Abstract
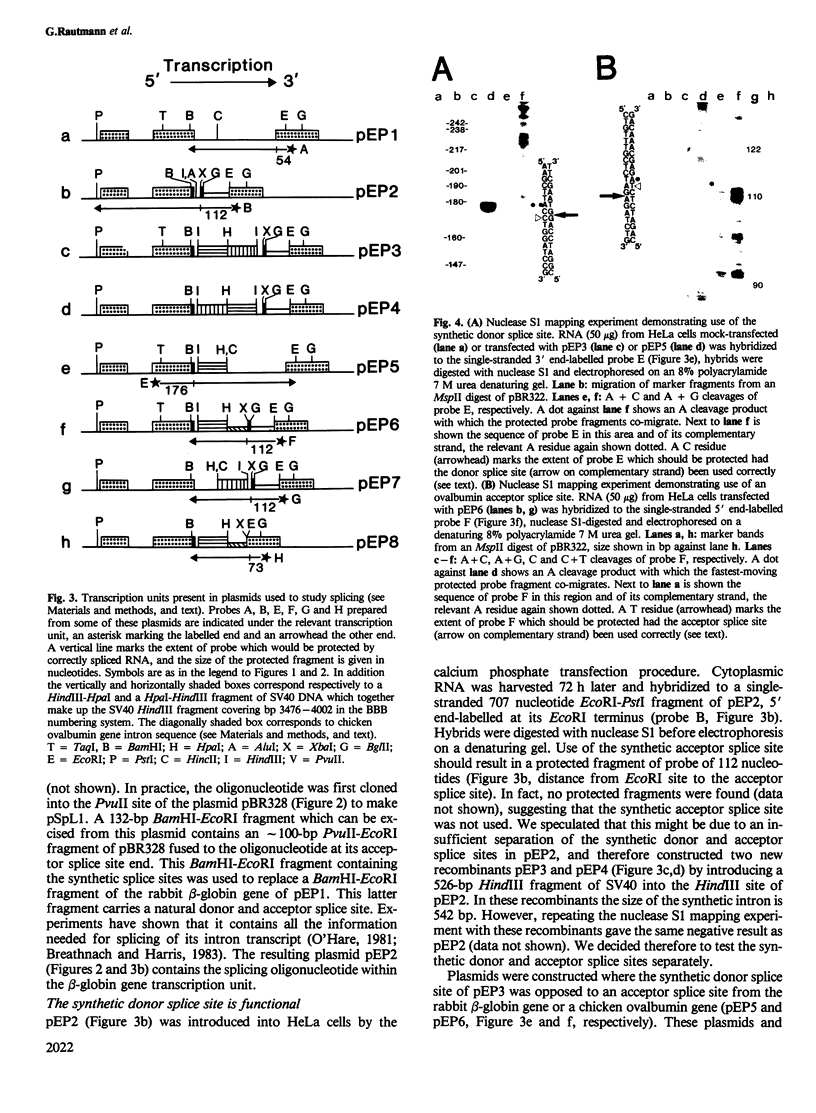
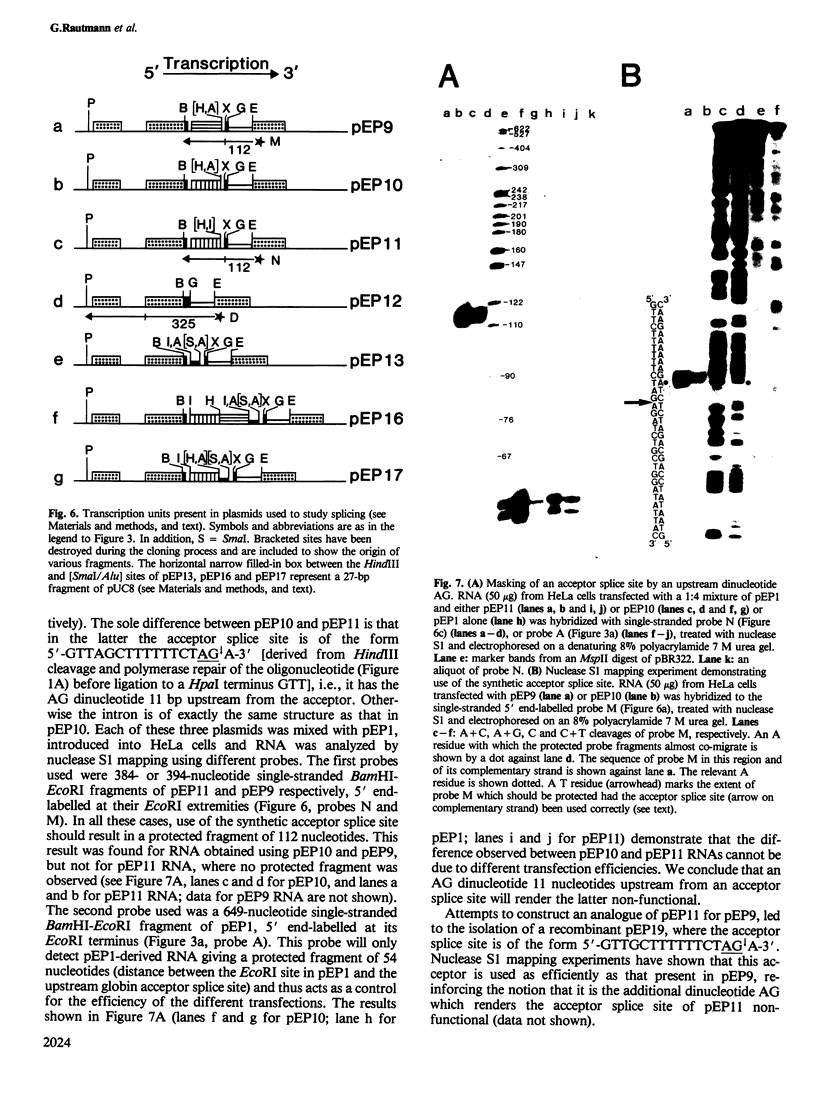
We have synthesised a 32-bp oligonucleotide containing sequences conforming to the consensus sequences for donor and acceptor splice sites. The oligonucleotide has been inserted into an RNA polymerase B (II) transcription unit and the resulting recombinant used to study the splicing mechanism. Our findings are as follows: (i) the synthetic sites function when separated by several different prokaryotic or eukaryotic DNA fragments providing bulk intron sequence, (ii) intron size need not be greater than 29 bp, (iii) an AG dinucleotide 11 bp upstream from the invariant AG of an acceptor splice site renders the latter non-functional, and (iv) sequence changes distant from splice sites can affect the efficiency of their utilisation.
Full text
PDF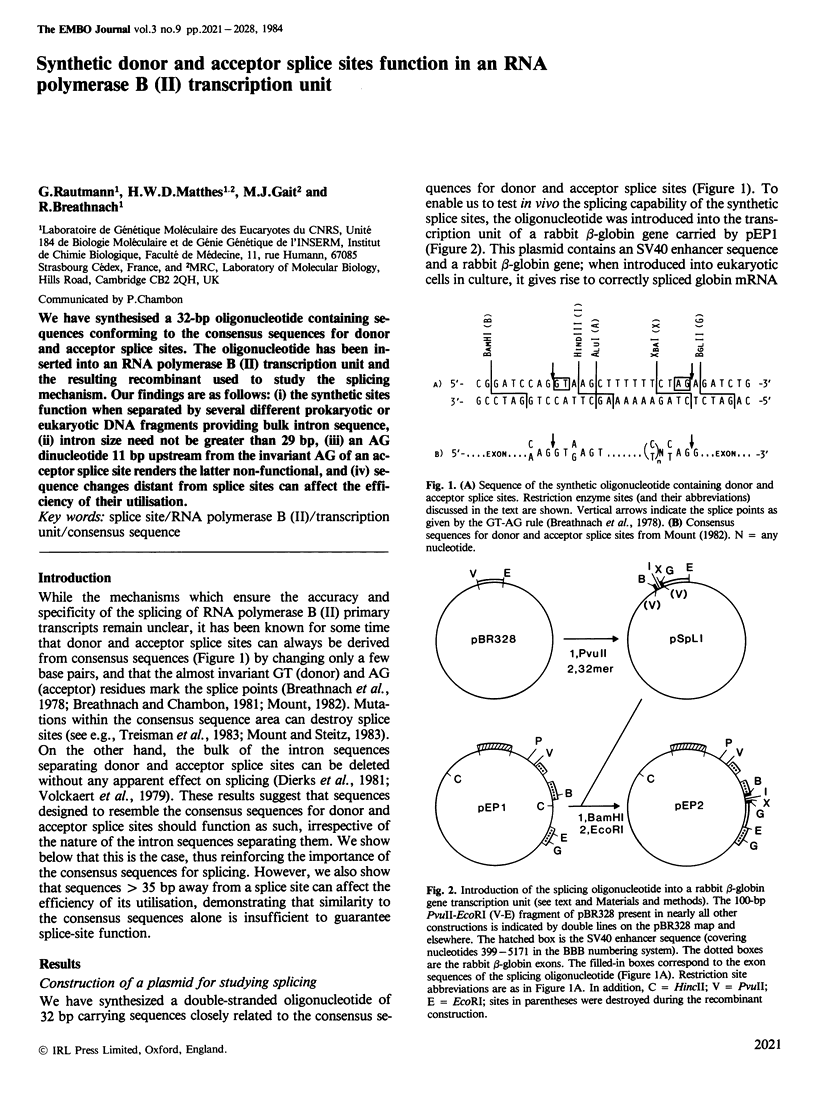




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerji J., Rusconi S., Schaffner W. Expression of a beta-globin gene is enhanced by remote SV40 DNA sequences. Cell. 1981 Dec;27(2 Pt 1):299–308. doi: 10.1016/0092-8674(81)90413-x. [DOI] [PubMed] [Google Scholar]
- Benoist C., O'Hare K., Breathnach R., Chambon P. The ovalbumin gene-sequence of putative control regions. Nucleic Acids Res. 1980 Jan 11;8(1):127–142. doi: 10.1093/nar/8.