with a minimum of 1000 units of enzyme activity per sample. Then the 96-well plate was covered with a cover mat and the samples were incubated at 37 °C for at least 6 h (typically overnight).
After the enzymatic hydrolysis, 800 μL of 2 % (v/v) formic acid in deionized water was added to each sample. Then the 96-well plate was placed on a TOMTEC Quadra4 semiautomated SPE station (Hamden, CT, USA). Urine samples, reagent blank, and calibration standards were pipette-mixed twice right before loading onto a 96-well format SPE cartridge (60 mg Strata XAW polymeric SPE packing with 1.5 mL liquid space, Phenomenex, Torrance, CA) which was previously conditioned with 2 × 430 μL of HPLC-grade 2 % (v/v) formic acid in methanol followed by 2 × 430 μL of 2 % (v/v) formic acid in deionized water. After sample loading (6 × 310 μL), the wells were washed with 2 × 430 μL of 2 % (v/v) formic acid in deionized water followed by 2 % (v/v) formic acid in methanol and dried under vacuum. The target analytes were eluted with 3 × 400 μL of 2 % (v/v) NH4OH in methanol. The eluates were evaporated to dryness under a stream of dry nitrogen (UHP grade) at 40 °C in a Turbovap 96 concentration workstation (Caliper Life Sciences, Hopkinton, MA, USA). The evaporated extracts were reconstituted with 50 μL of 1:1 (v/v) acetonitrile/water mixture.
Chromatographic separation and detection
HPLC was performed on an Agilent 1290 (Agilent Technologies, Santa Clara, CA, USA) system equipped with a binary pump, an autosampler with a cooling thermostat module, and a temperature-controlled column compartment. Chromatographic separation was performed on a ZORBAX Eclipse XDB-C8 column (4.6 × 150 mm, 5 μm) from Agilent Technologies kept at 45 °C during analysis and operated at a flow rate of 0.7 mL min−1. The reconstituted SPE extracts were kept in the autosampler at 4 °C and an injection volume of 10 μL was used for the analysis. Analytes were separated with the gradient shown in Table 1 using 20 mM ammonium acetate in deionized water as mobile phase A and acetonitrile as mobile phase B. All analytes eluted within 8 min.
Table 1.
HPLC gradient program
| Time (min) | A% | B% |
|---|---|---|
| 0.0 | 95 | 5 |
| 0.5 | 95 | 5 |
| 7.0 | 25 | 75 |
| 8.5 | 0 | 100 |
| 9.3 | 0 | 100 |
| 10.3 | 95 | 5 |
| 14.0 | 95 | 5 |
A—20 mM ammonium acetate in water; B—acetonitrile
Mass spectrometry analysis was performed on an AB Sciex 5500 Qtrap mass spectrometer (Applied Biosystems, Foster City, CA, USA) equipped with a turbo ion spray (ESI) ionization probe. The parameters were set as follows: curtain gas 20 psi, collision gas medium option, ionspray voltage −4500 V, temperature 450 °C, and ion source gases 45 psi. The mass spectrometer was operated in scheduled multiple reaction monitoring (s-MRM) mode using negative polarity. Table 2 shows the transitions and collision energies used for each analyte.
Table 2.
Flame retardant metabolites and their isotope labeled analogs, quantitation and confirmation ions, and collision energies (CE)
| Metabolite | Quantitation ion
|
Confirmation ion
|
||||
|---|---|---|---|---|---|---|
| Precursor ion (m/z) | Product ion (m/z) | CE (eV) | Precursor ion (m/z) | Product ion (m/z) | CE (eV) | |
| BCEtP | 221 | 35 | 25 | 223 | 37 | 31 |
| BCEtP-d8 | 229 | 35 | 27 | |||
| BCPP | 249 | 35 | 33 | 251 | 37 | 27 |
| BCPP-d12 | 261 | 35 | 33 | |||
| BDCPP | 319 | 35 | 40 | 319 | 37 | 39 |
| BDCPP-d10 | 329 | 35 | 40 | |||
| DBuP | 209 | 79 | 28 | 209 | 153 | 19 |
| DBuP-d18 | 227 | 79 | 30 | |||
| DBzP | 277 | 79 | 33 | 277 | 63 | 30 |
| DBzP-d14 | 291 | 79 | 36 | |||
| DPhP | 249 | 93 | 33 | 249 | 155 | 28 |
| DPhP-d10 | 259 | 98 | 33 | |||
| DoCP | 277 | 107 | 34 | 277 | 169 | 31 |
| DoCP-d14 | 291 | 114 | 34 | |||
| DpCP | 277 | 107 | 35 | 277 | 169 | 30 |
| DpCP-d14 | 291 | 114 | 35 | |||
| TBBA | 436.7 | 392.7 | 14 | 434.7 | 390.7 | 13 |
| TBBA-13C6 | 442.7 | 398.7 | 14 | |||
Analyst software version 1.6.2 (Applied Biosystems) was used to control all system components, the data collection, and analysis. To evaluate the difference between the urinary concentrations of the target analytes with and without enzymatic treatment, we performed a parametric analysis of variance (ANOVA) test. Statistical significance was set at p < 0.05.
