Abstract
The roof plate resident BMPs have sequential functions in the developing spinal cord, establishing cell fate and orienting axonal trajectories. These activities are, however, restricted to the dI1–dI3 neurons in the most dorsal region of the spinal cord. What limits the extent of the action of the BMPs to these neurons? To address this question, we have examined both the distribution of the inhibitory Smads (I-Smads), Smad6 and Smad7 in the spinal cord and the consequence of ectopically expressing the I-Smads in chicken embryos. Our studies suggest that the I-Smads function in vivo to restrict the action of BMP signaling in the dorsal spinal cord. Moreover, the I-Smads have distinct roles in regulating the diverse activities of the BMPs. Thus, the ectopic expression of Smad7 suppresses the dI1 and dI3 neural fates and concomitantly increases the number of dI4–dI6 spinal neurons. In contrast, Smad6 most potently functions to block dI1 axon outgrowth. Taken together, these experiments suggest that the I-Smads have distinct roles in spatially limiting the response of cells to BMP signaling.
Keywords: Spinal cord, Cell fate specification, Axon outgrowth, Dorsal spinal neurons, Inhibitory Smads, Bone morphogenetic proteins
Introduction
A critical paradigm in developmental biology is the use of graded distributions of inductive growth factors, or morphogens, to pattern fields of cells. Morphogens are used reiteratively, in a concentration dependent manner, to specify cellular identity (Ulloa and Briscoe, 2007). Recent studies have also demonstrated that, in addition to the specification of diverse cell fates, morphogens can also direct multiple cellular processes within a developing organism (Augsburger et al., 1999; Charron et al., 2003; Irving et al., 2002; Lyuksyutova et al., 2003). An example of this paradigm is the role of members of the Bone Morphogenetic Protein (BMP) family in establishing neural identity in the dorsal spinal cord (Lee et al., 1998; Liem et al., 1997) and then directing the orientation (Butler and Dodd, 2003) and growth rate (Phan et al., 2010) of axons extending from the dorsal-most commissural neurons. BMPs, in combination with activins, both members of the Transforming Growth Factor (TGF) β superfamily, act from the roof plate at the dorsal midline of the spinal cord to specify the fate of the dorsal-most spinal neurons, dorsal interneuron (dI) 1 to dI3 (Chizhikov and Millen, 2005). The remainder of the dorsal spinal cord is thought to form independently of signals from the roof plate: the dI4–dI6 neurons are still present after either ablation of the roof plate (Lee et al., 2000) or BMP receptor (Bmpr) signaling (Timmer et al., 2002; Wine-Lee et al., 2004). However, Bmprs are present in the dorsal progenitor (dP) 4–dP6 neurons (Yamauchi et al., 2008) and explants taken from the early intermediate chick spinal cord can be directed by the BMPs to form dI1 neurons (Liem et al., 1997) suggesting that the intermediate progenitors are initially competent to respond to signals from the roof plate. These studies raise the question, what limits the action of signals from the roof plate to specific groups of dorsal neurons? Here, we present evidence that the endogenous inhibitors of TGFβ signaling, the inhibitory Smad (I-Smad) proteins, Smad6 and Smad7, have a role in this process.
The activities of the BMPs are mediated by a heterodimeric complex of type I and type II BMP serine/threonine kinase receptors. On BMP binding, the type I Bmprs activate the receptor-activated Smads (R-Smads) (Moustakas and Heldin, 2009) which oligomerize with the common-mediator Smad (Co-Smad) Smad4 (Chesnutt et al., 2004; Feng and Derynck, 2005). The Smad complex then translocates to the nucleus to act as a transcriptional regulator (Heldin et al., 1997). BMP signaling can be blocked by the action of Smad6 and Smad7 (Imamura et al., 1997; Nakao et al., 1997). I-Smads have been shown to inhibit BMP or TGFβ signaling in vitro by several mechanisms (Moustakas and Heldin, 2009), which include competing with the R-Smads for binding to the type I BMP receptor (Goto et al., 2007; Hayashi et al., 1997; Imamura et al., 1997; Kamiya et al., 2010; Nakao et al., 1997; Souchelnytskyi et al., 1998), competing with Smad4 for R-Smad binding (Hata et al., 1998) or cooperating in the induction of type I BMP receptor degradation (Kavsak et al., 2000; Murakami et al., 2003). The extent to which certain activities are common to the I-Smads or unique to a specific I-Smad remains unclear.
The canonical Bmpr complex has been shown to mediate both the cell fate specification and guidance activities of the BMPs in the dorsal spinal cord (Lee et al., 1998; Liem et al., 1997; Yamauchi et al., 2008). Although the Smads have been implicated in the regulation of both cell fate specification and neurite outgrowth/regeneration in other systems (Moustakas and Heldin, 2009; Parikh et al., 2011; Yanagisawa et al., 2001; Zou et al., 2009), their role in mediating BMP signaling in the developing spinal cord remains unresolved. Previous studies have shown that Smad4 is critical for pattern formation in the dorsal neural tube (Chesnutt et al., 2004) however the function of the R-Smads and I-Smads has not been determined. Here, we investigate whether Smad6 and Smad7 regulate BMP signaling in vivo. Our studies in developing chicken embryos have shown that the I-Smads have different expression patterns in the spinal cord and are able to block distinct activities of the BMPs. Smad7 is present in newly differentiating neurons in the intermediate spinal cord and, when mis-expressed dorsally, blocks the acquisition of the dorsal interneuron (dI)1 and dI3 fates and results in a dorsal expansion of dI4–dI6 fates. In contrast, Smad6 is most highly expressed in mature neurons in the dorsal and intermediate spinal cord and, when ectopically expressed, inhibits dI1 axon outgrowth. Together, these studies suggest that the I-Smads act to endogenously limit the extent of BMP/activin signaling in the developing spinal cord and that different I-Smads can block specific activities of the BMPs.
Materials and methods
In situ hybridization and immunohistochemistry
Antibody staining and in situ hybridization histochemistry were performed on 20–30 μm transverse sections from Hamburger and Hamilton (HH) stages 10–27 (Hamburger and Hamilton, 1992) chick and Embryonic (E)13 rat spinal cords and dissociated dorsal neurons from E13 rat embryos as previously described (Augsburger et al., 1999). Fluorescence and differential interference contrast images were collected on a Carl Zeiss LSM510 confocal and Axiovert 200 M and Axioplan 2 microscopes. Images were quantified and processed using Adobe Photoshop CS4.
Chick Cath1 (Ben-Arie et al., 1996), Cash1 (Jasoni et al., 1994), Smad6, Smad7a and Smad7b (Vargesson and Laufer, 2009) in situ probes were made from linearized plasmids using a DIG RNA labeling kit (Roche).
