Abstract
HCN4 is a hyperpolarization-activated nucleotide-gated cation channel involved in the generation of the If current that drives cardiac pacemaker activity. Previous studies have demonstrated that HCN4 is highly expressed in a restricted manner in adult sinoatrial (SA) node [Eur. J. Biochem. 268 (2001) 1646]. However, its developmental expression pattern is unknown. We have examined expression of HCN4 mRNA during mouse heart development. HCN4 mRNA was first detected in the cardiac crescent at embryonic day (ED) 7.5. At ED 8 it was symmetrically located in the most caudal portion of the heart tube, the sinus venosus where pacemaker activity has previously been reported [Am. J. Physiol. 212 (1967) 407]. With further development, HCN4 expression became asymmetrically distributed, occupying the dorsal wall of the right atria, and was progressively restricted to the junction of the right atrial appendage and the superior vena cava. The site of HCN4 expression in late embryonic heart coincided with the location of the SA node in postnatal and adult heart [Cardiovasc. Res. 52 (2001) 51]. Our results suggest that HCN4 may be a unique marker of the developing SA node
Keywords: Hyperpolarization-activated cyclic nucleotide-gated cation channels, Sinus venosus, Development, Pacemaker
Pacemaker activity in the heart sinoatrial (SA) node is controlled by a depolarizing, Na+/K+ current named If or Ih (Accili et al., 2002; DiFrancesco, 1993, 1995). Although the pacemaker current has been known for more than 20 years, cloning of the pacemaker channels responsible for the If current occurred only recently (Ludwig et al., 1998; Santoro et al., 1998). Today, four members of the HCN (hyperpolarization-activated cyclic nucleotide-gated cation channels) family have been reported, including HCN1, HCN2, HCN3 and HCN4.
Both HCN2 and HCN4 are expressed in the murine adult SA node. HCN2 shows a moderate level of expression within the node, and a broader distribution that includes ventricular myocardium; HCN4 is restricted to the node with a high level of expression, constituting a good molecular marker for the adult cardiac pacemaker (Moosmang et al., 2001).
The earliest stage at which cardiac pacemaker activity has been recorded is in the nonbeating heart of an 8 (sp) chick embryo (which would correspond to a 5–10 (sp) mouse embryo (Van Mierop, 1967). At this stage, an impulse generated in the prospective SA region is conducted to the bulboventricular portion of the heart. Later on in development, the impulse that triggers the heartbeat likewise originates from the caudalmost region of the heart tube, the sinus venosus.
Based on the previously reported specific expression of the pacemaker channel HCN4 in the SA node in adult mouse heart (Moosmang et al., 2001) we have studied its pattern of expression during development. Our studies reveal an intriguing early appearance of HCN4 mRNA in the cardiac crescent, and also suggest that HCN4 is a unique marker for the developing cardiac pacemaker.
1. Results and discussion
HCN4 expression was detected at embryonic day (ED) 7.5, in dispersed cells throughout the precardiac mesoderm (cardiac crescent) (Fig. 1A). As development progressed, and the bilateral regions of the cardiogenic mesoderm had fused to form the heart tube, expression became confined to the caudalmost portion of the heart. At ED 8 (9 somite pairs (sp)), HCN4 mRNA was symmetrically distributed and restricted to the sinus venosus, including the right and left horns, as well as the transverse middle portion (Fig. 1B,C). Sections of these early hearts also revealed bilaterally symmetric expression in the junction between the common cardinal veins and the sinus venosus (data not shown). The first morphological descriptions of the developing mouse SA node reported its location in this junctional region, although in asymmetric fashion, confined only to the right horn of the sinus venosus (Van Mierop and Gessner, 1970).
Fig. 1.