Results and discussion
Selection of ionization mode
We tested negative ESI and negative atmospheric pressure chemical ionization (APCI) to evaluate the best ionization strategy. All compounds ionized under both ionization modes; however, some analytes, especially the chlorinated compounds and TBBA, were more sensitive in ESI than they were in APCI. Therefore, considering overall efficiency, we selected negative ESI as the ionization method.
Selection of the analytical column
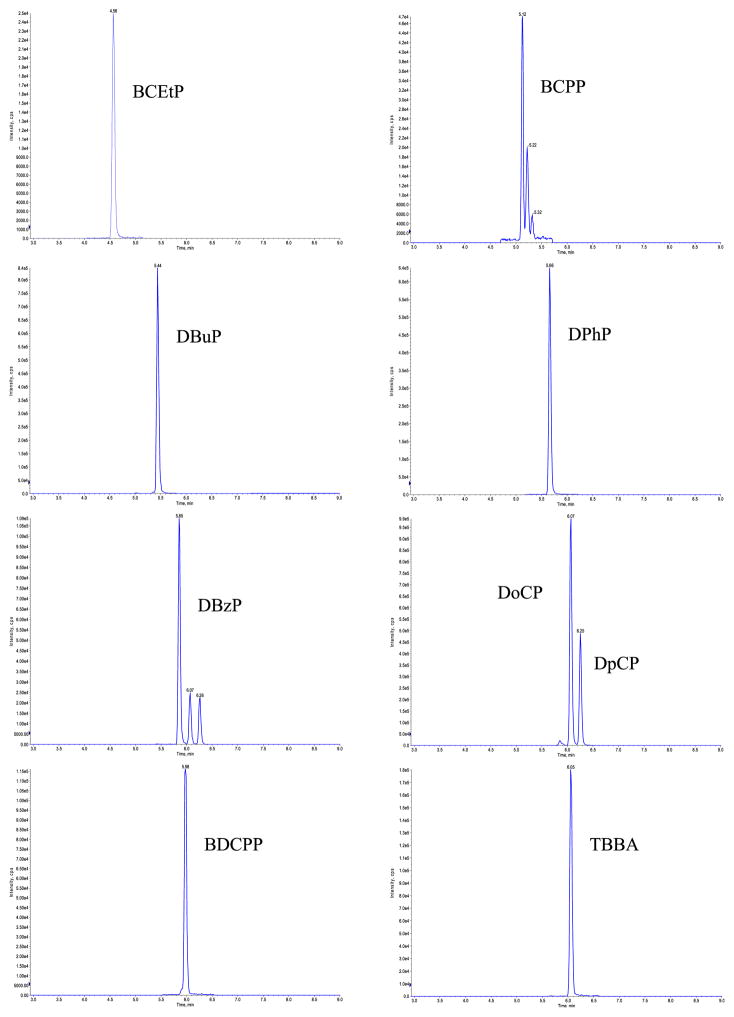
We tested several reversed phase C8 and C18 columns with different dimensions and particle sizes. One of our main targets was to have a short analytical run time (in order to analyze all 96 samples within a 24-h period). Some columns we examined could not completely separate DoCP and DpCP, and some needed comparably longer run times to elute all analytes and completely resolve cresol isomers peaks. We selected ZORBAX Eclipse XDB-C8 column (4.6 × 150 mm, 5 μm) because it eluted all nine analytes in less than 8 min, and completely resolved DoCP and DpCP. The ion chromatograms for native and labeled BCPP appeared as a cluster of three peaks, and the full cluster was integrated for quantitation purposes. Figure 2 shows a typical ion chromatograms for all target analytes in urine.
Fig. 2.
Typical chromatograms of the target analytes in urine (0. 3 ng on column)
Optimization of SPE conditions
Previous studies have shown better recoveries for some of the flame retardant biomarkers using weak anion exchange sorbents than other sorbents [25, 27]. Therefore, we chose Strata-X-AW to evaluate recoveries of all nine target analytes. Recoveries were evaluated by using pre- and post-extraction spiked aliquots from the blank urine pool. Portions from breakthrough and wash steps were also tested to evaluate potential losses during sample loading and washing steps. Samples were eluted with 1–5 % of NH4OH in 80–100 % methanol and in 80–100 % acetonitrile. Care was taken to control the sample pass through flow rate to as low as possible by regulating the negative vacuum supply to the SPE plate between no vacuum and −2 in Hg. As expected, drying time of the eluents increased when the solvent composition was less than 100 %. Significant improvement of recoveries was not observed with the increase of NH4OH above 2 %. Elution with methanol improved recovery compared with acetonitrile (data not shown). Because Strata-X-AW extracts eluted with 2 % NH4OH in methanol provided satisfying recoveries for all target analytes, we did not evaluate the performance of other weak anion exchange sorbents.