Antibodies against the following proteins were used. Rabbit: Lhx2/9 (pan Lh2a/b), 1:1000 (Liem et al., 1997); Islet1/2 (K5), 1:2000 (Tsuchida et al., 1994); GFP 1:1000 (Invitrogen); Pax2, 1:250 (Invitrogen); Smad6 1:100 (Cell Signaling Technology); pSmad1/5/8, 1:1000 (a generous gift from Ed Laufer), Mafb 1:2000 (Eichmann et al., 1997). Mouse: Msx1/2 1:5 (Developmental Studies Hybridoma Bank); Pax6, 1:5 (Developmental Studies Hybridoma Bank); p27 (Kip1), 1:250 (BD Transduction Laboratories), FLAG (M2), 1:1000 (Sigma). Goat: Pax3, 1:250 (R&D Systems); Sox2, 1:1000 (Santa Cruz Biotechnology); Isl1 1:2000 (R&D Systems); Guinea pig: Ngn2, 1:16,000 (Skaggs et al., 2011). Sheep: GFP 1:2000 (Biogenesis). Species appropriate Cyanine 3 and Fluorescein conjugated secondary antibodies were used (Jackson ImmunoResearch Laboratories).
Expression constructs and in ovo electroporation
Expression constructs encoding Smad6 or Smad7 under the control of the CMV enhancer have been described by the Miyazono group (Ebisawa et al., 2001; Imamura et al., 1997). Expression constructs containing either Smad6 or Smad7 were fused to the Math1 enhancer (Helms et al., 2000) in combination with an IRES-farnesylated (f) GFP cassette as previously described (Yamauchi et al., 2008).
Fertile White Leghorn eggs (AA Laboratory Eggs, Westminster, CA) were incubated to HH stages 10–16. The following expression constructs were electroporated into the developing neural tube as previously described (Briscoe et al., 2000): CMV::GFP (0.2 μg/μl), CMV::Smad6 (1.8 μg/μl), CMV::Smad7 (1.8 μg/μl), Math1::fGFP (0.2 μg/μl), Math1::Smad6-IRES-fGFP (3 μg/μl), and Math1::Smad7-IRES-fGFP (3 μg/μl). All statistical analyses were performed using a two-tailed Student’s t-test.
Results
I-Smads are expressed in dynamic patterns in the developing spinal cord
The chicken genome contains a single Smad6 gene and two Smad7 genes, Smad7a and Smad7b (Vargesson and Laufer, 2009) which are 96% identical at the amino acid level. To investigate whether these I-Smads have a role in the specification of the dorsal spinal cord, we first examined whether Smad6 and Smad7a/b are present in the developing embryonic chicken spinal cord. We examined the expression pattern of the I-Smad genes from Hamburger Hamilton (HH) stages 10 to 27 (Hamburger and Hamilton, 1992) the period during which the fate of the dorsal populations of neurons, dI1–dI6 are specified (Gross et al., 2002; Helms and Johnson, 2003; Muller et al., 2002) and the dI1 commissural axon trajectory is established (Holley et al., 1982).
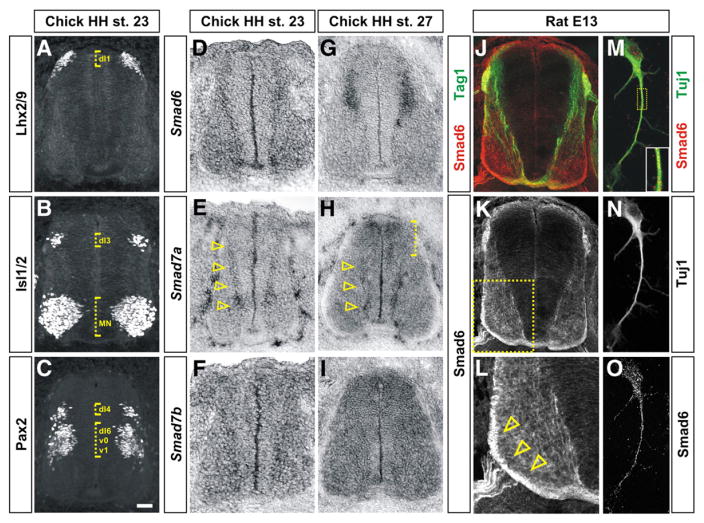
Using in situ hybridization and immunohistochemistry, we were unable to detect I-Smad gene expression during the early stages of the specification of the dorsal spinal cord, HH stages 10–18 (data not shown), suggesting that the I-Smads have no functional role at these stages. However, both Smad6 and Smad7 were present at slightly later stages of dorsal neural development, from HH stage 22 on, albeit in distinct patterns. At HH stage 23, Smad6 is expressed at low levels in many post-mitotic cells in the spinal cord (Fig. 1D). By HH stage 27, Smad6 resolves to be present at high levels only in differentiated neurons in the dorsal spinal cord (Fig. 1G). At this stage, the expression pattern of Smad6 substantially overlaps with the migrating Lhx2/9+ dI1 neurons (Supplemental Fig. 4), suggesting that Smad6 is present in dI1 neurons. The protein distribution of Smad6 in both the rodent spinal cord (Figs. 1J–L) and dissociated dorsal neurons (Figs. 1M–O) confirms that it is present on the processes of spinal neurons (Figs. 1K and O) including the Tag1+ dI1 axons (Fig. 1J). Intriguingly, Smad6 appears to be present at the highest levels on axons in the ventral funiculus (arrowheads, Fig. 1L), a position consistent with the contralaterally projecting dI1 commissural axons (arrowhead, Supplemental Fig. 4B).
Fig. 1.
Inhibitory Smads have dynamic expression patterns during early spinal cord development. (A–C) Transverse sections of the spinal cord taken from HH stage 23 chicken embryos labeled with antibodies against Lhx2/9 (A), Isl1/2 (B) and Pax2 (C) to establish the positions of dI1, dI3 and dI4 populations of dorsal interneurons (dotted brackets). (D–I) In situ hybridization experiments for Smad6 (D and G), Smad7a (E and H) and Smad7b (F and I) on sections of the spinal cord taken from either HH stage 23 (D, E and F) or HH stage 27 (G, H and I) chicken embryos. (D and G) Although Smad6 is broadly expressed in post-mitotic spinal neurons at HH stage 23, expression resolves to dorsal post-mitotic neurons by HH stage 27. (E and H) Smad7a is expressed at the highest levels in differentiating spinal neurons in the intermediate and ventral spinal cord at both HH stages 23 and 27 (arrowheads). At HH stage 27, Smad7a is also expressed in dorsal neural progenitors (dotted bracket, H). (F and I) Smad7b is expressed throughout the spinal cord with the highest levels at HH stage 27. (J–O) Antibodies against Smad6 (red, J–M and O) label neural processes in transverse sections (J–L) and Tuj1+ (green, M and N) dissociated dorsal neurons (M–O) taken from E13 rat spinal cords. Smad6 is co-expressed in the Tag1+ (green) dI1 (commissural) axons in the dorsal spinal cord (J) and is present at the highest levels on the contralaterally projecting commissural axons (compare arrowheads in L to the arrowhead in Supplemental Fig. 4B). Panel L is a higher magnification view of the boxed region in K. Scale bar: A–L: 25 μm, M–O: 5 μm.
