Abstract
Certain per- and polyfluoroalkyl substances (PFASs) are suspected developmental toxicants, but data on PFAS concentrations and exposure routes in children are limited. We measured plasma PFASs in children aged 6–10 years from the Boston-area Project Viva prebirth cohort, and used multivariable linear regression to estimate associations with sociodemographic, behavioral, and health-related factors, and maternal PFASs measured during pregnancy. PFAS concentrations in Project Viva children (sampled 2007–2010) were similar to concentrations among youth participants (aged 12–19 years) in the 2007–8 and 2009–10 National Health and Nutrition Examination Survey (NHANES); mean concentrations of most PFASs declined from 2007 to 2010 in Project Viva and NHANES. In mutually adjusted models, predictors of higher PFAS concentrations included older child age, lower adiposity, carpeting or a rug in the child's bedroom, higher maternal education, and higher neighborhood income. Concentrations of perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), perfluorohexanesulfonate (PFHxS), and 2-(N-methyl-perfluorooctane sulfonamido) acetate (Me-PFOSA-AcOH) were 26-36% lower in children of black mothers compared to children of white mothers and increased 12–21% per interquartile range increase in maternal pregnancy PFASs. Breastfeeding duration did not predict childhood PFAS concentrations in adjusted multivariable models. Together, the studied predictors explained the observed variability in PFAS concentrations to only a modest degree.
Graphical abstract
Per- and polyfluoroalkyl substances (PFASs) are a class of synthetic compounds employed since the 1950s in a range of industrial and consumer products, including stain-resistant fabric and carpet treatments and oil-resistant coatings for food packaging.1–3 Certain PFASs are persistent in the environment, accumulate in the human body, and have been widely detected in human biosamples.2,4,5 Though evidence on the developmental health effects of PFASs is mixed, early life exposure to PFASs has been linked in some studies to neurobehavioral problems,6–9 immune suppression,10,11 and increased adiposity12–14 in children.
Exposure to PFASs for the general population can occur through use of PFAS-containing products and contact with PFASs in household dust and air.2,15–17 The use of PFASs in food packaging as well as their presence in some foods suggests dietary exposures may also be important.2,4,15,16 Young children, who have increased hand-to-mouth activity and exposure to dust on household surfaces and greater food consumption relative to body weight, may have higher uptake of PFAS than adults.15 Breastfeeding may also be an important exposure pathway for infants and toddlers.18–20
Evidence on factors influencing PFAS concentrations in children is limited. PFASs are known to cross the placenta,21 and concentrations of PFASs in maternal peripheral blood correlate highly with infant cord blood concentrations,22–24 suggesting that placental transfer could represent an exposure route for children. Prior studies have also reported higher concentrations of PFASs among children with longer duration of breastfeeding.18,25,26 In studies that examined PFAS concentrations in mothers and children aged 2–826 and 6–1127 years, mother and child levels measured at the same time point were moderately to highly correlated, with concentrations higher among children.
Though some studies have reported higher PFAS concentrations in boys28,29 and older children,18,28,30 others have not observed consistent patterns by sex18,30 or age.25,26 A small study investigating potential predictors of serum PFASs in young children (age 2–8 years, n = 68) in California identified residential dust PFAS levels and frequency of wearing waterproof clothes as significant predictors; dietary predictors varied across measured PFAS analytes, but included consumption of canned and fresh fish, French fries, hot dogs, chips, and microwave popcorn.26
Further research is needed to characterize factors affecting PFAS concentrations in children, who appear to have higher PFAS body burdens than adults and may also be particularly sensitive to potential developmental toxicity. In a large prospective cohort of mothers and children followed from pregnancy onward, we measured plasma concentrations of PFASs in children (aged 6–10 years), and examined associations with potential predictors including demographic characteristics, behavioral, and health-related factors, and maternal PFAS concentrations measured in pregnancy.
Materials and Methods
Study Population
Participants were drawn from Project Viva, a prospective prebirth cohort that enrolled pregnant mothers from 1999 to 2002 at their first prenatal visits at Atrius Harvard Vanguard Medical Associates, a multispecialty group practice in urban and suburban Eastern Massachusetts.31 Of 2128 enrolled mothers with live single births, 1668 contributed blood samples during early pregnancy (median 9.7 weeks gestation, range 4.8–21.4) in 1999–2000. At visits in midchildhood (median age 7.7 years, range 6.6–10.6) in 2007–2010, a subset of children (n = 702) contributed blood samples. The Human Subjects Committees of participating institutions approved all study protocols. All mothers provided written informed consent, and children provided verbal assent at the midchildhood visit. The involvement of the Centers for Disease Control and Prevention (CDC) laboratory did not constitute engagement in human subjects research. All study forms are available at https://www.hms.harvard.edu/viva/.
Maternal and Child PFAS Measurements
Maternal and child plasma samples were stored in cryovial (non-PFAS containing) tubes in liquid nitrogen freezers. Samples were subsequently thawed, aliquoted, shipped to the Division of Laboratory Sciences at the Centers for Disease Control and Prevention (CDC) and analyzed for concentrations of 8 PFAS analytes: perfluorooctanesulfonate (PFOS), perfluorooctanoate (PFOA), perfluorohexanesulfonate (PFHxS), perfluorononanoate (PFNA), 2-(N-ethyl-perfluorooctane sulfonamido) acetate (Et-PFOSA-AcOH; also known as EtFOSAA), 2-(N-methyl-perfluorooctane sulfonamido) acetate (Me-PFOSA-AcOH; also known as MeFOSAA), perfluorodecanoate (PFDeA; also known as PFDA), and perfluorooctane sulfonamide (PFOSA; also known as FOSA). Analyses of maternal plasma were conducted in 2014 and have been previously described. Child plasma samples were analyzed in 2015 using online solid-phase extraction coupled with isotope dilution high-performance liquid chromatography-tandem mass spectrometry. Low-concentration quality control materials (QCs) and high-concentration QCs, prepared from a calf serum pool, were analyzed with the study samples, analytical standards, and with reagent and serum blanks to ensure the accuracy and reliability of the data. Analytical methods for the maternal and child samples were the same as those used to analyze PFAS concentrations in NHANES samples for the 2011–2012 and 2013–2014 NHANES cycles, respectively. 4, Child plasma samples were also analyzed for concentrations of linear and branched isomers of PFOA [n-perfluorooctanoate (n-PFOA), branched perfluorooctanoates (Sb-PFOA) ], and PFOS [n-perfluorooctanesulfonate (n-PFOS), perfluoromethylheptanesulfonates (Sm-PFOS), perfluorodimethylhexanesulfonates (Sm2-PFOS) ]. A total of 653 child plasma samples had sufficient volume for PFAS quantification and limits of detection (LOD) were 0.1 ng/mL; values below the LOD were estimated as LOD/√2 (0.0707 ng/mL).
Predictor Data
Project Viva study staff collected data on participant demographics and health-related behaviors via study questionnaires and interviews. We included data on socio-demographic characteristics, health history, and behavioral factors that we hypothesized might serve as potential predictors of PFAS plasma concentrations in childhood.
At the midchildhood visit, mothers were asked about child participants' fast food consumption and the presence of carpeting or rugs in the rooms where the child “usually sleeps” and “spends most awake time”. Mothers also reported on children's outdoor activities in summer and winter (“In the past year, how much do your child's activities (playing outdoors, playing sports, spectator sports, etc.) take him/her outside?”) with possible responses “1. not that often,” “2. a moderate amount,” “3. quite a lot,” and “4. virtually all the time.” Based on response frequency, we categorized outdoor activity time as low (1 or 2 for summer/1 for winter), medium (3 for summer/2 for winter) or high (4 for summer/3 or 4 for winter).
Project Viva staff measured children's height and weight at the midchildhood visit. We calculated body mass index (BMI) as weight in kilograms/(height in meters)2 and calculated BMI percentiles for age and sex using 2000 CDC reference data. Per CDC guidelines, we categorized BMI as underweight/normal weight (<85%), overweight (85-<95%), or obese (>95%). Median household income for the census tract of residence at the time of the midchildhood visit was determined using geocoded home addresses and data from the 2000 U.S. Census.