Enzymatic deconjugation
Many metabolites eliminated in urine are present in their conjugated form [32]. A commonly used approach to quantify the urinary concentrations of these compounds is to first hydrolyze the conjugates to report total (conjugated plus unconjugated) concentrations [33, 34]. Also, while many authors have examined the use of enzymatic hydrolysis as the OPFR biomarkers may be eliminated as conjugates in urine [35], only a few recently published methods utilized enzymatic deconjugation [36, 37]. To evaluate the optimal deconjugation conditions, we treated 12 urine samples (in triplicate) separately with three types of enzymes, β-glucuronidase (Escherichia coli K-12), β-glucuronidase (E. coli, recombinant), and β-glucuronidase/sulfatase (Helix Pomatia, type H-1), and compared the concentrations of the target analytes to those obtained without enzymatic treatment. We observed no noticeable differences regardless of the type of enzyme used (data not shown), and selected β-glucuronidase/sulfatase (Helix Pomatia, type H-1) to hydrolyze the conjugates. In Table 3, we present the concentrations detected with and without β-glucuronidase/sulfatase (Helix Pomatia, type H-1) treatment. None of the samples tested had detectable concentrations of DBzP, DoCP, DpCP, or TBBA. For the other analytes, the concentrations in enzymatically treated and non-treated samples differed significantly for DPhP (p = 0.002) and DBuP (p = 0.004), but not for BDCPP, BCPP, or BCEtP (all p > 0.05).
Table 3.
Concentrations of select analytes with and without β-glucuronidase/sulfatase (Helix Pomatia, type H-1) treatment
| Sample | Mean concentration ± standard deviation (ng mL−1)a
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| BCEtP
|
BCPP
|
BDCPP
|
DBuP
|
DPhP
|
||||||
| Without enzyme | With enzyme | Without enzyme | With enzyme | Without enzyme | With enzyme | Without enzyme | With enzyme | Without enzyme | With enzyme | |
| 1 | 2.37 ± 0.02 | 2.40 ± 0.01 | 0.54 ± 0.03 | 0.49 ± 0.03 | 5.69 ± 0.18 | 5.85 ± 0.23 | 1.04 ± 0.03 | 1.11 ± 0.07 | 16.5 ± 0.15 | 20.5 ± 0.21 |
| 2 | 0.83 ± 0.01 | 0.94 ± 0.05 | 0.23 ± 0.02 | 0.28 ± 0.02 | 4.26 ± 0.04 | 4.50 ± 0.17 | 0.68 ± 0.06 | 0.95 ± 0.03 | 9.10 ± 0.25 | 10.4 ± 0.34 |
| 3 | 7.47 ± 0.14 | 7.80 ± 0.02 | 0.24 ± 0.02 | 0.21 ± 0.01 | 1.45 ± 0.14 | 1.75 ± 0.54 | 0.29 ± 0.02 | 0.49 ± 0.04 | 6.79 ± 0.20 | 7.86 ± 0.15 |
| 4 | 0.73 ± 0.01 | 0.74 ± 0 | 2.20 ± 0.02 | 2.24 ± 0.04 | 3.61 ± 0.01 | 3.68 ± 0.09 | 0.23 ± 0.02 | 0.29 ± 0.02 | 4.64 ± 0.05 | 4.90 ± 0.18 |
| 5 | 1.20 ± 0.01 | 1.28 ± 0.01 | 1.98 ± 0.03 | 1.96 ± 0.08 | 0.85 ± 0.03 | 0.91 ± 0.02 | ** | ** | 1.32 ± 0.03 | 1.49 ± 0.05 |
| 6 | 4.67 ± 0.10 | 4.81 ± 0.10 | 0.07 ± 0 * | 0.07 ± 0 * | 2.73 ± 0.15 | 2.76 ± 0.14 | 1.76 ± 0.04 | 1.98 ± 0.01 | 19.4 ± 0.17 | 21.3 ± 0.15 |
| 7 | 1.93 ± 0.02 | 1.95 ± 0.02 | 0.46 ± 0.02 | 0.44 ± 0.04 | 6.89 ± 0.46 | 7.50 ± 0.20 | 1.09 ± 0.05 | 1.33 ± 0.04 | 18.3 ± 0.46 | 22.4 ± 0.40 |