In contrast, at HH stage 23, Smad7a is expressed at the highest levels in the intermediate spinal cord in newly born neurons at the boundary between the ventricular zone, where the progenitor cells are differentiating, and the mantle layer, where the post-mitotic neurons reside (arrowheads, Fig. 1E). These newly differentiated neurons have positions consistent with being from the dP4–dP6 and ventral p0–p2 progenitor cell populations (Figs. 1C and E). At HH stage 27, Smad7a is still present in newly born neurons in the ventral spinal cord (arrowheads, Fig. 1H) but is also up-regulated in the dorsal-most region of the ventricular zone (bracket, Fig. 1H). Smad7b is expressed at very low levels throughout the spinal cord at HH stage 23 (Fig. 1F) and at higher levels throughout the spinal cord at HH stage 27 (Fig. 1I). The dynamic expression patterns of the I-Smads suggest that they may inhibit the disparate effects of BMP mediated signaling, cell fate specification and axon guidance.
Ectopic expression of I-Smads in chicken embryos inhibits the acquisition of dorsal cell fate
Since little is known about the ability of the I-Smads to terminate BMP signaling in vivo, we examined the consequence of mis-expressing either Smad6 or Smad7 by in ovo electroporation of chicken embryos. Mouse Smad6 and Smad7 were used in these experiments; these proteins are 71% and 88%/87% identical to chicken Smad6 and Smad7a/b at the amino acid level. Since the mouse genome contains only one copy of Smad7, use of the mouse Smad7 gene circumvented the need to mis-express two nearly identical forms of chicken Smad7.
We have previously found that modulating the activity of the type I BMP receptors (Bmprs) altered the specification of dorsal cell fate only when Bmpr signaling is disrupted at HH stages 10–12 (Yamauchi et al., 2008). No fate changes were observed when Bmpr signaling was disrupted at HH stages 14/15 (Yamauchi et al., 2008). This result suggests that the “window” of competence for dorsal spinal neural progenitors to be re-specified by Bmpr signaling is closed by HH stage 15. Thus, we assessed whether the I-Smads could block the acquisition of dorsal cell fate in a similar time frame by ectopically expressing Smad6 or Smad7 throughout the spinal cord at either HH stages 10–12 (Fig. 2E) or HH stages 14–16 (Fig. 2F). Both I-Smad proteins were observed to be present at high levels throughout the spinal cord following electroporation at either stage (Supplemental Fig. 1 and data not shown). Electroporated embryos were permitted to develop to HH stages 24/25 and then examined for dorsal cell fate defects by assessing the distribution of Cath1 (Atoh1) in dP1 cells (Helms and Johnson, 1998) and Cash1 in dP3–dP5 cells (Gowan et al., 2001) as well as the number of Lhx2/9+ dI1 neurons (Lee and Jessell, 1999; Liem et al., 1997) and Isl1/2+ dI3 neurons (Liem et al., 1997), two of the dorsal cell types that are dependent on BMP/activin signaling from the roof plate (Lee et al., 1998; Liem et al., 1997; Timmer et al., 2005). As a control for this series of experiments, chicken embryos were also electroporated with CMV::GFP. This construct was never observed to have a significant effect on the development of the spinal cord (Figs. 2–5 and data not shown).
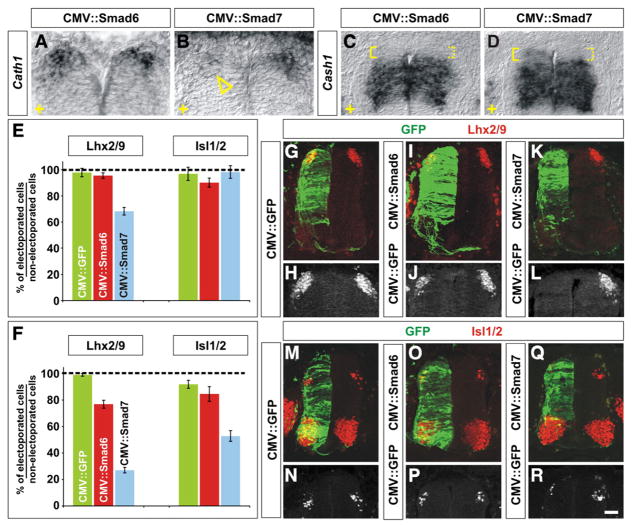
Fig. 2.
Mis-expression of I-Smads differentially affects the fate of dorsal spinal neurons. (A–R) GFP (G, H, M and N), Smad6 (A, C, I, J, O and P) or Smad7 (B, D, K, L, Q and R) were ectopically expressed throughout the spinal cord under the control of the CMV enhancer by in ovo electroporation at either HH stages 10–12 (E) or HH stages 14–16 (A–D, F–R). Embryos were harvested at HH stages 24/25 and examined for the extent of Cath1 (A and B) and Cash1 (C and D) expression and the number of Lhx2/9+ commissural (dI1) neurons (red, G–L) or Isl1/2+ association (dI3) neurons (red, M–R). (A–D) In situ hybridization experiments demonstrated that in ovo electroporation of Smad7 (arrowhead, B), but not Smad6 (A), greatly reduces the extent of expression of Cath1 expression (A and B) suggesting that dP1 cells have been lost. Cash1 levels were also affected by Smad7 (D), but Smad6 (C), mis-expression, low levels of Cash1 expression were now observed adjacent to the roof plate (compare expression levels in solid brackets to dotted brackets, C and D). The electroporated side is indicted by +. (E) The ectopic expression of Smad6 from HH stages 10–12 had no effect on the number of either dI1 or dI3 neurons compared to the electroporation of GFP (dI1, Student’s t-test, p>0.53 probability different from GFP+ control, n=107 sections from 8 embryos; dI3, p>0.29, n=50 sections from 5 embryos). In contrast, the mis-expression of Smad7 resulted in a 30% loss of dI1 neurons (p<4.3×10−11, n=72 sections from 4 embryos) but had no effect on the number of dI3 neurons (p>0.70, n=47 sections from 6 embryos). (F–R) A CMV::GFP construct electroporated into the chick spinal cord at HH stages 14–16 had no observable effect on the number of either Lhx2/9+ dI1 (F, G and H; n=316 sections from 8 embryos) or Isl1/2+ dI3 neurons (F, M and N; n=72 sections from 5 embryos). The mis-expression of Smad6 construct had a weak effect on cell fate: 20% of dI1 neurons were lost (F, I and J; p<6.4×10−17 probability different from GFP+ control, n=69 sections from 5 embryos) but there was no significant effect of mis-expressing Smad6 on the number of dI3 neurons (F, O and P; p>0.74, n=45 sections from 5 embryos). In contrast, the mis-expression of Smad7 resulted in a profound loss of both dI1 and dI3 neurons. Over 70% of dI1 neurons were lost (F, K and L; p<2.7×10−131, n=105 sections from 9 embryos) whereas about 50% of dI3 neurons were lost (F, Q and R; p<2.2×10−11, n=80 sections from 5 embryos) from HH stage 24/25 spinal cords. Scale bar: 25 μm.