Statistical Analysis
We assessed PFOS and PFOA as total concentrations (sum of linear and branched isomers), and also examined predictors of n-PFOS and Sm-PFOS (Sm2-PFOS was excluded due to low detection frequency; n-PFOA and Sb-PFOA were not analyzed separately because Sb-PFOA represented only a small share (4% on average) of total PFOA). We calculated descriptive statistics and evaluated correlations among PFASs by calculating Spearman rank correlation coefficients. We also assessed collinearity among model covariates by calculating variance inflation factors.
We ran bivariable linear regression models examining the independent influence of potential predictors on natural-log transformed concentrations of total PFOS, n-PFOS, Sm-PFOS, total PFOA, PFHxS, PFNA, PFDeA, and Me-PFOSA-AcOH. We also ran bivariable models examining the associations of child concentrations of each PFAS analyte with maternal pregnancy concentrations of the same PFAS analyte.
We fitted multivariable linear regression models to examine associations of predictors with log-transformed child PFAS concentrations, adjusted for all other predictors. Because we hypothesized that maternal PFAS concentrations might lie on the causal pathway between some prenatal predictors (such as maternal education level) and childhood PFAS concentrations, primary multivariable models excluded maternal PFAS concentrations in pregnancy. In secondary analyses, we fitted multivariable linear regression models for each childhood PFAS analyte with additional adjustment for maternal pregnancy concentrations for the corresponding PFAS analyte. Given the low frequency of detection for PFDeA in maternal plasma (45%), we did not run models including maternal PFAS for PFDeA; isomers of PFOA and PFOS were not measured in maternal plasma, so we did not examine associations with childhood PFAS isomers. To maximize sample size and improve model precision, all multivariable models excluded child race and paternal education; these variables were closely related to maternal race and maternal education, respectively, but maternal race and maternal education had lower percentages of missing data. For all linear regression results, we estimated percent difference in PFAS concentrations associated with each predictor by exponentiating regression coefficients, subtracting 1, and multiplying by 100%. We performed analyses in SAS Version 9.4 (SAS Institute Inc., Cary, NC).
Results
PFAS Concentrations
PFAS concentrations had skewed distributions, so we present geometric means (Table 1). We detected PFOS, PFOA, PFHxS, and PFNA in >99% of samples. PFDeA and Me-PFOSA-AcOH were detectable in most samples (88.2% and 65.9%, respectively), whereas only a small percentage of samples had detectable levels of Et-PFOSA-AcOH (5.2%) and PFOSA (0.9%). Given low detection frequencies, Et-PFOSA-AcOH and PFOSA measures were excluded from further analyses. Correlations among measured PFASs varied (Spearman correlation coefficients 0.14-0.78), with PFOS and PFOA most highly correlated (Table 2).
Table 1. Summary Statistics for PFAS Concentrations (ng/mL) Measured in Project Viva Midchildhood (Age 6–10 Years) Plasma Samples (N = 653)a.
| PFAS analyte (ng/mL) | N (%) detect | geometric mean | minimum | 10th percentile | median | 90th percentile | maximum |
|---|---|---|---|---|---|---|---|
| PFOS (sum) | 650 (99.5) | 6.2 | <LOD | 2.8 | 6.2 | 13.7 | 51.4 |
| n-PFOS | 650 (99.5) | 4.5 | <LOD | 2.0 | 4.4 | 9.9 | 34.2 |
| Sm-PFOS | 650 (99.5) | 1.7 | <LOD | 0.7 | 1.7 | 4.0 | 16.8 |
| Sm2-PFOS | 9 (1.4) | <LOD | <LOD | <LOD | <LOD | <LOD | 0.4 |
| PFOA (sum) | 650 (99.5) | 4.2 | <LOD | 2.3 | 4.4 | 7.9 | 14.3 |
| n-PFOA | 650 (99.5) | 4.0 | <LOD | 2.3 | 4.1 | 7.4 | 13.8 |
| Sb-PFOA | 376 (57.6) | 0.2 | <LOD | <LOD | 0.2 | 0.6 | 2.4 |
| PFHxS | 650 (99.5) | 2.2 | <LOD | 0.8 | 1.9 | 7.0 | 56.8 |
| PFNA | 650 (99.5) | 1.7 | <LOD | 0.8 | 1.5 | 3.8 | 25.7 |
| Et-PFOSA-AcOH | 34 (5.2) | <LOD | <LOD | <LOD | <LOD | <LOD | 1.4 |
| Me-PFOSA-AcOH | 424 (64.9) | 0.3 | <LOD | <LOD | 0.3 | 1.3 | 6.7 |
| PFDeA | 576 (88.2) | 0.3 | <LOD | <LOD | 0.3 | 0.6 | 1.9 |
| FOSA | 6 (0.9) | <LOD | <LOD | <LOD | <LOD | <LOD | 0.5 |
Limit of detection (LOD) = 0.1 ng/mL.
Table 2. Spearman Correlation Coefficients for PFAS Analytes Measured in Project Viva Midchildhood (Age 6–10 Years) Plasma Samples (N = 653)a.
| PFOS | PFOA | PFHxS | PFNA | Me-PFOSA-AcOH | PFDeA | |
|---|---|---|---|---|---|---|
| PFOS | 1 | |||||
| PFOA | 0.78 | 1 | ||||
| PFHxS | 0.66 | 0.60 | 1 | |||
| PFNA | 0.34 | 0.43 | 0.14 | 1 | ||
| Me-PFOSA-AcOH | 0.62 | 0.50 | 0.36 | 0.23 | 1 | |
| PFDeA | 0.59 | 0.69 | 0.34 | 0.56 | 0.31 | 1 |
All correlation coefficient p-values <0.0001.
Sociodemographic, Behavioral, and Health-Related Predictors
Studied potential predictors and participant characteristics are outlined in Table 3. Supporting Information (SI) Table S1 presents unadjusted associations of potential predictors with child PFAS concentrations. Unadjusted associations of predictors with linear and branched PFOS isomers (n-PFOS and sm-PFOS) were very similar to those for total PFOS (SI Table S2). In multivariable models mutually adjusted for all sociodemographic, behavioral, and health-related predictors (Table 4), concentrations of PFOA, PFOS, and PFHxS were lower in later sampling years, while PFNA concentrations were lowest in 2007 (the first year of sampling). PFOA and PFDeA concentrations were lower among children with higher BMI. Children who were older at blood draw had higher concentrations of PFOS and PFHxS. Older maternal age was associated with higher Me-PFOSA-AcOH concentrations, but not with other PFASs. PFOA, PFOS, PFHxS, and MeFOSAA concentrations were lower among children of black mothers. PFOS and PFHxS concentrations were higher among mothers with higher educational attainment. Living in a higher income census tract was associated with higher PFOS, PFDeA, and Me-PFOSA-AcOH, but concentrations of PFHxS were lower among those with higher family income (family income was not strongly associated with concentrations of other PFASs).