| 8 | 1.79 ± 0.03 | 1.84 ± 0.03 | 1.99 ± 0.01 | 1.98 ± 0.04 | 4.01 ± 0.08 | 3.97 ± 0.19 | 0.34 ± 0.02 | 0.39 ± 0.02 | 7.33 ± 0.27 | 8.22 ± 0.22 |
| 9 | 1.10 ± 0.01 | 1.18 ± 0.03 | 0.17 ± 0.01 | 0.22 ± 0.01 | 20.3 ± 0.06 | 20.8 ± 0.32 | 0.40 ± 0 | 0.45 ± 0.02 | 8.68 ± 0.12 | 10.7 ± 0.35 |
| 10 | 1.48 ± 0.03 | 1.51 ± 0.06 | 0.38 ± 0.06 | 0.33 ± 0.04 | 7.29 ± 0.11 | 7.48 ± 0.20 | 0.88 ± 0.07 | 1.02 ± 0.04 | 13.7 ± 0.38 | 16.4 ± 0.15 |
| 11 | 1.30 ± 0.05 | 1.31 ± 0.01 | 0.07 ± 0 * | 0.13 ± 0 | 2.77 ± 0.02 | 2.84 ± 0.03 | 0.38 ± 0.01 | 0.45 ± 0.01 | 7.43 ± 0.57 | 8.07 ± 0.06 |
| 12 | 4.04 ± 0.14 | 4.45 ± 0.07 | 1.34 ± 0.08 | 1.36 ± 0.04 | ** | ** | 0.54 ± 0.02 | 0.55 ± 0.01 | 11.1 ± 0 | 11.7 ± 0.23 |
| 13 | 1.21 ± 0.05 | 1.25 ± 0.04 | 0.85 ± 0.03 | 0.91 ± 0.01 | 6.65 ± 0.06 | 7.00 ± 0.25 | 0.16 ± 0.01 | 0.20 ± 0.01 | 3.21 ± 0.04 | 3.31 ± 0.03 |
| 14 | 0.99 ± 0.01 | 0.99 ± 0.02 | 0.66 ± 0.04 | 0.71 ± 0.03 | 5.73 ± 0.08 | 5.88 ± 0.17 | 0.39 ± 0.03 | 0.58 ± 0 | 6.54 ± 0.37 | 6.83 ± 0.12 |
Concentrations below limit of detection (LOD) were replaced with LOD/√2;
unable to quantify chromatographic interference
Calculated from three replicate measurements
Next, we evaluated the optimal conditions for the enzymatic treatment with β-glucuronidase/sulfatase (Helix Pomatia, type H-1) by changing the amount of enzyme and by changing the incubation time and analyzing in duplicate four samples with detectable concentrations of DBuP, DPhP, BDCPP, and BCEtP. For all analytes, the concentration increased with increasing amounts of enzyme with a maximum at 400 μL. Only DBuP concentrations increased for at least 6 h while concentrations of the other analytes did not increase noticeably after 5 h. For convenience, we chose overnight incubation with 400 μL of enzyme (about 1000 units of enzyme activity per sample).
Matrix effects
The composition of urine varies considerably from person to person, and even within a person, with regard to types and concentrations of solutes. This complexity may cause matrix-dependent ion enhancement or ion suppression [38, 39]. Matrix effects can be accounted for, at least in part, by utilizing stable isotope labeled internal standards or by preparing calibration standards in the same matrix as study samples. Each target compound in this method is quantified with its own deuterium or 13C labeled internal standard. However, even with such provisions, matrix effects may exist. To evaluate matrix effects, we analyzed ten sets of calibration curves constructed in ten different urines or deionized water. The mean slope ± standard deviation in urine and in water for each analyte, and the percent difference between the slopes are shown in Table 4. Within our experimental conditions, none of the urines tested was free of all target analytes, but mean slopes in urine for every analyte were not considerably different from their mean slopes in water; therefore, a water-based calibration curve was chosen for quantification.
Table 4.