Whole mount in situ hybridization showing HCN4 mRNA expression at early developmental stages. (A) ED 7.5 embryo where HCN4 expression is observed in the cardiac mesoderm (arrowheads); (B,C) Show an ED 8 (9 sp) embryo with HCN4 symmetric expression in the sinus venosus (arrowheads); (D,E,F) Frontal, right and left views, respectively, of an ED 8.5 (12 sp) embryo. Black arrowheads point to the sinus venosus. White arrowhead indicates a group of HCN4 positive cells localized in the left atria; (G,H,I) Right, left and frontal left views, respectively, of an ED 9 (14 sp) embryo. Black arrowheads show the sinus venosus and the septum transversum. a–atria; cm–cardiac mesoderm; lhsv–left horn sinus venosus; lv–left ventricle; rhsv–right horn sinus venosus; st–septum transversum; sv–sinus venosus; v–ventricle. (J,K,L,M). Paraffin sections at different cardiac levels of an ED 8.5 (12sp) embryo. Arrowheads indicate HCN4 positive cells in the left atrial wall. ccv–common cardinal vein; la–left atrium; lv–left ventricle; nt–neural tube; st–septum transversum; sv–sinus venosus; v–ventricle.
By ED 8.5 (12 sp) (Fig. 1D,E), a group of HCN4 positive cells was observed extending from the left portion of the sinus venosus to the left atrial wall (Fig. 1F). Serial sections of these embryos at the atrial level demonstrated the presence of HCN4 positive cells only in the left atrial wall but not in the right, confirming the asymmetry of this phenomenon (Fig. 1J–M).
At ED 8.75 (14 sp) (Fig. 1G–I), HCN4 mRNA was also observed in the septum transversum, which appears caudal to the sinus venosus and contains hepatic precursor cells that will migrate and incorporate into the future liver (Fig. 1I,M). Among cell types found in this structure are endothelial cell precursors that will give rise to the hepatic plexus. In agreement with this finding, HCN4 has previously been reported to participate in the generation of the If current recorded in the adult rabbit portal vein (Greenwood and Prestwich, 2002).
From ED 10 (27 sp) (Fig. 2A,B), HCN4 expression occurred asymmetrically in the region of the sinus venosus. The wall of the right common cardinal vein as it enters the right horn of the sinus venosus maintained high HCN4 expression, while the same portion of the left common cardinal vein exhibited reduced expression (Fig. 2E–H). At this stage there were also some positive cells in the dorsal wall of the right atria (Fig. 2C), as well as a strong signal in the septum transversum (Fig. 2D).
Fig. 2.

Whole mount in situ hybridization showing HCN4 expression on an ED 10 (27sp) embryo. (A) Right lateral view where expression in the central nervous system can be appreciated (black arrowheads) together with the expression in the heart. White arrowhead indicates the sinus venosus portion of the heart. (B) Left lateral view of the same embryo shown in A. (C) Higher magnification of the right side of the embryonic heart where the signal in the dorsal wall of the right atria, prospective SA node, can be observed (black arrowhead). White arrowhead points to the right horn of the sinus venosus. (D) Higher magnification of the left side of the embryonic heart, where the expression in the septum transversum (black arrowhead) and the left horn of the sinus venosus (white arrowhead) is indicated. la–left atrium; lhsv–left horn sinus venosus; lv–left ventricle; oft–outflow tract; ra–right atrium; rhsv–right horn sinus venosus; rv–right ventricle; sa–prospective sinoatrial region; st–septum transversum; sv–sinus venosus. Paraffin sections obtained at different levels of the ED 10 embryo (Heart: E–H), (CNS: I) following whole mount in situ hybridization to detect HCN4 mRNA. The asymmetric signal in the dorsal wall of the right atrium and the rostral portion of the right horn of the sinus venosus is observed in (E) and (F). (G) and (H) show the asymmetric signal only in the right side, at the junction between the common cardinal vein and the sinus venosus. Signal in the septum transversum is also observed. acv–anterior cardinal vein; ccv–common cardinal vein; la–left atrium; lv–left ventricle; nt–neural tube; ra–right atrium; rv–right ventricle; st–septum transversum; sv–sinus venosus. Central nervous system staining is shown in I, where the forebrain and hindbrain neuroepithelia are observed. d–diencephalon; hn–hindbrain neuroepithelium; hv–primary head vein; os–optic stalk; ov–optic vesicle; r–hindbrain roof; tv–telencephalic vesicles; vp–preoptic vascular plexus.