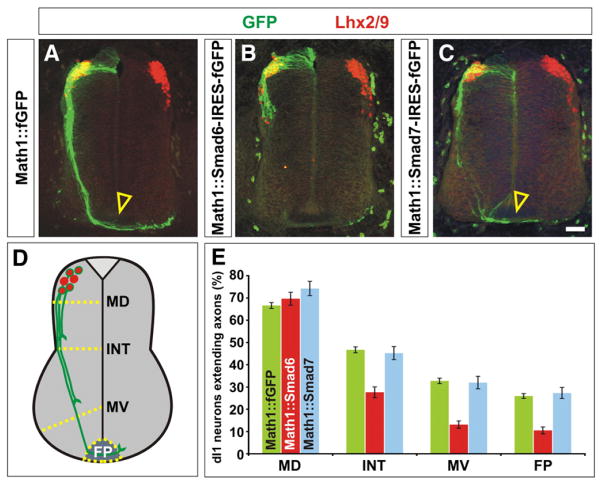
Fig. 5.
Mis-expression of Smad6, but not Smad7, resulted in decreased dI1 commissural axon outgrowth. (A) Lhx2/9+ dI1 commissural neurons (red) electroporated with farnesylated (f) GFP under the control of the Math1 enhancer (Math1::fGFP) at HH stage 15, extend GFP+ axons (green) normally to the floor plate (FP, arrowhead) by HH stage 23. (B) In contrast, Lhx2/9+ dI1 neurons (red) expressing a Smad6-IRES-fGFP cassette (green) have dramatically reduced axon outgrowth by HH stage 23. (C) Lhx2/9+ dI1 axons electroporated with a Smad7-IRES-fGFP cassette (green) extend normally to the FP (arrowhead) by HH stage 23. (D) The extent of the dI1 axon outgrowth was quantified by determining whether dI1 axons had crossed one of four arbitrary lines in the spinal cord: mid-dorsal (MD), intermediate (INT), mid-ventral (MV) or the FP. (E) By HH stage 23, 65–75% of Lhx2/9+ neurons electroporated with GFP, Smad6-IRES-fGFP or Smad7-IRES-fGFP had extended GFP+ axons. Over 35% of these axons had reached the FP in both the GFP (n=465 sections, taken from 10 embryos) or Smad7 (n=116 sections taken from 5 embryos) mis-expression experiments (probability of similarity, p>0.57). In contrast, only 15% of Lhx2/9+ neurons electroporated with Smad6-IRES-fGFP (n=105 sections, taken from 5 embryos) had extended to axons to the FP (probability of similarity to GFP control, p<9.4×10−11). Scale bar: 25 μm.
Despite the importance of BMP signaling in the early development of the spinal cord, the mis-expression of the I-Smads throughout the spinal cord at HH stages 10–12 had surprisingly modest effects on the specification of dorsal cell fate (Fig. 2E). No significant loss of dorsal spinal neurons was observed following the ectopic expression of Smad6. Smad7 mis-expression resulted in the loss of about 30% of dI1 neurons, but did not have a measurable effect on the number of dI3 neurons (Fig. 2E). These observations may stem from the I-Smads being unexpectedly ineffective at blocking endogenous R-Smad signaling at HH stages 10–12 since the electroporation of either I-Smad did not result in a significant loss of R-Smad activity (Supplemental Fig. 1I).
In contrast, the mis-expression of Smad6 and Smad7 at HH stages 14–16 had far more severe consequences for the identity of the dorsal spinal cord. Both I-Smads were able to equivalently inhibit the activity of the R-Smads (Supplemental Figs. 1D, H and I). However, only the ectopic expression of Smad7 significantly affected the domains of Cath1 and Cash1 expression. The extent of Cath1 was greatly diminished (arrowhead, Fig. 2B), and low levels of Cash1 now extended dorsally to the roof plate (solid bracket, Fig. 2D), suggesting that Smad7 can suppress the dP1 cell fate, while promoting dP3–dP5 fates. Examination of post-mitotic neural dorsal markers confirmed that the ectopic expression of Smad7 more profoundly affects the identity of the dorsal spinal cord compared to Smad6. The mis-expression of Smad6 resulted in the loss of 20% of the dI1 neurons (Figs. 2F, I and J) but had no apparent effect on the number of dI3 neurons (Figs. 2F, O and P). In contrast, the mis-expression of Smad7 caused major changes in the numbers of both dI1 and dI3 neurons. Nearly 80% of the dI1 neurons were missing (Figs. 2F, K and L) and 50% of the dI3 neurons were absent (Figs. 2F, Q and R).
To summarize these results, the I-Smads appear to be most effective in blocking BMP/activin signaling during a later time period than the one in which the type I Bmprs specify dorsal fate (Yamauchi et al., 2008). The I-Smads also appear to have different activities when mis-expressed, with Smad7 being more successful than Smad6 at blocking the acquisition of the dorsal-most cell fates in the spinal cord. Taken together with the endogenous expression pattern of Smad7a, these results suggest a model in which the presence of Smad7 acts to limit the action of dorsalizing BMP/activin signals from the roof plate on newly differentiating neurons in the intermediate spinal cord as well as later born neural progenitors in the dorsal ventricular zone.
Smad7, but not Smad6, misexpression results in an increase in Pax2+ dI4 neurons
To further assess whether Smad6 or Smad7 has a role in mediating dorsal cell fate, we determined whether there were compensatory fate changes in the spinal cord following the electroporation of the I-Smads at HH stages 14–16 that indicated the fate of the presumptive dI1 and dI3 neurons. We first determined whether there were increased levels of cell death in the spinal cord following electroporation of CMV::Smad6 and CMV::Smad7. In both cases, the introduction of either of these constructs had no effect on the number of apoptotic cells detected by the TUNEL reaction (Supplemental Fig. 2 and data not shown).