The mean slope of calibration curve ± standard deviation in water and in urine for each flame retardant metabolite, and % difference between the slopes
| Analyte | Slope ± standard deviationa
|
% Difference | |
|---|---|---|---|
| Water curve | Urine curve | ||
| BCEtP | 0.1048 ± 0.0034 | 0.1058 ± 0.0022 | 1.0 |
| BCPP | 0.0921 ± 0.0005 | 0.0894 ± 0.0025 | 2.9 |
| BDCPP | 0.1001 ± 0.0013 | 0.0956 ± 0.0027 | 4.6 |
| DBuP | 0.1130 ± 0.0008 | 0.1173 ± 0.0073 | 3.7 |
| DBzP | 0.1065 ± 0.0026 | 0.1048 ± 0.0040 | 1.7 |
| DPhP | 0.1185 ± 0.0019 | 0.1198 ± 0.0097 | 1.0 |
| DoCP | 0.0912 ± 0.0008 | 0.0870 ± 0.0018 | 4.7 |
| DpCP | 0.0986 ± 0.0031 | 0.0942 ± 0.0021 | 4.6 |
| TBBA | 0.0806 ± 0.0016 | 0.0780 ± 0.0017 | 3.2 |
N = 10
Recoveries
Recoveries were evaluated at four concentrations (2, 8, 16, 30 ng mL−1) by using pre- and post-extraction spiked aliquots from the blank urine pool. Each level was prepared and analyzed in triplicate for five different days. Relative recoveries, calculated as the ratio of response ratios (native/label) for pre- and post-spiked extractions, are shown in Table 5. Recoveries of 90–113 % were obtained for all analytes at all concentrations considered.
Table 5.
Relative recoveries (recovery [%]) of off-line SPE and relative standard deviations of recovery (RSD [%]) for flame retardant metabolites at four different concentration levels (2, 8, 16, and 30 ng mL−1)
| Analyte | 2 ng mL−1
|
8 ng mL−1
|
16 ng mL−1
|
30 ng mL−1
|
||||
|---|---|---|---|---|---|---|---|---|
| Recovery | RSD | Recovery | RSD | Recovery | RSD | Recovery | RSD | |
| BCEtP | 94 | 14 | 105 | 14 | 92 | 23 | 97 | 10 |
| BCPP | 96 | 14 | 100 | 7 | 104 | 4 | 100 | 8 |
| BDCPP | 99 | 14 | 95 | 9 | 101 | 13 | 102 | 17 |
| DBuP | 97 | 7 | 103 | 8 | 99 | 11 | 98 | 9 |
| DBzP | 99 | 9 | 98 | 10 | 113 | 21 | 105 | 8 |
| DPhP | 102 | 9 | 106 | 15 | 108 | 8 | 98 | 11 |
| DoCP | 96 | 14 | 95 | 14 | 102 | 13 | 93 | 9 |
| DpCP | 93 | 9 | 103 | 10 | 98 | 14 | 90 | 14 |
| TBBA | 101 | 17 | 102 | 17 | 103 | 20 | 103 | 14 |
Precision and accuracy
The precision [40] was calculated as the coefficient of variation (%CV) of repeat measurements (N = 40) of the QCL and QCH materials prepared in duplicate. Samples were prepared by two analysts and analyzed in two instruments over the course of 1 month. CVs ranged from 2.7 to 7.5 % (Table 6) with both interday and intraday imprecisions <7 %. Accuracy was calculated at three different concentrations with 20 repeat measurements. Accuracy, expressed as percent error of measured value to its nominal value, ranged from 94 to 108 % (Table 6).
Table 6.
Method validation data for flame retardants metabolites
| Analyte | LOD (ng mL−1) | Accuracy (%)a
|
Precision (%)b
|
|||
|---|---|---|---|---|---|---|
| 0.5 (ng mL−1) | 5 (ng mL−1) | 25 (ng mL−1) | QCL 4 (ng mL−1) | QCH 15 (ng mL−1) | ||
| BCEtP | 0.08 | 96.5 | 100.3 | 100.5 | 3.4 | 3.4 |
| BCPP | 0.1 | 96.9 | 98.7 | 100.7 | 3.4 | 3.0 |
| BDCPP | 0.11 | 96.3 | 106.3 | 99.0 | 4.3 | 3.0 |
| DBuP | 0.05 | 98.1 | 102.4 | 102.0 | 7.5 | 5.7 |
| DBzP | 0.05 | 101.6 | 101.0 | 99.0 | 3.4 | 3.9 |
| DPhP | 0.16 | 105.0 | 101.9 | 97.9 | 3.5 | 2.9 |
| DoCP | 0.05 | 96.2 | 98.4 | 105.1 | 3.4 | 3.6 |
| DpCP | 0.05 | 97.2 | 93.5 | 107.9 | 4.2 | 3.9 |
| TBBA | 0.05 | 99.1 | 99.8 | 100.0 | 2.7 | 3.0 |
N = 20
N = 40, two instruments used by two analysts over 1 month
Analytical sensitivity
The limits of detection (LODs) were assessed by 20 repeated measurements of low concentration standards and by plotting the standard deviation of the measured concentration versus the concentration of the standard. The expected standard deviation at the zero concentration, S0, was determined by the y-intercept of a linear regression analysis of the above plot. The LODs were calculated as three times S0 [41] and are shown in Table 6.