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Harris B. A. Plasmids for the cloning and expression of full-length double-stranded cDNAs under control of the SV40 early or late gene promoter. Nucleic Acids Res. 1983 Oct 25;11(20):7119–7136. doi: 10.1093/nar/11.20.7119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobkin C., Pergolizzi R. G., Bahre P., Bank A. Abnormal splice in a mutant human beta-globin gene not at the site of a mutation. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1184–1188. doi: 10.1073/pnas.80.5.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth M. L., Gait M. J., Goelet P., Hong G. F., Singh M., Titmas R. C. Rapid synthesis of oligodeoxyribonucleotides VI. Efficient, mechanised synthesis of heptadecadeoxyribonucleotides by an improved solid phase phosphotriester route. Nucleic Acids Res. 1981 Apr 10;9(7):1691–1706. doi: 10.1093/nar/9.7.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hen R., Sassone-Corsi P., Corden J., Gaub M. P., Chambon P. Sequences upstream from the T-A-T-A box are required in vivo and in vitro for efficient transcription from the adenovirus serotype 2 major late promoter. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7132–7136. doi: 10.1073/pnas.79.23.7132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoury G., Gruss P., Dhar R., Lai C. J. Processing and expression of early SV40 mRNA: a role for RNA conformation in splicing. Cell. 1979 Sep;18(1):85–92. doi: 10.1016/0092-8674(79)90356-8. [DOI] [PubMed] [Google Scholar]