Table 3. Characteristics of Study Participants (n = 653).
| mean ± SD or N (%) | ||
|---|---|---|
| Child Characteristics | ||
| Child's Age at Blood Draw (years) | 7.9 ± 0.8 | |
| Year of Blood Draw | ||
| 2007 | 68 (10) | |
| 2008 | 223 (34) | |
| 2009 | 207 (32) | |
| 2010 | 155 (24) | |
| Sex | ||
| male | 354 (53) | |
| female | 308 (47) | |
| Race/Ethnicity | ||
| white | 366 (56) | |
| black | 115 (18) | |
| other | 111 (17) | |
| missing | 61 (9) | |
| Breastfeeding Duration | ||
| <3 months | 207 (32) | |
| 3-<6 months | 66 (10) | |
| ≥6 months | 325 (50) | |
| missing | 55 (8) | |
| BMI in Midchildhood (Percentile) | ||
| underweight/normal (<85th) | 464 (72) | |
| overweight (85-<95th) | 85 (13) | |
| obese (≥95th) | 96 (15) | |
| missing | 8 (1) | |
| fast food consumption | ||
| <1 per month | 120 (18) | |
| 1–3 times per month | 278 (43) | |
| once per week | 175 (27) | |
| ≥2 times per week | 46 (7) | |
| missing | 36 (6) | |
| Time Spent Outdoors (In Summer) | ||
| low | 110 (17) | |
| medium | 303 (46) | |
| high | 204 (31) | |
| missing | 36 (6) | |
| Time Spent Outdoors (In Winter) | ||
| low | 194 (30) | |
| medium | 338 (52) | |
| high | 84 (13) | |
| missing | 37 (6) | |
| Parental Characteristics | ||
| Maternal Age at Enrollment | 31.8 ± 5.6 | |
| Maternal Race/Ethnicity | ||
| white | 404 (62) | |
| black | 135 (21) | |
| other | 110 (17) | |
| missing | 4 (1) | |
| Maternal Education | ||
| <college | 229 (35) | |
| college | 235 (36) | |
| >college | 185 (28) | |
| missing | 4 (1) | |
| Paternal Education | ||
| <college | 202 (31) | |
| college | 203 (31) | |
| >college | 170 (26) | |
| missing | 78 (12) | |
| Mother Married or Cohabitating | ||
| no | 71 (11) | |
| yes | 577 (88) | |
| missing | 5 (1) | |
| Maternal Parity | ||
| 0 | 276 (42) | |
| ≥1 | 377 (58) | |
| Household Characteristics | ||
| Annual Household Income | ||
| ≤$40K | 90 (14) | |
| $>40—≤70K | 96 (15) | |
| $>70—≤150K | 274 (42) | |
| >$150K | 148 (23) | |
| missing | 45 (7) | |
| Carpeting or a Rug in Room Where Child Sleeps | ||
| no | 204 (31) | |
| yes | 412 (63) | |
| missing | 37 (6) | |
| Carpeting or a Rug in Room Where Child Spends Most Awake Time | ||
| no | 197 (30) | |
| yes | 415 (64) | |
| missing | 41 (6) | |
| Neighborhood Characteristics | ||
| Census Tract Median Household Income | $63,204 ± 23,695 | |
Table 4. Adjusted Associations from Multivariable Linear Regression Models for Predictors of PFAS Concentrations in Project Viva Midchildhood (age 6–10 years) Plasma Samples (n = 545).
| % difference in PFAS concentration (95% CI) | ||||||
|---|---|---|---|---|---|---|
|
|
||||||
| PFOA | PFOS | PFHxS | PFNA | PFDeA | Me-PFOSA-AcOH | |
| Child Characteristics | ||||||
| Child's Age at Blood Draw (years) | 2.5 (−4.4, 9.9) | 8.6 (−0.9, 18.9) | 15.3 (1.6, 30.9) | −0.4 (−9.9, 10.1) | −2.1 (−11.0, 7.6) | 5.8 (−10.0, 24.6) |
| Year of Blood Draw | ||||||
| 2007 | ref | ref | ref | ref | ref | ref |
| 2008 | 5.7 (−7.9, 21.3) | −4.1 (−19.9, 14.7) | 10.4 (−14.0, 41.7) | 40.5 (15.4, 71.1) | 12.8 (−6.5, 36.0) | 0.1 (−27.4, 37.9) |
| 2009 | −17.1 (−27.8, −4.8) | −28.8 (−40.6, −14.7) | −23.8 (−40.8, −2.1) | 34.5 (10.3, 64.1) | −1.8 (−18.6, 18.6) | −35.1 (−53.0, −10.4) |
| 2010 | −31.0 (−42.1, −17.8) | −38.5 (−51.1, −22.6) | −43.9 (−59.2, −22.8) | 29.1 (0.4, 66.0) | −5.3 (−25.4, 20.3) | −46.3 (−64.3, −19.1) |
| Sex | ||||||
| male | ref | ref | ref | ref | ref | ref |
| female | 2.7 (−5.4, 11.5) | −2.6 (−12.4, 8.5) | −12.5 (−24.6, 1.6) | 5.4 (−6.3, 18.5) | 4.8 (−6.2, 17.2) | −0.8 (−18.1, 20.1) |
| Breastfeeding Duration | ||||||
| <3 months | ref | ref | ref | ref | ref | ref |
| 3–<6 months | −4.4 (−17.1, 10.2) | −5.2 (−21.3, 14.1) | 0.4 (−22.5, 30.0) | −6.8 (−24, 14.2) | −8.1 (−24.3, 11.6) | −4.9 (−31.7, 32.6) |
| ≥6 months | 2.6 (−6.7, 12.9) | 8.4 (−4.3, 22.7) | 10.9 (−6.6, 31.8) | −12.1 (−23.3, 0.8) | −12.6 (−23.2, −0.5) | 3.9 (−16.7, 29.6) |
| BMI in Midchildhood (Percentile) | ||||||
| underweight/normal (<85th) | ref | ref | ref | ref | ref | ref |
| overweight (85−<95th) | −10.1 (−20.3, 1.5) | −9.7 (−22.9, 5.7) | −16.9 (−33.3, 3.5) | −0.9 (−16.7, 17.9) | −2.8 (−17.6, 14.6) | −6.7 (−29.6, 23.7) |
| obese (≥95fh) | −12.1 (−22.4, −0.5) | −7.2 (−21.1, 9.1) | −8.0 (−26.6, 15.2) | 3.2 (−13.6, 23.3) | −15.6 (−28.7, −0.1) | −1.2 (−26.0, 31.9) |
| Fast Food Consumption | ||||||
| <1 per month | ref | ref | ref | ref | ref | ref |
| 1—3 times per month | 3.9 (−6.8, 15.8) | −2.8 (−15.6, 11.9) | 6.4 (−12.6, 29.6) | 9.2 (−6.5, 27.6) | 1.2 (−12.6, 17.3) | 0.6 (−21.8, 29.5) |
| once per week | −1.9 (−13.0, 10.6) | −9.0 (−22.2, 6.4) | 3.4 (−16.8, 28.6) | 4.7 (−11.8, 24.4) | 1.1 (−14.2, 19.0) | −10.5 (−32.4, 18.3) |
| ≥2 times per week | 13.1 (−4.9, 34.4) | 4.3 (−16.8, 30.7) | −1.0 (−27.7, 35.6) | 18.3 (−7.7, 51.6) | 6.3 (−16.0, 34.6) | 35.0 (−9.9, 102.2) |
| Time Spent Outdoors (In Summer) | ||||||
| low | ref | ref | ref | ref | ref | ref |
| medium | 12.0 (−0.7, 26.4) | 17.4 (0.3, 37.4) | 17.1 (−6.0, 45.8) | 13.0 (−4.9, 34.4) | 18.1 (0.2, 39.2) | 14.5 (−13.6, 51.7) |
| high | 14.5 (0.2, 30.9) | 17.5 (−1.3, 39.9) | 6.2 (−16.7, 35.3) | 19.7 (−1.2, 45.0) | 26.9 (5.8, 52.2) | 29.5 (−5.2, 76.9) |
| Time Spent Outdoors (In Winter) | ||||||
| low | ref | ref | ref | ref | ref | ref |
| medium | −1.7 (−10.8, 8.3) | −4.4 (−15.8, 8.5) | −15.0 (−28.8, 1.5) | 2.8 (−10.6, 18.2) | −7.5 (−19.0, 5.6) | −13.9 (−31.4, 8.0) |