Method application
The applicability of the method was tested by analyzing 76 urine samples collected anonymously in 2015 from Atlanta adult residents with no known occupational exposure to flame retardants or their metabolites. All of the samples had detectable concentrations of DPhP and BDCPP, and none had detectable concentrations of DBzP, DoCP, or TBBA (Table 7).
Table 7.
Method application data for flame retardants metabolites in urine from anonymous adult volunteers (general population) and from firefighters (exposed population)
| Analyte | General population (N = 76)
|
Exposed population (N = 146)
|
||||
|---|---|---|---|---|---|---|
| Detection frequency (%) | Median (ng mL−1) | Range (ng mL−1) | Detection frequency (%) | Median (ng mL−1) | Range (ng mL−1) | |
| BCEtP | 10 | <LOD | <LOD–4.1 | 90 | 0.86 | <LOD–10 |
| BCPP | 5 | <LOD | <LOD–0.98 | 63 | 0.24 | <LOD–2.9 |
| BDCPP | 100 | 0.69 | 0.31–6.8 | 100 | 3.4 | 0.30–44 |
| DBuP | 5 | <LOD | <LOD–0.26 | 92 | 0.18 | <LOD–2.4 |
| DPhP | 100 | 0.89 | 0.26–5.6 | 100 | 2.9 | 0.24–28 |
| DpCP | 0 | <LOD | 34 | <LOD | <LOD–0.31 | |
| TBBA | 0 | <LOD | 5 | <LOD | <LOD–0.21 | |
DBzP and DoCP were not detected in any of the samples tested
The method was also tested by analyzing 146 urine samples collected from firefighters after structural firefighting performed while wearing full protective clothing and SCBA respirators. Again, all of the urine samples had detectable concentrations of DPhP and BDCPP. None of the samples tested had detectable concentrations of DBzP or DoCP (Table 7).
Median concentrations of BDCPP and DPhP in the firefighters’ samples were approximately five and three times higher, respectively, than the median from the general population samples suggesting that occupational exposures may be higher than background exposures.
Together, these results show that the current method is sensitive enough to detect urinary concentrations of flame retardant biomarkers from background exposures in the general population as well as higher concentrations encountered in occupationally exposed populations.
Conclusions
We developed a sensitive HPLC-isotope dilution tandem mass spectrometry method with a semiautomated SPE sample cleanup for the quantification of biomarkers of eight chlorinated and non-chlorinated organophosphates, and one non-PBDE brominated compound in urine. Two major advantages of this method are the use of a relatively small sample volume and the high sample throughput. Our preliminary data suggest that the method is sensitive, precise, and accurate enough to detect trace-level concentrations of these compounds in urine. Potential applications may include obtaining reference range concentrations of these biomarkers for large-scale general population studies such as the National Health and Nutrition Examination Survey. Nevertheless, additional considerations, such as adequate collection protocols, handling and storage of the samples, and data on the temporal stability of the analytes in urine, are needed to demonstrate the utility of these measures for exposure and risk assessment purposes.
Acknowledgments
We gratefully acknowledge Dr. Kenneth Fent (NIOSH, CDC) for providing firefighters’ de-identified urine specimens for method validation, and Dr. Sam Caudill for helping with statistical analysis. This work was supported in part by an appointment (LW) to the Research Participation Program at the CDC, administered by the Oak Ridge Institute for Science and Education through an inter-agency agreement between the U.S. Department of Energy and CDC.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention (CDC). Use of trade names is for identification only and does not imply endorsement by the CDC, the Public Health Service, or the US Department of Health and Human Services
Compliance with ethical standards
Conflict of interest
The authors declare they have no competing financial or other conflicts of interest.
References
- 1.de Wit CA. An overview of brominated flame retardants in the environment. Chemosphere. 2002;46(5):583–624. doi: 10.1016/s0045-6535(01)00225-9. [DOI] [PubMed] [Google Scholar]
- 2.Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, et al. Novel and high volume use flame retardants in us couches reflective of the 2005 PentaBDE phase out. Environ Sci Technol. 2012;46(24):13432–9. doi: 10.1021/es303471d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tullo A. Great lakes to phase out two flame retardants. Chem Eng News. 2003;81(45):13. [Google Scholar]
- 4.USEPA. DecaBDE phase-out initiative. 2010 http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/deccadbe.html.