At ED 10, HCN4 expression appeared also in the central nervous system (Fig. 2A,B). Briefly, HCN4 mRNA was confined to proliferating neuroepithelia lining the brain ventricles. Sections of ED 10 stained embryos revealed expression of HCN4 mRNA in forebrain neuroepithelia, including telencephalic vesicles, optic vesicles and optic stalk, and the diencephalon (Fig. 2I). As previously reported, HCN4 is expressed in adult brain regions that originate from these precursor populations, including the striatum (Santoro et al., 2000), the retina (Moosmang et al., 2001) and thalamic nuclei (Santoro et al., 2000). Low levels of HCN4 mRNA could also be found in the midbrain neuroepithelium (data not shown). Highest expression levels, however, were seen in hindbrain neuroepithelium, especially at the hindbrain roof (Fig. 2I). Hindbrain precursor cells differentiate and form the cerebellum and medulla, both of which show HCN4 expressing cells in the adult (Santoro et al., 2000).
At ED 12 (50 sp), the disposition of the sinus venosus has changed, the left SA sulcus has deepened and the left side of the SA orifice is displaced toward the right atrium with the result that the right horn of the sinus venosus is incorporated into the dorsal wall of the right atrium. These developmental changes could be followed by the expression pattern of HCN4. At ED 12, HCN4 mRNA was found surrounding the orifice of the right common cardinal vein (Fig. 3C) (prospective superior vena cava) and extended along the junction of the right common cardinal vein and the right atrial appendage in the dorsal wall of the right atrium corresponding to the region where the anatomic SA node has been located during embryonic development (Van Mierop and Gessner, 1970) (Fig. 3C–E). Low levels of HCN4 expression were present in the left common cardinal vein and in the left horn of the sinus venosus (Fig. 3B–E) that will give rise to the coronary sinus and the common pulmonary vein (Blom et al., 2001).
Fig. 3.

HCN4 mRNA expression in whole ED 12 heart (from 50 sp embryo). (A) Frontal view of the whole heart where no signal is observed. (B) Dorsal view of the heart showing asymmetrical HCN4 expression in the dorsal wall of the right atrium. At this stage, some signal was also observed in the coronary sinus extending to the left atria and in the left common cardinal vein. (C) Lateral view of the right side of same heart, where strong expression surrounding the orifice of the right common cardinal vein (future superior vena cava) is indicated (white arrowhead). Strong HCN4 mRNA expression also occupied the dorsal wall of the right atrium. (D) Coronal section of an ED 12 heart at the atrial level showing the presence of HCN4 mRNA in the junction between the right common cardinal vein and the right atrial appendage. Some signal was also observed in the left common cardinal vein. (E) High magnification of a coronal section at the level where the right common cardinal vein enters the right atrium (small arrowhead indicating the venous valves), where HCN4 signal is observed at the junction between the right atrium and the common cardinal vein (large arrowhead). cs–coronary sinus; la–left atrium; lccv–left common cardinal vein; lv–left ventricle; ra–right atrial appendage; rccv–right common cardinal vein; rv–right ventricle; v–ventricle; vv–venous valves.
After ED 12 HCN4 expression became progressively more restricted. At early postnatal stages (postnatal day 4) positive cells were confined to the SA node region enclosed by the orifices of the superior and inferior vena cava, the crista terminalis and the interatrial septum (Mangoni and Nargeot, 2001) (Fig. 4A–D). The intensity of the signal was very strong, suggesting a high level of expression.
Fig. 4.

Postnatal day 4 mouse hearts showing HCN4 expression in the SA node. (A) Frontal top view of the heart, where HCN4 signal was observed surrounding the portion of the superior vena cava in close proximity to the right atrial appendage. (B) Right lateral top view of the same heart as in A, where HCN4 positive cells occupied the junction between the superior vena cava and the right atrial appendage. (C) Dorsal view of the heart showing HCN4 mRNA located in the previously described SA node region, between the orifices of the superior and inferior vena cava, in the dorsal wall of the right atrium (arrowheads). (D) Longitudinal section of a postnatal day 4 heart at the level of the superior vena cava, where HCN4 mRNA was observed in the junction between the superior vena cava and the right atrial appendage. ao–aorta; ivc–inferior vena cava; la–left atrium; pa–pulmonary artery; ra–right atrium; raa – right atrial appendage; sa–sinoatrial node; svc–superior vena cava.