| high | −9.3 (−21.3, 4.4) | −20.5 (−33.8, −4.4) | −0.5 (−23.0, 28.6) | −0.1 (−18.4, 22.2) | −11.6 (−27.1, 7.1) | −25.6 (−46.5, 3.4) |
| Maternal Characteristics | ||||||
| Age at Enrollment (per 5 years) | −3.0 (−7.5, 1.7) | −1.0 (−6.9, 5.3) | −1.1 (−9.3, 7.8) | −0.8 (−7.3, 6.2) | −1.9 (−8.0, 4.7) | 12.5 (0.7, 25.6) |
| Race/Ethnicity | ||||||
| white | ref | ref | ref | ref | ref | ref |
| black | −26.0 (−34.7, −16.2) | −27.4 (−38.3, −14.5) | −36.2 (−49.2, −20.0) | −3.7 (−19.5, 15.1) | −6.9 (−21.5, 10.3) | −32.7 (−49.7, −10.0) |
| other | −12.6 (−23.1, −0.8) | −11.2 (−24.8, 4.8) | −36.3 (−49.4, −19.8) | 11.2 (−7.3, 33.4) | −1.8 (−17.4, 16.7) | −23.7 (−43.3, 2.6) |
| Education | ||||||
| <college | ref | ref | ref | ref | ref | ref |
| college | 7.0 (−4.6, 20.0) | 12.3 (−3.3, 30.4) | 24.3 (0.9, 53.0) | 10.3 (−6.4, 29.9) | 6.2 (−9.2, 24.1) | −13.9 (−31.4, 8.0) |
| Education | ||||||
| >college | 12.3 (−1.1, 27.5) | 20.3 (1.8, 42.0) | 38.2 (9.7, 74.2) | 3.5 (−13.7, 24.3) | 11.0 (− 6.6, 32.1) | −25.6 (−46.5, 3.4) |
| Married or Cohabitating | ||||||
| no | ref | ref | ref | ref | ref | ref |
| yes | 8.1 (−7.9, 26.9) | 2.1 (−17.1, 25.8) | −1.4 (−26.3, 31.8) | −4.4 (−24.0, 20.2) | 2.9 (−17.2, 28.0) | −3.4 (−33.5, 40.4) |
| Parity | ||||||
| 0 | ref | ref | ref | ref | ref | ref |
| ≥1 | 8.7 (−0.5, 18.7) | 2.0 (−9.0, 14.5) | −0.5 (−15.2, 16.8) | 4.6 (−7.8, 18.7) | 3.8 (−7.9, 17.1) | 2.8 (−16.3, 26.3) |
| Household Characteristics | ||||||
| Annual Household Income | ||||||
| ≤$40K | ref | ref | ref | ref | ref | ref |
| $>40-≤70K | −8.6 (−22.4, 7.7) | 4.8 (−15.4, 29.8) | −3.9 (−28.6, 29.5) | 0.5 (−20.6, 27.1) | 0.3 (−19.8, 25.4) | 18.8 (−18.9, 74.2) |
| $>70−≤150K | −5.4 (−19.2, 10.7) | −10.7 (−27.3, 9.6) | −20.5 (−40.3, 5.8) | 1.9 (−18.7, 27.8) | −8.9 (−26.5, 13.0) | −11.4 (−38.6, 27.9) |
| >$1S0K | −6.7 (−22, 11.5) | −13.8 (−31.8, 8.9) | −27.7 (−47.8, 0.1) | 3.3 (−20.1, 33.6) | −8.4 (−28.2, 16.9) | −9.0 (−40.0, 38.2) |
| Carpeting or a Rug in Room Where Child Sleeps | ||||||
| no | ref | ref | ref | ref | ref | ref |
| yes | 8.1 (−1.7, 18.8) | 18.5 (4.8, 34.0) | 14.5 (−3.5, 35.9) | −0.2 (−12.9, 14.2) | 10.0 (−3.2, 25.1) | 23.8 (−0.6, 54.3) |
| Carpeting or a Rug in Room Where Child Spends Most Awake Time | ||||||
| no | ref | ref | ref | ref | ref | ref |
| yes | −1.4 (−10.6, 8.7) | −1.2 (−13.0, 12.2) | 1.2 (−15.3, 20.7) | −7.1 (−19.2, 6.8) | −13.9 (−24.6, −1.7) | −6.5 (−25.5, 17.3) |
| Neighborhood Characteristics | ||||||
| Census Tract Median Household Income (per $10,000 increase) | 1.5 (−0.5, 3.6) | 2.8 (0.2, 5.6) | 0.7 (−2.9, 4.4) | 0.9 (−2.0, 3.8) | 4.1 (1.3, 7.0) | 4.6 (−0.2, 9.6) |
| Model R2 | 0.25 | 0.22 | 0.19 | 0.05 | 0.08 | 0.16 |
Sleeping in a room with carpet or a rug was associated with higher levels of PFOS, PFHxS, and Me-PFOSA-AcOH, but spending awake time in a room with carpet or a rug was not predictive of higher PFASs; PFDeA concentrations were actually lower among these children. Longer breastfeeding duration predicted somewhat lower concentrations of PFNA and PFDeA, but higher concentrations of sm-PFOS (see below). PFOA, PFNA, and Me-PFOSA-AcOH concentrations were somewhat elevated among those with high intake of fast food (≥2 times weekly), but measures of association were imprecise due to the small number of participants in this group (7%).
Maternal marital/cohabitation status, parity, and child sex did not predict PFAS concentrations. Adjusted associations of predictors with linear and branched PFOS isomers (n-PFOS and sm-PFOS) were generally similar to those for total PFOS, though breastfeeding ≥6 months versus <3 months was associated with an increase in sm-PFOS concentrations of 14.7% (95% confidence interval (CI): 2.0, 28.9) (see SI Table S2), while only a suggestive increase was observed for total PFOS (8.4%; 95% CI: −4.3, 22.7).
Multivariable model R2 values are presented in Table 4. Together, sociodemographic, behavioral and health-related predictors explained between 5% (PFNA) and 25% (PFOA) of variance in childhood PFAS concentrations. Variance inflation factors were <2.5 for all covariates included in multivariable models, suggesting that collinearity among covariates did not substantially reduce precision of effect estimates.39
Maternal PFAS Concentrations in Pregnancy As Predictors of Childhood PFASs
In the subset of the cohort for whom measures of PFAS concentrations in maternal plasma from pregnancy were available (n = 440) (SI Table S3), maternal PFAS concentrations in pregnancy were positively associated with concentrations of the corresponding PFAS in children for all studied PFASs except PFNA (Table 5). In multivariable models, interquartile range (IQR) increases in maternal pregnancy PFASs were associated with increases in childhood PFASs ranging from 12.0% (95% CI: 3.9–20.6) for PFOS to 21.2% (95% CI: 9.9–33.7) for Me-PFOSA-AcOH. Associations of other studied predictors with PFASs did not differ meaningfully between primary multivariable models and those including maternal PFAS concentrations.
Table 5. Adjusted Associations from Multivariable Linear Regression Models for Predictors of PFAS Concentrations in Project Viva Midchildhood (Age 6–10 years) Plasma Samples, Additionally Adjusted for PFAS Concentrations in Maternal Plasma from Pregnancy (n = 439).