- 5.Betts K. Court bans widely used flame retardant. Environ Sci Technol. 2008;42(11):3910. doi: 10.1021/es0871199. [DOI] [PubMed] [Google Scholar]
- 6.van der Veen I, de Boer J. Phosphorus flame retardants: properties, production, environmental occurrence, toxicity and analysis. Chemosphere. 2012;88(10):1119–53. doi: 10.1016/j.chemosphere.2012.03.067. [DOI] [PubMed] [Google Scholar]
- 7.Covaci A, Harrad S, Abdallah MA, Ali N, Law RJ, Herzke D, et al. Novel brominated flame retardants: a review of their analysis, environmental fate and behavior. Environ Int. 2011;37(2):532–56. doi: 10.1016/j.envint.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 8.Solbu K, Thorud S, Hersson M, Ovrebo S, Ellingsen D, Lundanes E, et al. Determination of airborne trialkyl and triaryl organophosphates originating from hydraulic fluids by gas chromatography-mass spectrometry—development of methodology for combined aerosol and vapor sampling. J Chromatogr A. 2007;1161(1–2):275–83. doi: 10.1016/j.chroma.2007.05.087. [DOI] [PubMed] [Google Scholar]
- 9.Andresen JA, Grundmann A, Bester K. Organophosphorus flame retardants and plasticisers in surface waters. Sci Total Environ. 2004;332(1–3):155–66. doi: 10.1016/j.scitotenv.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 10.Stapleton HM, Allen JG, Kelly SM, Konstantinov A, Klosterhaus S, Watkins D, et al. Alternate and new brominated flame retardants detected in US house dust. Environ Sci Technol. 2008;42(18):6910–6. doi: 10.1021/es801070p. [DOI] [PubMed] [Google Scholar]
- 11.Butt CM, Congleton J, Hoffman K, Fang ML, Stapleton HM. Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ Sci Technol. 2014;48(17):10432–8. doi: 10.1021/es5025299. [DOI] [PubMed] [Google Scholar]
- 12.Stapleton HM, Klosterhaus S, Keller A, Ferguson PL, van Bergen S, Cooper E, et al. Identification of flame retardants in polyurethane foam collected from baby products. Environ Sci Technol. 2011;45(12):5323–31. doi: 10.1021/es2007462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carignan CC, McClean MD, Cooper EM, Watkins DJ, Fraser AJ, Heiger-Bernays W, et al. Predictors of tris(1,3-dichloro-2-propyl) phosphate metabolite in the urine of office workers. Environ Int. 2013;55:56–61. doi: 10.1016/j.envint.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dodson RE, Perovich LJ, Covaci A, Van den Eede N, Ionas AC, Dirtu AC, et al. After the PBDE phase-out: a broad suite of flame retardants in repeat house dust samples from California. Environ Sci Technol. 2012;46(24):13056–66. doi: 10.1021/es303879n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stapleton HM, Klosterhaus S, Eagle S, Fuh J, Meeker JD, Blum A, et al. Detection of organophosphate flame retardants in furniture foam and US house dust. Environ Sci Technol. 2009;43(19):7490–5. doi: 10.1021/es9014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stapleton HM, Misenheimer J, Hoffman K, Webster TF. Flame retardant associations between children’s handwipes and house dust. Chemosphere. 2014;116:54–60. doi: 10.1016/j.chemosphere.2013.12.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dodson RE, Van den Eede N, Covaci A, Perovich LJ, Brody JG, Rudel RA. Urinary biomonitoring of phosphate flame retardants: levels in California adults and recommendations for future studies. Environ Sci Technol. 2014;48(23):13625–33. doi: 10.1021/es503445c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Babich MA. CPSC staff preliminary risk assessment of flame retardant (FR) chemicals in upholstered furniture foam. US Consumer Product Safety Commission; 2006. [Google Scholar]
- 19.Dishaw LV, Powers CM, Ryde IT, Roberts SC, Seidler FJ, Slotkin TA, et al. Is the PentaBDE replacement, tris (1,3-dichloropropyl) phosphate (TDCPP), a developmental neurotoxicant? Studies in PC12 cells. Toxicol Appl Pharmacol. 2011;256(3):281–9. doi: 10.1016/j.taap.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meeker JD, Cooper EM, Stapleton HM, Hauser R. Exploratory analysis of urinary metabolites of phosphorus-containing flame retardants in relation to markers of male reproductive health. Endocr Disruptors. 2013;1(1):e26306. doi: 10.4161/endo.26306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meeker JD, Stapleton HM. House dust concentrations of organophosphate flame retardants in relation to hormone levels and semen quality parameters. Environ Health Perspect. 2010;118(3):318–23. doi: 10.1289/ehp.0901332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, et al. Accumulation and endocrine disrupting effects of the flame retardant mixture firemaster (R) 550 in rats: an exploratory assessment. J Biochem Mol Toxicol. 2013;27(2):124–36. doi: 10.1002/jbt.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van den Eede N, Maho W, Erratico C, Neels H, Covaci A. First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions. Toxicol Lett. 2013;223(1):9–15. doi: 10.1016/j.toxlet.