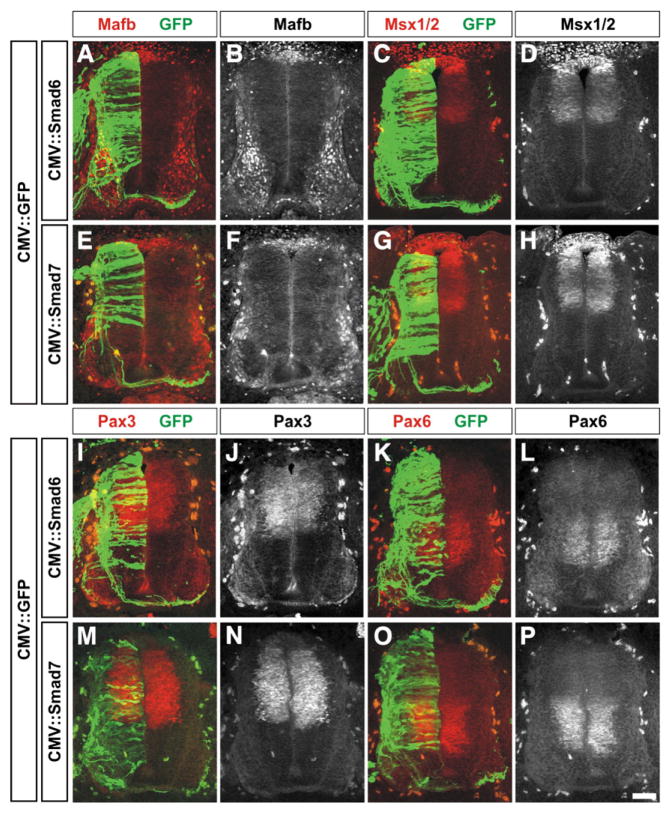
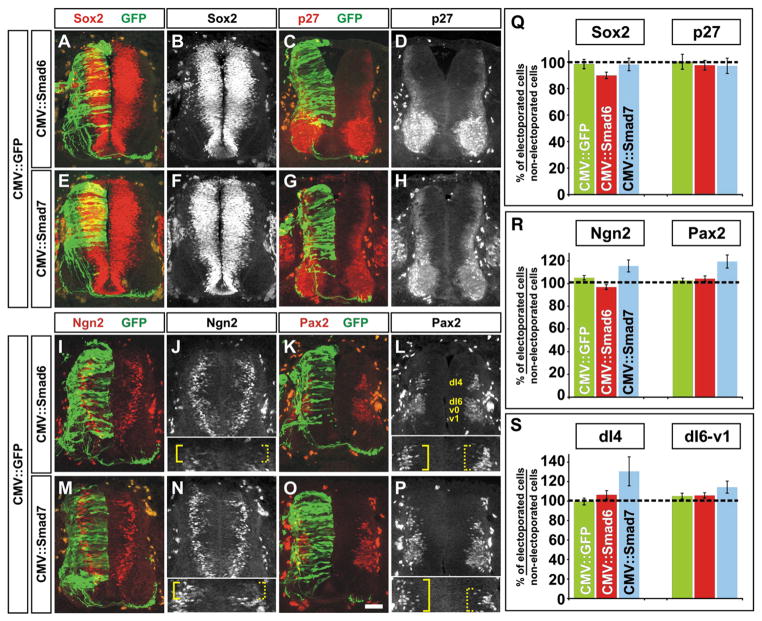
We then examined whether the overall pattern of the dorsal half of the spinal cord was altered. We found that there was no observable effect of electroporation with either CMV::Smad6 or CMV::Smad7 on the distribution of Mafb, Msx1/2, Pax3 and Pax6, markers of the dorsal and intermediate spinal cord (Fig. 3). Thus, there did not appear to be a gross defect in either the formation of the roof plate or the patterning of dorsal or intermediate spinal neural progenitors. We also assessed the overall number of both Sox2+ neural progenitors (Bylund et al., 2003) and p27+ post-mitotic neurons (Novitch et al., 2001) following mis-expression of the I-Smads. Supporting the conclusion that the mis-expression of the I-Smads did not result in ectopic cell death, the over-expression of either Smad6 (Figs. 4A–D) or Smad7 (Figs. 4E–H) had no observable effect on the number of Sox2+ or p27+ cells compared to the GFP control (Fig. 4Q).
Fig. 3.
Mis-expression of Smad6 and Smad7 does not affect the distribution of many markers of the roof plate and dorsal and intermediate spinal cord. (A–P) GFP (green) in combination with either Smad6 (A–D and I–L) or Smad7 (E–H and M–P) were ectopically expressed throughout the spinal cord under the control of the CMV enhancer at HH stages 14/15. Embryos were harvested at HH stages 24/25 and examined for the distribution of markers that broadly demarcate the dorsal and intermediate spinal cord: Mafb (A, B, E and F; roof plate cells and post-mitotic MNs, Augsburger et al., 1999), Msx1/2 (C, D, G and H: roof plate and dorsal progenitor neurons, Timmer et al., 2002), Pax3 (I, J, M and N; dP1–dP6 progenitor neurons, Goulding et al., 1991) or Pax6 (K, L, O and P; p0–p2, pMN progenitor neurons, Ericson et al., 1997). There was no observable difference between the distribution of these markers on the electroporated or non-electroporated side of the spinal cord. Scale bar: 25 μm.
Fig. 4.
Smad7, but not Smad6, mis-expression leads to a compensatory increase in the number of dI4 neurons. (A–P) GFP (green) in combination with either Smad6 (A–D and I–L) or Smad7 (E–H and M–P) were ectopically expressed throughout the spinal cord under the control of the CMV enhancer at HH stages 14–16. Embryos were harvested at HH stages 24/25 and examined for the number of Sox2+ (A, B, E and F; neural progenitors), p27+ (C, D, G and H; post-mitotic neurons), Ngn2+ (I, J, M and N; dP2–5 progenitors) and Pax2+ (K, L, O and P; post-mitotic dI4 and dI6-v1 neurons) cells in the dorsal half of the spinal cord. The inserts in panels J and N show a magnified view of the most dorsal region of the spinal cord. Ngn2+ cells are present more dorsally only on the Smad7 electroporated side of the spinal cord (compare solid brackets, J and N). The inserts in panels L and P show a magnified view of the dI4 population of Pax2+ neurons. Pax2+ cells were present in a more dorsal location only after Smad7 mis-expression (compare solid brackets, L and P). (Q) There was no significant difference in the number of Sox2+ or p27+ cells following electroporation of either CMV::Smad6 (Student’s t-test, Sox2: p>0.18, n=19 sections taken from 5 embryos; p27: p>0.45, n=19 sections taken from 4 embryos) or CMV::Smad7 (Sox2: p>0.59, n=18 sections taken from 4 embryos ; p27: p>0.76, n=13 sections taken from 4 embryos) compared to the CMV::GFP+ control (Sox2: n=20 sections taken from 3 embryos; p27: n=19 sections taken from 3 embryos). (R) In contrast, there was a significant increase in the number of both Ngn2+ and Pax2+ cells following electroporation of CMV::Smad7 (Student’s t-test, Ngn2: p<0.027, n=26 sections taken from 7 embryos; Pax2: p<0.0012, n=33 sections taken from 7 embryos) but not CMV::Smad6 (Ngn2: p>0.13, n=51 sections taken from 11 embryos; Pax2: p>0.61, n=68 sections taken from 11 embryos) compared to the CMV::GFP+ control (Ngn2: n=54 sections taken from 8 embryos; Pax2: n=66 sections taken from 6 embryos). (S) Further examination of the Pax2+ population showed that the mis-expression of Smad7 resulted in significantly more neurons in the more dorsal dI4 Pax2+ cell population compared to the more ventral dI6-v1 Pax2+ population (dI4: p<0.0014 probability of similarity with GFP+ control, n=24 sections from 7 embryos; dI6: p>0.13, n=26 sections from 7 embryos). Scale bar: 25 μm.