In the adult heart, HCN4 mRNA was detected in the reported SA node region but at lower levels and in a more restricted location than at previous stages (Fig. 5). The highest level of expression was found in the junction between the orifice of the superior vena cava and the right atrial appendage.
Fig. 5.
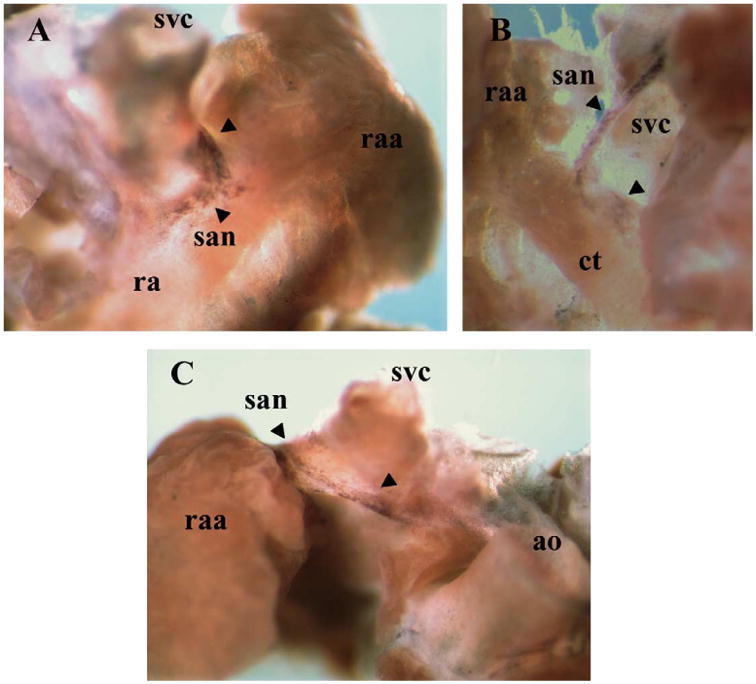
HCN4 expression in the adult heart SA node. (A) Dorsal top view of the right atrium where HCN4 signal was observed at the junction between the superior vena cava and the right atrial appendage (arrowheads). (B) Frontal view of the right atrium dorsal wall, where HCN4 positive cells were observed surrounding the orifice of the superior vena cava, and extending caudally, following the crista terminalis as a border (arrowheads). (C) Frontal view of the right atrium dissected out from the whole heart, where HCN4 positive cells are located surrounding the superior vena cava at the junction with the right atrial appendage (indicated by the arrowheads). ao–aorta; ct–crista terminalis; ra–right atrium; raa–right atrial appendage; san–sinoatrial node; svc–superior vena cava.
2. Experimental procedures
2.1. Whole mount in situ hybridization
Timed pregnant females, as well as, postnatal and adult CD1 mice were euthanized by cervical dislocation and their embryos or hearts, respectively, were dissected out, rinsed in ice-cold PBS and fixed overnight in 4% paraformaldehyde pH 7.4 at 4 °C. Embryos and whole hearts were then dehydrated and rehydrated in a graded series of methanol, treated with proteinase K (10 μg/ml) for 5– 15 min depending on the developmental stage and post-fixed in 4% paraformaldehyde, 0.2% glutaraldehyde. Mouse HCN4 antisense and sense riboprobes were labeled with digoxigenin-d-UTP (Roche) by in vitro transcription using T7 and T3 RNA polymerases (Roche) respectively. A cDNA fragment corresponding to HCN4 amino acids 400–690 was used as template. Probes were added to the hybridization solution at a concentration of 2 μg/ml, and incubated overnight at 68 °C. Tissue was then washed in 5 × SSC, 50% formamide, digested with RNAseA (100 mg/ml) and washed again in 2 × SSC, 50% formamide. For the immunodetection of digoxigenin, tissue was blocked in 10% sheep serum for 2 h and incubated overnight at 4 °C with the anti-digoxigenin antibody (Roche) (1:2000). Enzymatic development of the signal was performed by addition of NBT/BCIP at room temperature. Control hybridizations in the presence of HCN4 sense probe did not yield any detectable signals (data not shown).