| % difference in PFAS concentration (95% CI) | |||||
|---|---|---|---|---|---|
|
|
|||||
| PFOA | PFOS | PFHxS | PFNA | Me-PFOSA-AcOH | |
| Maternal PFAS Concentration (per interquartile range increase) | 14.3 (7.2, 22.0) | 12.0 (3.9, 20.6) | 18.7 (13.4, 24.3) | 3.0 (−4.6, 11.3) | 21.2 (9.9, 33.7) |
| Child Characteristics | |||||
| Cliild'S Age at Blood Draw (years) | 2.3 (−5.7, 11.0) | 9.3 (−2.0, 21.9) | 18.6 (3.5, 35.9) | 0.1 (−10.8, 12.4) | 10.1 (−8.4, 32.3) |
| Year of Blood Draw | |||||
| 2007 | ref | ref | ref | ref | ref |
| 2008 | 2.1 (−12.2, 18.6) | −3.9 (−21.3, 17.2) | 2.4 (−20.6, 32.1) | 31.9 (6.5, 63.3) | 5.1 (−25.3, 48.0) |
| 2009 | −18.0 (−29.6, −4.3) | −25.8 (−39.6, −8.9) | −21.9 (−39.8, 1.2) | 41.1 (13.3, 75.7) | −27.6 (−49.3, 3.4) |
| 2010 | −30.9 (−43.4, −15.5) | −38.7 (−53.2, −19.8) | −45.2 (−60.9, −23.2) | 28.1 (−3.8, 70.5) | −45.7 (−65.8, −13.8) |
| Sex | |||||
| male | ref | ref | ref | ref | ref |
| female | 3.7 (−5.8, 14.1) | −1.2 (−12.9, 12.2) | −12.0 (−25.1, 3.4) | 7.7 (−6.0, 23.5) | −3.2 (−22.1, 20.3) |
| Breastfeeding Duration | |||||
| <3 months | ref | ref | ref | ref | ref |
| 3—<6 months | −8.9 (−22.7, 7.4) | −3.3 (−22.2, 20.2) | −12.2 (−33.6, 16.2) | −13.1 (−31.2, 9.8) | 6.8 (−26.5, 55.2) |
| ≥6 months | 2.2 (−8.6, 14.3) | 9.4 (−5.5, 26.7) | 2.2 (−15.0, 23.0) | −17.3 (−29.3, −3.3) | 3.1 (−19.7, 32.3) |
| BMI in Midchildhood (Percentile) | |||||
| underweight/normal (<85th) | ref | ref | ref | ref | ref |
| overweight (85−<95th) | −12.0 (−23.1, 0.8) | −13.9 (−28.1, 3.1) | −12.0 (−30.0, 10.7) | −2.2 (−19.4, 18.6) | −10.9 (−34.5, 21.3) |
| obese (≥95fh) | −16.4 (−27.3, −3.8) | −11.7 (−26.6, 6.3) | −8.5 (−27.7, 15.8) | 0.5 (−17.7, 22.6) | −10.8 (−35.2, 22.7) |
| Fast Food Consumption | |||||
| <1 per month | ref | ref | ref | ref | ref |
| 1—3 times per month | 3.1 (−8.9, 16.6) | −2.8 (−17.4, 14.5) | 3.4 (−16.1, 27.4) | 10.6 (−7.3, 31.8) | −2.0 (−25.9, 29.8) |
| once per week | 1.5 (−11.4, 16.4) | −8.6 (−23.7, 9.5) | −8.4 (−27.3, 15.4) | 9.0 (−10.3, 32.3) | −17.4 (−39.4, 12.8) |
| ≥2 times per week | 17.9 (−4.2, 45.0) | 3.9 (−21.0, 36.8) | −9.3 (−36.1, 28.7) | 27.5 (−5.1, 71.3) | 51.3 (−5.5, 142.3) |
| Time Spent Outdoors (In Summer) | |||||
| low | ref | ref | ref | ref | ref |
| medium | 8.1 (−6.2, 24.6) | 12.4 (−6.8, 35.6) | 6.8 (−15.9, 35.6) | 9.3 (−10.6, 33.8) | 11.7 (−19.1, 54.1) |
| high | 11.0 (−4.9, 29.5) | 13.6 (−7.4, 39.4) | 7.4 (−17.2, 39.4) | 21.4 (−2.6, 51.2) | 22.1 (−14.0, 73.4) |
| Time Spent Outdoors (In Winter) | |||||
| low | ref | ref | ref | ref | ref |
| medium | 0.3 (−10.3, 12.2) | −0.1 (−13.9, 16.0) | −17.0 (−31.2, 0.2) | 11.3 (−5.2, 30.6) | −4.2 (−25.6, 23.5) |
| high | −6.0 (−19.8, 10.3) | −18.5 (−34.1, 0.7) | −9.8 (−31.1, 18.2) | 7.8 (−14.2, 35.4) | −19.2 (−43.8, 16.2) |
| Maternal Characteristics | |||||
| Age at Enrollment (per 5 years) | −1.8 (−7.1, 3.7) | 0.5 (−6.5, 8.1) | 1.2 (−7.7, 11.1) | 1.4 (−6.2, 9.7) | 17.8 (3.9, 33.6) |
| Race/Ethnicity | |||||
| white | ref | ref | ref | ref | ref |
| black | −22.3 (−32.9, −10.0) | −23.9 (−37.2, −7.7) | −22.9 (−39.8, −1.2) | 0.5 (−18.3, 23.6) | −34.7 (−53.1, −9.2) |
| other | −10.0 (−22.6, 4.6) | −7.2 (−24.0, 13.4) | −29.3 (−45.2, −8.9) | 11.9 (−9.7, 38.6) | −15.2 (−39.7, 19.3) |
| Education | |||||
| <college | ref | ref | ref | ref | ref |
| college | 7.5 (−5.8, 22.7) | 10.3 (−7.4, 31.3) | 22.3 (−2.0, 52.6) | 5.1 (−12.8, 26.7) | 7.2 (−20.4, 44.4) |
| >college | 16.2 (0.1, 34.8) | 17.8 (−3.2, 43.5) | 31.9 (2.8, 69.2) | 1.5 (−17.7, 25.3) | 10.6 (−20.9, 54.7) |
| Married or Coliabitating | |||||
| no | ref | ref | ref | ref | ref |
| yes | −0.3 (−16.9, 19.6) | −3.5 (−24.0, 22.7) | −4.2 (−29.3, 30.0) | −8.8 (−29.4, 17.8) | −16.7 (−44.7, 25.4) |
| Parity | |||||
| 0 | ref | ref | ref | ref | ref |
| ≥1 | 15.6 (4.3, 28.0) | 6.1 (−7.2, 21.3) | 9.2 (−7.8, 29.3) | 5.8 (−8.8, 22.6) | 2.0 (−18.6, 27.8) |
| Household Characteristics | |||||
| Annual Household Income | |||||
| ≤$40K | ref | ref | ref | ref | ref |
| $>40−≤70K | −17.7 (−31.8, −0.6) | −1.2 (−23.0, 26.7) | −9.6 (−34.1, 24.1) | −5.2 (−27.4, 23.9) | 11.7 (−27.0, 70.9) |
| $>70−≤150K | −13.0 (−27.3, 4.2) | −17.3 (−34.8, 5.0) | −26.7 (−45.9, −0.7) | −2.0 (−24.2, 26.7) | −20.3 (−47.0, 19.9) |
| >$1S0K | −16.3 (−31.5, 2.2) | −19.2 (−37.9, 5.2) | −32.3 (−51.6, −5.3) | 2.0 (−23.3, 35.5) | −8.7 (−41.9, 43.5) |
| Carpeting or a Rug in Room Where Child Sleeps | |||||
| no | ref | ref | ref | ref | ref |
| yes | 7.1 (−4.0, 19.5) | 19.0 (2.8, 37.7) | 21.6 (1.0, 46.4) | 7.9 (−7.7, 26.2) | 26.4 (−1.6, 62.4) |
| Carpeting or a Rug in Room Where Child Spends Most Awake Time | |||||
| no | ref | ref | ref | ref | ref |
| yes | 5.3 (−5.9, 17.9) | 5.0 (−9.6, 21.8) | 5.0 (−13.2, 27.0) | −4.5 (−18.6, 12.1) | −3.2 (−25.0, 25.0) |
| Neighborhood Characteristics | |||||
| Census Tract Median Household Income (per $10,000 increase) | 2.2 (−0.2, 4.6) | 3.4 (0.2, 6.6) | 3.0 (−1.0, 7.1) | 0.8 (−2.6, 4.2) | 4.7 (−0.8, 10.4) |
| Model R2 | 0.27a | 0.21a | 0.27a | 0.07a | 0.19a |
R2 values for multivariable model excluding maternal pregnancy PFAS levels but restricted to n = 439 participants with available maternal PFAS levels were PFOA — 0.24, PFOS — 0.19, PFHxS — 0.18, PFNA − 0.07, Me-PFOSA-AcOH − 0.16.
R2 values for multivariable models including maternal PFAS concentrations in pregnancy are presented in Table 5. When compared to R2 values for multivariable models excluding maternal pregnancy PFAS concentrations but restricted to the 440 participants with available maternal PFAS measures (noted in Table 5), R2 values for the models including maternal pregnancy PFASs showed the greatest increase for PFHxS (from 0.18 to 0.27). For other PFASs, R2 increases due to inclusion of maternal pregnancy PFAS concentrations were more modest.