Finally, we assessed whether there was a compensatory increase in the number of dorsal neurons thought to form independently of BMP signaling. Previous studies have shown that the dI4–dI6 populations of neurons are generated in the absence of either the roof plate or Bmpr signaling (Lee et al., 2000; Millonig et al., 2000; Wine-Lee et al., 2004). We thus examined whether there was an alteration in the distribution of Ngn2, present in newly differentiating dP2–dP5 neural progenitors, or Pax2, present in differentiated dI4 and dI6-v1 cells (Burrill et al., 1997; Gowan et al., 2001; Helms and Johnson, 2003; Zhuang and Sockanathan, 2006). The mis-expression of either GFP or Smad6 had no effect on the distribution of Ngn2 or Pax2 (Figs. 4I–L and R). In sharp contrast, the mis-expression of Smad7 resulted in a 15% increase in the number of Ngn2+ progenitors (solid bracket, Figs. 4M, N and R) and a 20% increase in the overall number of Pax2+ neurons (Figs. 4O, P and R). Further inspection showed that the additional Pax2+ neurons predominately came from the dI4 population of neurons (Fig. 4S) and that these neurons expanded into a dorsal location that overlapped with the Isl1+ dI3 population of neurons (solid bracket, Fig. 4P and Supplemental Fig. 3). There was no significant difference in the number of Pax2+ dI6-v1 neurons following electroporation with Smad7, Smad6 or GFP (Fig. 4S).
Thus, the mis-expression of Smad7, but not Smad6, results in the loss of the BMP/activin dependent spinal neurons and an increase in the number of dorsal spinal neurons generated independently of signals from the roof plate. This result strongly supports a model in which Smad7 acts in newly differentiating neurons in the intermediate spinal cord to block BMP/activin signaling from the roof plate and thereby enables the generation of dI4–dI6 spinal neurons.
Smad6, but not Smad7, regulates dI1 axon outgrowth
As development proceeds, Smad6 gene expression is strongly upregulated in a population of dorsal post-mitotic neurons (Fig. 1G) and Smad6 protein is present on processes, most notably on the contralaterally projecting commissural axons (Figs. 1J–L). The position of many of the Smad6+ cells overlaps with the early-born Lhx2/9+ dI1 commissural neurons, which by HH stage 27 are in the process of migrating to a deeper location in the intermediate spinal cord (Supplemental Fig. 4). In contrast, the expression pattern of Smad7a in the dorsal spinal cord is limited to progenitors in the ventricular zone and Smad7b is ubiquitously expressed at low levels. Since BMP signaling from the roof plate is also required to guide dI1 axons away from the dorsal midline (Augsburger et al., 1999; Butler and Dodd, 2003), we assessed whether the distinct expression patterns of the I-Smads might have a functional consequence in the formation of the dI1 axonal circuit.
Smad6 and Smad7 were ectopically expressed in chicken embryos under the control of Math1 (Atoh1) enhancer, which specifically drives the expression of heterologous genes in differentiated dI1 neurons (Helms et al., 2000). An IRES-farnesylated (f) GFP cassette was included in the construct so that the extent of axon outgrowth could be assessed. Math1::fGFP and Math1::Smad6/7-IRES-fGFP were electroporated at HH stages 14–16 and the resulting embryos examined at HH stages 22/23. The extent of dI1 axon outgrowth was quantified by assessing the number of Lhx2/9+ GFP+ dI1 axons that had reached four arbitrarily drawn boundaries along the dorsal–ventral axis of the spinal cord, mid-dorsal (MD), intermediate (INT), mid-ventral (MV) and floor plate (FP) (Fig. 5D, Phan et al., 2010).
By HH stage 23, 65% of the control GFP+ dI1 neurons had extended axons and approximately 40% of these axons had reached the floor plate (arrowhead, Figs. 5A and E). Similarly, 75% of the Smad7+ dI1 neurons extended axons of which 35% had reached the floor plate (arrowhead, Figs. 5C and E). In contrast, when Smad6 was ectopically expressed in dI1 neurons, the axons showed significant outgrowth defects. Although 70% of Smad6+ dI1 neurons extended axons, far fewer of these axons, only 15%, had reached the floor plate by HH stage 23 (Figs. 5B and E). This result suggests that a key activity of Smad6 in the developing spinal cord is to regulate axon outgrowth, a previously unknown function for I-Smad signaling in vivo. Interestingly, this phenotype was not manifested by all classes of commissural axons, since GFP+ axons were observed to cross the floor plate following electroporation of CMV:: Smad6 (Figs. 2I and O, 3A, C, I and K, 4A, C, I and K). Thus, the ability of Smad6 to block axonal outgrowth may be restricted to the dI1 neurons.
In summary, this study suggests that the I-Smads can regulate the diverse effects of BMP signaling in the spinal cord. However, whereas Smad7 may primarily inhibit the specification of dI1–dI3 cell fate, Smad6 regulates axonal outgrowth in post-mitotic dI1 neurons.
Discussion
The I-Smads can function to block BMP/activin signaling in the spinal cord
Although the type I Bmprs (Dewulf et al., 1995; Roelen et al., 1997; Yamauchi et al., 2008) and Smads (Arnold et al., 2006; Faure et al., 2002; Flanders et al., 2001 and VMH and SJB, unpublished data) are present throughout the dorsal spinal cord, only the dorsal-most populations of spinal neurons require BMP/activin signaling for their identity. What limits the action of BMP/activin signals from the roof plate to these neurons? Our studies have suggested a role for Smad7a in this process. Smad7a is expressed in newly differentiating dorsal neurons in the intermediate spinal cord from HH stages 22/23 (~3.5 days). The timing by which dI4–dI6 neurons arise in the chick spinal cord has not been precisely established, however genetic studies in mouse (Glasgow et al., 2005; Gross et al., 2002; Helms et al., 2005; Muller et al., 2002) have suggested that dI4–dI6 neurons start to be born around embryonic (E) stage 10.5 and continue to be born through stage E11.5. The roughly analogous period in chicken embryonic development is from HH stages 18/19 (~3 days) to HH stage 24 (~4 days). Thus, Smad7a starts to be expressed immediately as the dI4–dI6 neurons are exiting the cell cycle and is present in differentiating neurons as they emerge from the intermediate ventricular zone. The timing of Smad7a expression is therefore consistent with Smad7a having a role ending the ability of dI4–dI6 neurons to receive patterning information from the roof plate. Moreover, this result suggests the final integration of dorsal patterning information may occur at the time of cell cycle exit. Our studies have also suggested that Smad7 may function endogenously to attenuate the ability of the BMPs to promote the dI1 fate, which is dependent on the patterned expression of Atoh1 (Cath1, (Gowan et al., 2001). Atoh1 expression starts to diminish around E12.5 (roughly equivalent to HH stage 27, (Helms et al., 2000). Thus, the expression of Smad7a in the dorsal-most neural progenitors from HH stage 27 together with the ability of Smad7 to potently suppress Cath1 expression strongly suggests that Smad7a functions to limit the production of dI1 neurons.