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Roberts SC, Macaulay LJ, Stapleton HM. In vitro metabolism of the brominated flame retardants 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (TBB) and bis(2-ethylhexyl) 2,3,4,5-tetrabromophthalate (TBPH) in human and rat tissues. Chem Res Toxicol. 2012;25(7):1435–41. doi: 10.1021/tx300086x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper EM, Covaci A, van Nuijs ALN, Webster TF, Stapleton HM. Analysis of the flame retardant metabolites bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011;401(7):2123–32. doi: 10.1007/s00216-011-5294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schindler BK, Foerster K, Angerer J. Determination of human urinary organophosphate flame retardant metabolites by solid-phase extraction and gas chromatography-tandem mass spectrometry. J Chromatogr B Anal Technol Biomed Life Sci. 2009;877(4):375–81. doi: 10.1016/j.jchromb.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 27.Van den Eede N, Neels H, Jorens PG, Covaci A. Analysis of organophosphate flame retardant diester metabolites in human urine by liquid chromatography electrospray ionisation tandem mass spectrometry. J Chromatogr A. 2013;1303:48–53. doi: 10.1016/j.chroma.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 28.Reemtsma T, Lingott J, Roegler S. Determination of 14 monoalkyl phosphates, dialkyl phosphates and dialkyl thiophosphates by LC-MS/MS in human urinary samples. Sci Total Environ. 2011;409(10):1990–3. doi: 10.1016/j.scitotenv.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 29.Schindler BK, Foerster K, Angerer J. Quantification of two urinary metabolites of organophosphorus flame retardants by solid-phase extraction and gas chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2009;395(4):1167–71. doi: 10.1007/s00216-009-3064-6. [DOI] [PubMed] [Google Scholar]
- 30.Pleil JD, Stiegel MA, Fent KW. Exploratory breath analyses for assessing toxic dermal exposures of firefighters during suppression of structural burns. J Breath Res. 2014;8(3):037107. doi: 10.1088/1752-7155/8/3/037107. [DOI] [PubMed] [Google Scholar]
- 31.Fent KW, Eisenberg J, Snawder J, Sammons D, Pleil JD, Stiegel MA, et al. Systemic exposure to PAHs and benzene in firefighters suppressing controlled structure fires. Ann Occup Hyg. 2014;58(7):830–45. doi: 10.1093/annhyg/meu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Casarett and Doull’s toxicology: the basic science of poisons. Vol. 8. New York: McGraw-Hill Education; 2013. [Google Scholar]
- 33.Davis MD, Wade EL, Restrepo PR, Roman-Esteva W, Bravo R, Kuklenyik P, et al. Semi-automated solid phase extraction method for the mass spectrometric quantification of 12 specific metabolites of organophosphorus pesticides, synthetic pyrethroids, and select herbicides in human urine. J Chromatogr B. 2013;929:18–26. doi: 10.1016/j.jchromb.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 34.Kuklenyik P, Baker SE, Bishop AM, Morales-A P, Calafat AM. Online solid phase extraction-high performance liquid chromatography–isotope dilution–tandem mass spectrometry approach to quantify N, N-diethyl-m-toluamide and oxidative metabolites in urine. Anal Chim Acta. 2013;787:267–73. doi: 10.1016/j.aca.2013.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petropoulou SSE, Petreas M, Park JS. Analytical methodology using ion-pair liquid chromatography-tandem mass spectrometry for the determination of four di-ester metabolites of organophosphate flame retardants in California human urine. J Chromatogr A. 2016;1434:70–80. doi: 10.1016/j.chroma.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 36.Butt CM, Hoffman K, Chen A, Lorenzo A, Congleton J, Stapleton HM. Regional comparison of organophosphate flame retardant (PFR) urinary metabolites and tetrabromobenzoic acid (TBBA) in mother-toddler pairs from California and New Jersey. Environ Int. 2016 doi: 10.1016/j.envint.2016.06.029. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Su G, Letcher RJ, Yu H, Gooden DM, Stapleton HM. Determination of glucuronide conjugates of hydroxyl triphenyl phosphate (OH-TPHP) metabolites in human urine and its use as a biomarker of TPHP exposure. Chemosphere. 2016;149:314–9. doi: 10.1016/j.chemosphere.2016.01.114. [DOI] [PubMed] [Google Scholar]
- 38.Matuszewski BK. Standard line slopes as a measure of a relative matrix effect in quantitative HPLC–MS bioanalysis. J Chromatogr B. 2006;830(2006):293–300. doi: 10.1016/j.jchromb.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003;75(13):3019–30. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 40.Caudill SP, Schleicher RL, Pirkle JL. Multi-rule quality control for the age-related eye disease study. Stat Med. 2008;27(20):4094–106. doi: 10.1002/sim.3222. [DOI] [PubMed] [Google Scholar]
- 41.Taylor JK. Quality assurance of chemical measurements. Chelsea: Lewis Publishers; 1987. [Google Scholar]