Discussion
In our study of children (aged 6–10 years) sampled in 2007–2010, sampling year was a strong predictor of PFAS levels, with mean concentrations of PFOA, PFOS, PFHxS, and MeFOSAA declining over our study period. Maternal levels of PFOA, PFOS, PFHxS, and MeFOSAA from pregnancy were associated with children's PFAS concentrations, though longer duration of breastfeeding did not predict higher childhood PFAS concentrations in multivariable models adjusted for potential confounders. Concentrations of certain PFASs were also higher among older child participants and those with lower BMI, carpeting or a rug in their bedrooms, higher maternal education level, and higher median income in the census tract of residence; maternal race/ethnicity was also related to child PFAS levels (concentrations were generally lowest in children of black mothers). High consumption of fast food (≥twice weekly) also predicted somewhat higher concentrations of PFOA, PFNA, and Me-PFOSA-AcOH, though these estimates were imprecise.
PFAS concentrations in Project Viva children aged 6–10 years (sampled from 2007 to 2010) were similar to concentrations observed in adolescent participants (aged 12–19 years) in the 2007–8 and 2009–10 cycles of the National Health and Nutrition Examination Survey (NHANES)40 (Figure 1; SI Table S4); PFASs were not assessed in younger NHANES participants. PFOS concentrations were somewhat lower in our cohort compared to corresponding NHANES sampling years among adolescents, whereas PFOA and PFNA concentrations were somewhat higher. The declines in mean PFOA, PFOS, PFHxS, and Me-PFOSA-AcOH concentrations that we observed over our 2007–2010 sampling period are generally consistent with temporal trends observed in NHANES and other populations.5,16 These declines likely relate to changes in PFAS production; the major U.S. manufacturer of PFOS completed a voluntary phase-out from 2000 to 2002 of PFOS and precursor chemicals that break down to PFOS, and U.S. manufacturers agreed to greatly reduce production of PFOA and precursors by 2010 and end production by 2015.1,5,16 The observed differences in PFAS concentrations over time highlight the importance of adjusting for sampling year to reduce potential confounding by temporal trends in studies of PFAS predictors or associations of PFASs with health outcomes.
Figure 1.
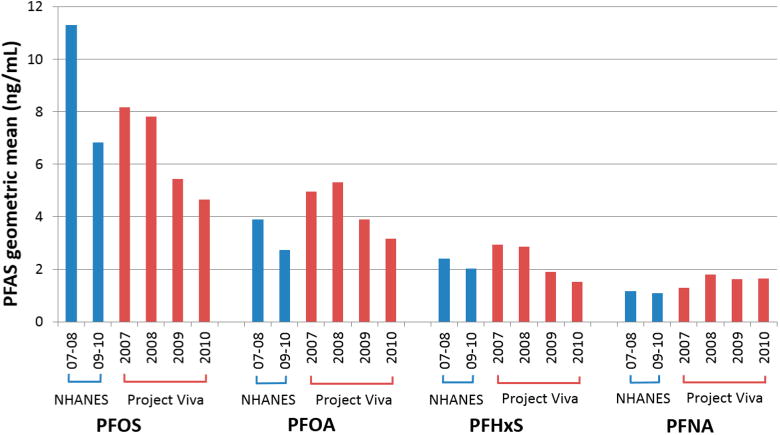
PFAS concentration geometric means by year of collection for Project Viva midchildhood (age 6–10 years) samples, and PFAS concentration geometric means for NHANES participants aged 12–19 years in 2007–2008 and 2009–2010 cycles.
In multivariable models adjusted for year of sampling and all studied sociodemographic, behavioral, and health-related predictors (Table 4), older age at blood draw was associated with higher PFHxS concentrations and suggestive increases in PFOS concentrations, but clear associations with age were not apparent for other PFASs. A study of 5–13 year olds in South Korea similarly observed higher concentrations of PFHxS and PFOS in older children,18 and other studies with sampling periods in the late 2000s have also reported trends of PFAS concentrations increasing with age in children.28,30 Among the studied PFASs, PFHxS has the longest estimated half-life in human serum (median 7.1 years, versus median 4.6 years for PFOS and median 3.4 years for PFOA);3 the higher concentrations of PFHxS and PFOS that we observed in older children may relate to those compounds' slower elimination rates and greater potential for bioaccumulation.
We observed lower concentrations of PFOA and PFDeA among obese children and trends also suggested somewhat lower concentrations of PFOS and PFHxS among overweight and obese children compared to normal/underweight children. Prior studies in South Korea18 and the United States25 also reported lower PFAS concentrations in children with higher BMI. Conversely, among mothers in our cohort, PFAS concentrations in pregnancy were higher in women with higher prepregnancy BMI.32 It should be noted that the cross-sectional associations we observed between midchildhood PFAS concentrations and BMI do not provide insight into potential causal relationships between early life PFAS exposure and later adiposity.
We observed evidence of higher PFAS concentrations among children of more highly educated mothers (PFOS and PFHxS) and those living in wealthier census tracts (PFOS and PFDeA), though family income was not consistently associated with PFAS levels (PFHxS concentrations were lower among those with higher family income). Higher PFAS concentrations have been observed among more socioeconomically advantaged participants in NHANES, which prior investigators have hypothesized may relate to patterns of use of PFAS-containing products and differences in dietary exposures to PFASs.4,45 In Project Viva, children of black and other race/ethnicity mothers also tended to have lower PFAS concentrations than children of white mothers. PFAS concentrations in NHANES were lowest among Mexican Americans, and PFOA concentrations were also lower among black participants compared to white participants; these associations were robust to adjustment for family income.45 Among a cohort of California girls (age 6–8 years), PFOA concentrations were lower among black, Asian, and Hispanic participants, but patterns by race were less consistent for other PFASs.25
Concentrations of PFOS, PFHxS, and Me-PFOSA-AcOH were somewhat higher among children who slept in a room with carpeting or a rug. Some PFASs were employed in carpet and upholstery stain protectants, and a case report documented high concentrations of PFHxS (range 27.5–423 ng/mL) and PFOS (range 15.2–108 ng/mL) in a Canadian family who frequently treated the carpets in their home, with the highest concentrations observed in the family's young children.41 In Project Viva, we also observed trends suggesting that PFOA, PFNA and MeFOSAA concentrations were higher among children with frequent consumption of fast food (≥twice weekly), although the small number of children in this highest consumption category limited the precision of effect estimates. Our finding is broadly consistent with research suggesting that consumption of food with oil-resistant packaging was associated with PFAS concentrations in adults42–44 and a study in which PFAS concentrations were higher among California children with greater consumption of hot dogs, French fries, chips, and microwave popcorn.26
In multivariable models, PFOS concentrations were higher among Viva children spending less time outdoors in winter; it is possible that time spent indoors could increase exposure to indoor sources of PFASs (such as fabric and carpet treatments). Conversely, spending less time outdoors during summer was associated with lower PFOA and PFDeA concentrations; the reason for this observed association is unclear. We are not aware of prior studies examining time spent indoors as a predictor of PFASs in children; further research could help elucidate the relationship of time spent outdoors versus indoors with PFAS exposure.
Maternal PFAS concentrations from pregnancy were associated with concentrations of the same PFASs measured in children. The magnitudes of these effect estimates were similar in bivariable and multivariable models, suggesting that the associations were not explained by other studied predictors with the potential to influence both child and maternal pregnancy PFAS concentrations (for example, maternal education), though shared dietary predictors or other shared exposures not accounted for in our multivariable models could be influential (if there was stability in shared exposures over the period between the pregnancy and childhood measures). The most substantial improvement in the multivariable model predictive power (R2) with the addition of maternal PFAS concentrations occurred in the model for PFHxS, which may relate to the relatively long half-life of PFHxS versus other studied PFASs. While prior studies have reported associations of maternal and child PFASs measured at the same time point,26,27 we are aware of no prior studies that have examined the relation of maternal PFAS concentrations from early pregnancy with later measures in children.