2.2. Paraffin sections
Stained embryos and whole hearts were dehydrated through a series of ethanol followed by xylene and a final overnight immersion in paraffin. Blocks were made the next day and 7–10 μm section s were obtained with a microtome.
Acknowledgments
We would like to thank Dr Bina Santoro who very kindly provided the cDNA that we have used for the synthesis of the HCN4 riboprobes. This study was supported by AHA and NIH grants to SE.
References
- Accili EA, Proenza C, Baruscotti M, DiFrancesco D. From funny current to HCN channels: 20 years of excitation. News Physiol Sci. 2002;17:32–37. doi: 10.1152/physiologyonline.2002.17.1.32. [DOI] [PubMed] [Google Scholar]
- Blom NA, Gittenbergerde Grot AC, Jongeneel TH, DeRuiter MC, Poelmann RE, Ottenkamp J. Normal development of the pulmonary veins in human embryos and formulation of a morphogenetic concept for sinus venosus defects. Am J Cardiol. 2001;87:305–309. doi: 10.1016/s0002-9149(00)01363-1. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. Pacemaker mechanisms in cardiac tissue. Annu Rev Physiol. 1993;58:299–327. doi: 10.1146/annurev.ph.55.030193.002323. [DOI] [PubMed] [Google Scholar]
- DiFrancesco D. The pacemaker current (If) plays an important role in regulating SA node pacemaker activity. Cardiovasc Res. 1995;30:307–308. [PubMed] [Google Scholar]
- Greenwood IA, Prestwich SA. Characteristics of hyperpolarization-activated cation currents in portal vein smooth muscle cells. Am J Physiol Cell Physiol. 2002;282:C744–C753. doi: 10.1152/ajpcell.00393.2001. [DOI] [PubMed] [Google Scholar]
- Ludwig A, Zong X, Jeglitsch M, Hofmann F, Biel M. A family of hyperpolarization-activated mammalian cation channels. Nature. 1998;393:587–591. doi: 10.1038/31255. [DOI] [PubMed] [Google Scholar]
- Mangoni ME, Nargeot J. Properties of hyperpolarization-activated current (If) in isolated mouse sino-atrial cells. Cardiovasc Res. 2001;52:51–64. doi: 10.1016/s0008-6363(01)00370-4. [DOI] [PubMed] [Google Scholar]
- Moosmang S, Stieber J, Zong X, Biel M, Hofmann F, Ludwig A. Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues Cellular expression and functional characterization of four hyperpolarization-activated pacemaker channels in cardiac and neuronal tissues. Eur J Biochem. 2001;268:1646–1652. doi: 10.1046/j.1432-1327.2001.02036.x. [DOI] [PubMed] [Google Scholar]
- Santoro B, Liu DT, Yao H, Bartsch D, Kandel ER, Siegelbaum SA, Tibbs GR. Identification of a gene encoding a hyperpolarization-activated pacemaker channel of brain. Cell. 1998;93:717–729. doi: 10.1016/s0092-8674(00)81434-8. [DOI] [PubMed] [Google Scholar]
- Santoro B, Chen S, Lüthi A, Pavlidis P, Shumyatsky GP, Tibbs GR, Siegelbaum SA. Molecular and functional heterogeneity of hyperpolarization-activated pacemaker channels in the mouse CNS. J Neurosci. 2000;20:5264–5275. doi: 10.1523/JNEUROSCI.20-14-05264.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Mierop LHS. Location of pacemaker in chick embryo heart at the time of initiation of heartbeat. Am J Physiol. 1967;212:407–415. doi: 10.1152/ajplegacy.1967.212.2.407. [DOI] [PubMed] [Google Scholar]
- Van Mierop LHS, Gessner IH. The morphologic development of the sinoatrial node in the mouse. J Cardiol. 1970;25:204–212. doi: 10.1016/0002-9149(70)90580-1. [DOI] [PubMed] [Google Scholar]