We examined the consequence of ectopically expressing the I-Smads throughout the dorsal spinal cord before they are normally present. These experiments revealed that Smad7 has the ability to potently suppress the acquisition of the dorsal-most fates, dI1 and dI3 while promoting the more intermediate dI4 fate. This activity is consistent with the model that Smad7a functions to establish the dI4 fate by blocking the acquisition of BMP-dependent fates. Moreover, the results from our experiments suggested Smad6 and Smad7 have specific roles regulating the different activities of the BMPs. Thus, Smad7 is an effective inhibitor of the acquisition of the dI1 and dI3 cell fates, whereas Smad6 only has a more modest effect on the specification of dI1 identity. In contrast, the mis-expression of Smad7 had no effect on the extension of axons, whereas over-expression of Smad6 inhibited dI1 commissural axon outgrowth. Taken together, these studies suggest that the key function of Smad7 in the intermediate spinal cord is to limit the action of BMP/activin signals from the roof plate, whereas Smad6 acts to regulate axon outgrowth.
I-Smads act later in development than Bmpr signaling to modulate dorsal cell fate
A noteworthy feature of our studies is that the effect of mis-expressing the I-Smads on cell fate is temporally dependent. Thus, we observed severe defects when the I-Smads were electroporated at HH stages 14–16 and modest to no defects when they were mis-expressed approximately 12 h earlier at HH stages 10–12. In contrast, in our previous studies, we found that constitutively active type I Bmprs (caBmprIs) are able to reprogram the dorsal spinal cord towards the dorsal-most fates only when ubiquitously expressed at HH stages 10–12 (Yamauchi et al., 2008). By HH stages 14/15, the mis-expression of caBmprIs had no effect on the fate of dorsal spinal neurons, but did have a profound effect on the extent of axon outgrowth (Yamauchi et al., 2008). Thus, the time frame over which the fate of dP1–dP3 cells can be re-specified by the I-Smads is later than the time frame over which the type I Bmprs can modulate fate in these cells. This result infers that, in the context of cell fate, the I-Smads are unlikely to act by directly blocking the function of the type I Bmprs in dI4–dI6 neurons, rather they act after the role of the type I Bmprs is over.
Our previous studies suggested that the “window” of competence during which the type I Bmprs can specify dorsal cell fate closes by HH stages 14/15 (Yamauchi et al., 2008). Although we have not identified when this window closes for I-Smad signaling, our results suggest that it does not open if dorsal spinal neurons are exposed to I-Smads too early in development. We had initially predicted that the earlier I-Smads were mis-expressed during development, the stronger their effect would be on the acquisition of cell fate. However, dorsal neural progenitors in the HH stage 10–12 spinal cord are, in fact, largely impervious to the ectopic expression of the I-Smads. Moreover the progenitors remain resistant, even though the expression of the I-Smads presumably persists a further 12 h until the spinal cord reaches HH stages 14–16, resulting in no change in the activity of the R-Smads by HH stages 24/25. In contrast, if the I-Smads, particularly Smad7, is electroporated into HH stages 14–16 spinal cords, dorsal cells are now competent to respond to the I-Smads, resulting in the loss of the most dorsal spinal cell fates and an increase in the number of dI4–dI6 neurons. These results are not a consequence of differences in the levels of I-Smad expression after electroporation at HH stages 10–12 and HH stages 14–16; the I-Smads were equivalently expressed at both stages. Rather, it appears that the window of competence must also open for dorsal cells to be able to respond to the I-Smads and that persistent early expression of the I-Smads results in the failure of that window to open.
Smad7 may function in the specification of dI4–dI6 fate
Our studies indicate that both of the I-Smads can function to block the specification of dorsal spinal neurons. However, Smad6 only affects 20% of dI1 neurons whereas the ectopic expression of Smad7 results in the loss of large numbers of dI1 and dI3 neurons. Moreover, the mis-expression of Smad7, but not Smad6, resulted in the formation of ectopic Cash1/Ngn2+ neural progenitors and Pax2+ neurons in the dorsal-most region of spinal cord. The remainder of the spinal cord appeared to develop normally with no change in the number of Mafb+ roof plate cells, Pax3+ and Pax6+ neural progenitors and Pax2+ dI6-v1 neurons. These results suggest that the neurons that would have adopted a dI1–dI3 fate under the influence of signals from the roof plate, instead became dI4–dI6 neurons, the dorsal neurons that form independently of signals from the roof plate. The precise identity of the ectopic Ngn2+ and Pax2+ neurons remains unclear. They formed closest to the position of the Pax2+ dI4 neurons, however in this case the location of the cell bodies along the dorsal–ventral axis of the spinal cord may not be indicative of their identity. Additionally, the number of ectopic Ngn2+/Pax2+ neurons does not account for the number of dI1 and dI3 neurons lost after Smad7 electroporation. On average, after Smad7 mis-expression, 20–25 dI1 neurons and 10 dI3 neurons were lost, however an average of only five ectopic Pax2+ cells were observed. It seems unlikely that the presumptive dI1/dI3 cells died: there was no increase in apoptotic cell death and the overall number of Sox2+ neural progenitors and p27+ neurons did not decrease. Thus, Smad7 does not interfere with the ability of BMP signaling to facilitate neurogenesis (Shi and Liu, 2011) rather, the Smad7+ cells continued to manifest general dorsal characteristics: they expressed Pax3 and Msx1/2, markers of the dorsal spinal cord (Goulding et al., 1991), but not Pax6 (Ericson et al., 1997) or Dbx1 (Pierani et al., 2001) markers of intermediate and ventral spinal fates (Fig. 3 and data not shown). It is possible that the presumptive dI1/dI3 cells were undetermined with respect to their identity and as development proceeded, more of them would have converted to a Pax2+ fate.
Our studies have shed some light on the mechanistic basis by which Smad6 and Smad7 inhibit dI1 and dI3 fate. Both BMP and activin signaling are important in the establishment of dorsal spinal cord identity (Liem et al., 1997; Timmer et al., 2005). Studies in vitro have suggested that Smad6 primarily inhibits BMP signaling (Goto et al., 2007) whereas Smad7 non-specifically blocks signaling within the TGFβ superfamily (Mochizuki et al., 2004) as well as regulating β-catenin (Han et al., 2006; Tang et al., 2008). Our in vivo studies examining the effect of the I-Smads on cell fate are consistent with this model; Smad6 may be less effective at inhibiting the dorsal-most cell fates because it only blocks BMP signaling, in contrast Smad7 can inhibit BMP, activin and Wnt signaling. Supporting this model, Smad6 has no effect on the dI3 fate, which has been shown to be dependent on activin signaling (Timmer et al., 2005). That Smad7 acts to modulate cell fate decisions some 12 h after the action of type I Bmpr signaling suggests that, in this case, Smad7 does not directly inhibit type I Bmpr signaling, but rather interferes with formation of the trimeric Smad complex (Zhang et al., 2007) or works in combination with Smurf1, a E3 ubiquitin protein ligase that targets the receptor complex for degradation (Ebisawa et al., 2001; Murakami et al., 2003). In either case, the ability of Smad7 to block BMP/TGFβ signaling locally in the intermediate spinal cord appears to be critical for the assumption of dI4–dI6 fate.