- Langford C. J., Klinz F. J., Donath C., Gallwitz D. Point mutations identify the conserved, intron-contained TACTAAC box as an essential splicing signal sequence in yeast. Cell. 1984 Mar;36(3):645–653. doi: 10.1016/0092-8674(84)90344-1. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Mount S., Steitz J. Lessons from mutant globins. Nature. 1983 Jun 2;303(5916):380–381. doi: 10.1038/303380a0. [DOI] [PubMed] [Google Scholar]
- Rautmann G., Glaichenhaus N., Nahgashfar Z., Breathnach R., Rassoulzadegan M. Complementation of a tsa mutant and replication of a recombinant DNA carrying the viral ori region in mouse cells transformed by polyoma virus. Virology. 1982 Oct 30;122(2):306–317. doi: 10.1016/0042-6822(82)90230-6. [DOI] [PubMed] [Google Scholar]
- Seif I., Khoury G., Dhar R. BKV splice sequences based on analysis of preferred donor and acceptor sites. Nucleic Acids Res. 1979 Jul 25;6(10):3387–3398. doi: 10.1093/nar/6.10.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soberon X., Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. IV. Deletion derivatives of pBR322 and pBR325. Gene. 1980 May;9(3-4):287–305. doi: 10.1016/0378-1119(90)90328-o. [DOI] [PubMed] [Google Scholar]
- Treisman R., Orkin S. H., Maniatis T. Specific transcription and RNA splicing defects in five cloned beta-thalassaemia genes. Nature. 1983 Apr 14;302(5909):591–596. doi: 10.1038/302591a0. [DOI] [PubMed] [Google Scholar]
- Volckaert G., Feunteun J., Crawford L. V., Berg P., Fiers W. Nucleotide sequence deletions within the coding region for small-t antigen of simian virus 40. J Virol. 1979 Jun;30(3):674–682. doi: 10.1128/jvi.30.3.674-682.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]