Although we observed associations of longer breastfeeding duration with higher PFASs in unadjusted bivariable models (SI Table S1), these associations generally were not observed in multivariable models, suggesting confounding by other studied variables. Branched isomers of PFOS (sm-PFOS) were positively associated with breastfeeding duration in multi-variable models, but total PFOS was only suggestively elevated among those with longer breastfeeding duration. While longer breastfeeding duration has been observed to increase PFAS concentrations in infants and young children,19,20 PFASs transferred through breastmilk may not persist in children's blood long enough to be detectable in older children. One prior study of PFAS predictors in South Korean children aged 5–13 years reported associations of higher PFASs with greater breastfeeding duration, but these analyses were not adjusted for other sociodemographic factors that may influence breastfeeding duration and PFAS concentrations.18
We observed no differences in PFAS concentrations by child sex in our population. In adult populations, PFAS concentrations are generally lower in females, but sex differences likely relate to PFAS excretion during pregnancy, breastfeeding,24 and menstruation,46 which are not relevant to prepubescent populations. Our results are consistent with those of other studies in young children where no sex differences in PFAS concentrations were observed,18,30 although higher PFASs among male children were observed in one study of older children (12–15 years)28 and another of children aged 2–12 years.29
The R2 values for our multivariable models were low, suggesting that factors beyond those included in our study may explain a substantial proportion of variation in PFAS concentrations among children; measurement error of our studied variables may also have limited the predictive power of our models. We lacked measures of PFAS concentrations in household dust and air, as well as other factors observed to predict PFAS concentrations in prior studies (such as frequency of wearing waterproof clothes). While Project Viva collected detailed longitudinal information on children's diet, due to the complexity of these data we plan to analyze dietary predictors separately.
In a population of children sampled in the late 2000s with plasma PFAS concentrations similar to those observed in youth participants of contemporaneous NHANES cycles, we observed variability in PFAS concentrations related to year of sampling, child age, child BMI, family sociodemographic factors, and maternal PFASs from pregnancy. Concentrations of certain PFASs were also higher among children with carpeting or a rug in their bedrooms and suggestively elevated in those frequently consuming fast food, suggesting that household furnishings and diet may represent potentially modifiable risk factors for PFAS exposure. Overall, however, the studied predictors explained the observed variability in PFAS concentrations to only a modest degree.
Supplementary Material
Acknowledgments
We thank the participants and staff of Project Viva. We also thank Kayoko Kato, Ayesha Patel, and Tao Jia for the PFAS measurements. This work was supported by grant funding from the National Institutes of Health: R01 ES021447, R01 HD034568, K24 HD069408, and P30 DK092924. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or any other body. The authors declare they have no competing financial interests.
Footnotes
Supporting Information: The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.est.6b05811.
Notes: The authors declare no competing financial interest.
References
- 1.Buck Rc, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SP. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr Environ Assess Manage. 2011;7(4):513–41. doi: 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lau C, Anitole K, Hodes C, Lai D, Pfahles-Hutchens A, Seed J. Perfluoroalkyl acids: a review of monitoring and toxicological findings. Toxicol Sci. 2007;99(2):366–94. doi: 10.1093/toxsci/kfm128. [DOI] [PubMed] [Google Scholar]
- 3.Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, Zobel LR. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect. 2007;115(9):1298–305. doi: 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jain RB. Contribution of diet and other factors to the levels of selected polyfluorinated compounds: data from NHANES 2003–2008. Int J Hyg Environ Health. 2014;217(1):52–61. doi: 10.1016/j.ijheh.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 5.Kato K, Wong LY, Jia LT, Kuklenyik Z, Calafat AM. Trends in exposure to polyfluoroalkyl chemicals in the U.S. Population: 1999–2008. Environ Sci Technol. 2011;45(19):8037–45. doi: 10.1021/es1043613. [DOI] [PubMed] [Google Scholar]
- 6.Gump BB, Wu Q, Dumas AK, Kannan K. Perfluorochemical (PFC) exposure in children: associations with impaired response inhibition. Environ Sci Technol. 2011;45(19):8151–9. doi: 10.1021/es103712g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoffman K, Webster TF, Weisskopf MG, Weinberg J, Vieira VM. Exposure to polyfluoroalkyl chemicals and attention deficit/hyperactivity disorder in U.S. children 12–15 years of age. Environ Health Perspect. 2010;118(12):1762–7. doi: 10.1289/ehp.1001898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Høyer BB, Ramlau-Hansen CH, Obel C, Pedersen HS, Hernik A, Ogniev V, Jonsson BA, Lindh CH, Rylander L, Rignell-Hydbom A, Bonde JP, Toft G. Pregnancy serum concentrations of perfluorinated alkyl substances and offspring behaviour and motor development at age 5–9 years–a prospective study. Environ Health. 2015;14:2. doi: 10.1186/1476-069X-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vuong AM, Yolton K, Webster GM, Sjodin A, Calafat AM, Braun JM, Dietrich KN, Lanphear BP, Chen A. Prenatal polybrominated diphenyl ether and perfluoroalkyl substance exposures and executive function in school-age children. Environ Res. 2016;147:556–64. doi: 10.1016/j.envres.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grandjean P, Andersen EW, Budtz-Jorgensen E, Nielsen F, Molbak K, Weihe P, Heilmann C. Serum vaccine antibody concentrations in children exposed to perfluorinated compounds. JAMA. 2012;307(4):391–7. doi: 10.1001/jama.2011.2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granum B, Haug LS, Namork E, Stolevik SB, Thomsen C, Aaberge IS, van Loveren H, Lovik M, Nygaard UC. Pre-natal exposure to perfluoroalkyl substances may be associated with altered vaccine antibody levels and immune-related health outcomes in early childhood. J Immunotoxicol. 2013;10(4):373–9. doi: 10.3109/1547691X.2012.755580. [DOI] [PubMed] [Google Scholar]
- 12.Braun JM, Chen A, Romano ME, Calafat AM, Webster GM, Yolton K, Lanphear BP. Prenatal perfluoroalkyl substance exposure and child adiposity at 8 years of age: The HOME study. Obesity. 2016;24(1):231–7. doi: 10.1002/oby.21258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Høyer BB, Ramlau-Hansen CH, Vrijheid M, Valvi D, Pedersen HS, Zviezdai V, Jonsson BA, Lindh CH, Bonde JP, Toft G. Anthropometry in 5- to 9-Year-Old Greenlandic and Ukrainian Children in Relation to Prenatal Exposure to Perfluorinated Alkyl Substances. Environ Health Perspect. 2015;123(8):841–6. doi: 10.1289/ehp.1408881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mora AM, Oken E, Rifas-Shiman SL, Webster TF, Gillman MW, Calafat AM, Ye X, Sagiv SK. Prenatal Exposure to Perfluoroalkyl Substances and Adiposity in Early and Mid-Childhood. Environ Health Perspect. 2017;125(3):467–73. doi: 10.1289/EHP246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trudel D, Horowitz L, Wormuth M, Scheringer M, Cousins IT, Hungerbuhler K. Estimating consumer exposure to PFOS and PFOA. Risk analysis: an official publication of the Society for Risk Analysis. 2008;28(2):251–69. doi: 10.1111/j.1539-6924.2008.01017.x. [DOI] [PubMed] [Google Scholar]
- 16.D'Eon J C, Mabury SA. Is indirect exposure a significant contributor to the burden of perfluorinated acids observed in humans? Environ Sci Technol. 2011;45(19):7974–84. doi: 10.1021/es200171y. [DOI] [PubMed] [Google Scholar]
- 17.Fraser AJ, Webster TF, Watkins DJ, Nelson JW, Stapleton HM, Calafat AM, Kato K, Shoeib M, Vieira VM, McClean MD. Polyfluorinated compounds in serum linked to indoor air in office environments. Environ Sci Technol. 2012;46(2):1209–15. doi: 10.1021/es2038257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim DH, Lee MY, Oh JE. Perfluorinated compounds in serum and urine samples from children aged 5–13 years in South Korea. Environ Pollut. 2014;192:171–8. doi: 10.1016/j.envpol.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 19.Mogensen UB, Grandjean P, Nielsen F, Weihe P, Budtz-Jorgensen E. Breastfeeding as an Exposure Pathway for Perfluorinated Alkylates. Environ Sci Technol. 2015;49(17):10466–73. doi: 10.1021/acs.est.5b02237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mondal D, Weldon RH, Armstrong BG, Gibson LJ, Lopez-Espinosa MJ, Shin HM, Fletcher T. Breastfeeding: a potential excretion route for mothers and implications for infant exposure to perfluoroalkyl acids. Environ Health Perspect. 2014;122(2):187–92. doi: 10.1289/ehp.1306613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang T, Qin X. Assessment of fetal exposure and maternal elimination of perfluoroalkyl substances. Environ Sci Process Impacts. 2014;16(8):1878–81. doi: 10.1039/c4em00129j. [DOI] [PubMed] [Google Scholar]
- 22.Fromme H, Mosch C, Morovitz M, Alba-Alejandre I, Boehmer S, Kiranoglu M, Faber F, Hannibal I, Genzel-Boroviczeny O, Koletzko B, Volkel W. Pre- and postnatal exposure to perfluorinated compounds (PFCs) Environ Sci Technol. 2010;44(18):7123–9. doi: 10.1021/es101184f. [DOI] [PubMed] [Google Scholar]
- 23.Kim S, Choi K, Ji K, Seo J, Kho Y, Park J, Kim S, Park S, Hwang I, Jeon J, Yang H, Giesy JP. Trans-placental transfer of thirteen perfluorinated compounds and relations with fetal thyroid hormones. Environ Sci Technol. 2011;45(17):7465–72. doi: 10.1021/es202408a. [DOI] [PubMed] [Google Scholar]
- 24.Kato K, Wong LY, Chen A, Dunbar C, Webster GM, Lanphear BP, Calafat AM. Changes in serum concentrations of maternal poly- and perfluoroalkyl substances over the course of pregnancy and predictors of exposure in a multiethnic cohort of Cincinnati, Ohio pregnant women during 2003–2006. Environ Sci Technol. 2014;48(16):9600–8. doi: 10.1021/es501811k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pinney SM, Biro FM, Windham GC, Herrick RL, Yaghjyan L, Calafat AM, Succop P, Sucharew H, Ball KM, Kato K, Kushi LH, Bornschein R. Serum biomarkers of polyfluoroalkyl compound exposure in young girls in Greater Cincinnati and the San Francisco Bay Area, USA. Environ Pollut. 2014;184:327–34. doi: 10.1016/j.envpol.2013.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu XM, Bennett DH, Calafat AM, Kato K, Strynar M, Andersen E, Moran RE, Tancredi DJ, Tulve NS, Hertz-Picciotto I. Serum concentrations of perfluorinated compounds (PFC) among selected populations of children and adults in California. Environ Res. 2015;136:264–73. doi: 10.1016/j.envres.2014.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morck TA, Nielsen F, Nielsen JK, Siersma VD, Grandjean P, Knudsen LE. PFAS concentrations in plasma samples from Danish school children and their mothers. Chemosphere. 2015;129:203–9. doi: 10.1016/j.chemosphere.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 28.Bao J, Lee YL, Chen PC, Jin YH, Dong GH. Perfluoroalkyl acids in blood serum samples from children in Taiwan. Environ Sci Pollut Res. 2014;21(12):7650–5. doi: 10.1007/s11356-014-2594-4. [DOI] [PubMed] [Google Scholar]
- 29.Olsen GW, Church TR, Hansen KJ, Burris JM, Butenhoff JL, Mandel JH, Zobel LR. Quantitative Evaluation of Perfluorooctanesulfonate (PFOS) and Other Fluorochemicals in the Serum of Children. Journal of Children's Health. 2004;2(1):53–76. [Google Scholar]
- 30.Schecter A, Malik-Bass N, Calafat AM, Kato K, Colacino JA, Gent TL, Hynan LS, Harris TR, Malla S, Birnbaum L. Polyfluoroalkyl compounds in Texas children from birth through 12 years of age. Environ Health Perspect. 2012;120(4):590–4. doi: 10.1289/ehp.1104325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oken E, Baccarelli AA, Gold DR, Kleinman KP, Litonjua AA, De Meo D, Rich-Edwards JW, Rifas-Shiman SL, Sagiv S, Taveras EM, Weiss ST, Belfort MB, Burris HH, Camargo CA, Jr, Huh SY, Mantzoros C, Parker MG, Gillman MW. Cohort profile: project viva. International Journal of Epidemiology. 2015;44(1):37–48. doi: 10.1093/ije/dyu008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sagiv SK, Rifas-Shiman SL, Webster TF, Mora AM, Harris MH, Calafat AM, Ye X, Gillman MW, Oken E. Sociodemographic and Perinatal Predictors of Early Pregnancy Per-and Polyfluoroalkyl Substance (PFAS) Concentrations. Environ Sci Technol. 2015;49(19):11849–58. doi: 10.1021/acs.est.5b02489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kato K, Basden BJ, Needham LL, Calafat AM. Improved selectivity for the analysis of maternal serum and cord serum for polyfluoroalkyl chemicals. Journal of chromatography A. 2011;1218(15):2133–7. doi: 10.1016/j.chroma.2010.10.051. [DOI] [PubMed] [Google Scholar]
- 34.Centers for Disease Control and Prevention (CDC) Laboratory Procedure Manual for Polyfluorinated Compounds in Serum (NHANES 2011–2012) (Method No. 6304.04) 2013 https://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/pfc_g_met.pdf.
- 35.Centers for Disease Control and Prevention (CDC) Laboratory Procedure Manual for Perfluoroalkyl and Polyfluoroalkyl Substances (NHANES 2013–2014) (Method No. 6304.06) https://www.cdc.gov/nchs/data/nhanes/nhanes_13_14/PFAS_H_MET.pdf.
- 36.Centers for Disease Control and Prevention (CDC) 2000 CDC Growth Charts for the United States: Methods and Development. 2000 http://www.cdc.gov/nchs/data/series/sr_11/sr11_246.pdf.
- 37.Centers for Disease Control and Prevention (CDC) Defining Childhood Obesity. 2015 http://www.cdc.gov/obesity/childhood/defining.html.
- 38.United States Census Bureau. US Census 2000: Summary File 3. 2000 http://www.census.gov/census2000/sumfile3.html.
- 39.Mansfield ER, Helms BP. Detecting multicollinearity. Am Stat. 1982;36(3):158–60. [Google Scholar]
- 40.Centers for Disease Control and Prevention (CDC) Fourth National Report on Human Exposure to Environmental Chemicals. 2015 https://www.cdc.gov/biomonitoring/pdf/fourthreport_updatedtables_feb2015.pdf.
- 41.Beesoon S, Genuis SJ, Benskin JP, Martin JW. Exceptionally high serum concentrations of perfluorohexanesulfonate in a Canadian family are linked to home carpet treatment applications. Environ Sci Technol. 2012;46(23):12960–7. doi: 10.1021/es3034654. [DOI] [PubMed] [Google Scholar]
- 42.Berg V, Nost TH, Huber S, Rylander C, Hansen S, Veyhe AS, Fuskevag OM, Odland JO, Sandanger TM. Maternal serum concentrations of per- and polyfluoroalkyl substances and their predictors in years with reduced production and use. Environ Int. 2014;69:58–66. doi: 10.1016/j.envint.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 43.Halldorsson TI, Fei C, Olsen J, Lipworth L, McLaughlin JK, Olsen SF. Dietary predictors of perfluorinated chemicals: a study from the Danish National Birth Cohort. Environ Sci Technol. 2008;42(23):8971–7. doi: 10.1021/es801907r. [DOI] [PubMed] [Google Scholar]
- 44.Ji K, Kim S, Kho Y, Paek D, Sakong J, Ha J, Kim S, Choi K. Serum concentrations of major perfluorinated compounds among the general population in Korea: dietary sources and potential impact on thyroid hormones. Environ Int. 2012;45:78–85. doi: 10.1016/j.envint.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 45.Nelson JW, Scammell MK, Hatch EE, Webster TF. Social disparities in exposures to bisphenol A and polyfluoroalkyl chemicals: a cross-sectional study within NHANES 2003–2006. Environ Health. 2012;11:10. doi: 10.1186/1476-069X-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu H, Yoon M, Verner MA, Xue J, Luo M, Andersen ME, Longnecker MP, Clewell HJ., 3rd Can the observed association between serum perfluoroalkyl substances and delayed menarche be explained on the basis of puberty-related changes in physiology and pharmacokinetics? Environ Int. 2015;82:61–8. doi: 10.1016/j.envint.2015.05.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