Smad6 regulates dI1 axon outgrowth
Our previous studies have demonstrated that the roof plate BMPs can also act as a chemorepellent, guiding dI1 commissural axons away from the dorsal midline (Augsburger et al., 1999; Butler and Dodd, 2003). We initially concentrated on the ability of the BMPs to orient dI1 axons, however our subsequent studies have shown that the BMPs also act to regulate the rate of dI1 axon growth as they grow through the dorsal spinal cord (Phan et al., 2010). These studies focused on the role of a non-canonical effector of BMP signaling Lim domain kinase (Limk) 1 acting locally to slow axon extension in the dorsal spinal cord. If the rate of outgrowth was not correctly regulated, dI1 axons made errors in their guidance decisions (Phan et al., 2010). Thus, the ability of the BMPs to control the rate of dI1 axon outgrowth is critical for stereotyped circuit formation. Moreover, an unusual topographical feature of the dI1 neurons is that their cell bodies remain near the source of BMPs for some days, thus in theory allowing BMP signaling to have a lingering effect on dI1 axon growth beyond the trajectory in the dorsal spinal cord.
Here we show that Smad6 is expressed in HH stage 27 post-mitotic migrating dI1 neurons (Fig. 1G) and Smad6 protein is present at high levels in contralaterally projecting dI1 axons (Figs. 1J–L). When Smad6 was mis-expressed at an earlier time in development axon growth was slowed in vivo, supporting the results from studies showing that the I-Smads can decrease neurite outgrowth in vitro (Yanagisawa et al., 2001) and that constitutively active or dominantly negative type I Bmprs inhibit dI1 axon outgrowth (Yamauchi et al., 2008 and KDP and SJB, unpublished data). Smad6 and the type I Bmprs affect dI1 axon outgrowth during the same time period, suggesting that Smad6 could regulate axon outgrowth by interacting with the type I Bmprs. However, we are observing the effects of Smad6 up to 40 h after electroporation, a period of time too long to conclude that Smad6 must act by directly interfering with BmprI receptor signaling. An alternate model is that Smad6 blocks the formation of the Smad complex. Smad6 has been shown to interact with Smad1 in Xenopus (Hata et al., 1998). Our studies have indicated that Smad6 can effectively inhibit the activity of the R-Smads (Supplemental Fig. 1A) and that Smad1 is present in neural processes (VMH and SJB, unpublished data). Recent work has shown that the activation of Smad1 can promote growth in regenerating axons (Parikh et al., 2011; Zou et al., 2009). Taken together, these observations suggest that presence of Smad6 could act to antagonize the intra-axonal Smad signaling, and thereby modulate axonal growth rate. The role of the Smads during axon guidance remains to be determined, however there has been elegant work showing that the R-Smads function as retrograde signals in the control of synaptic growth and homeostasis (Ball et al., 2010; Goold and Davis, 2007; McCabe et al., 2003).
Summary
These studies demonstrate a role for the I-Smads in the regulation of BMP/activin signaling, permitting the effect of BMP/activin signaling to be limited to specific areas of the dorsal spinal cord. They further shed light on functional differences between the action of Smad6 and Smad7 in the developing spinal cord, which permit them to inhibit different activities of the BMPs.
Acknowledgments
We are most grateful to Michele Frendo, Jan Smogorzewski and Ken Yamauchi for technical assistance, Kohei Miyazono for the Smad6 and Smad7 constructs, Ed Laufer for the Smad6, Smad7a and Smad7b in situ probes and phoso-Smad1/5/8 antibodies and Ben Novitch for the Cath1/Cash1 in situ probes and Ngn2 antibodies. We would also like to thank Michael Quick for continued support, members of the Butler and Novitch labs for helpful discussions and Ben Novitch and Ken Yamauchi for comments on the manuscript. This work was supported by grants from the James H. Zumberge Research and Innovation Fund, the March of Dimes Foundation (1-FY07-458) and the National Institutes of Health (NS 063999) to S.J.B.
Footnotes
Supplementary materials related to this article can be found online at doi:10.1016/j.ydbio.2011.06.017.
References
- Arnold SJ, Maretto S, Islam A, Bikoff EK, Robertson EJ. Dose-dependent Smad1, Smad5 and Smad8 signaling in the early mouse embryo. Dev Biol. 2006;296:104–118. doi: 10.1016/j.ydbio.2006.04.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augsburger A, Schuchardt A, Hoskins S, Dodd J, Butler S. BMPs as mediators of roof plate repulsion of commissural neurons. Neuron. 1999;24:127–141. doi: 10.1016/s0896-6273(00)80827-2. [DOI] [PubMed] [Google Scholar]
- Ball RW, Warren-Paquin M, Tsurudome K, Liao EH, Elazzouzi F, Cavanagh C, An BS, Wang TT, White JH, Haghighi AP. Retrograde BMP signaling controls synaptic growth at the NMJ by regulating trio expression in motor neurons. Neuron. 2010;66:536–549. doi: 10.1016/j.neuron.2010.04.011. [DOI] [PubMed] [Google Scholar]
- Ben-Arie N, McCall AE, Berkman S, Eichele G, Bellen HJ, Zoghbi HY. Evolutionary conservation of sequence and expression of the bHLH protein Atonal suggests a conserved role in neurogenesis. Hum Mol Genet. 1996;5:1207–1216. doi: 10.1093/hmg/5.9.1207. [DOI] [PubMed] [Google Scholar]
- Briscoe J, Pierani A, Jessell TM, Ericson J. A homeodomain protein code specifies progenitor cell identity and neuronal fate in the ventral neural tube. Cell. 2000;101:435–445. doi: 10.1016/s0092-8674(00)80853-3. [DOI] [PubMed] [Google Scholar]
- Burrill JD, Moran L, Goulding MD, Saueressig H. PAX2 is expressed in multiple spinal cord interneurons, including a population of EN1+ interneurons that require PAX6 for their development. Development. 1997;124:4493–4503. doi: 10.1242/dev.124.22.4493. [DOI] [PubMed] [Google Scholar]
- Butler SJ, Dodd J. A role for BMP heterodimers in roof plate-mediated repulsion of commissural axons. Neuron. 2003;38:389–401. doi: 10.1016/s0896-6273(03)00254-x. [DOI] [PubMed] [Google Scholar]
- Bylund M, Andersson E, Novitch BG, Muhr J. Vertebrate neurogenesis is counteracted by Sox1-3 activity. Nat Neurosci. 2003;6:1162–1168. doi: 10.1038/nn1131. [DOI] [PubMed] [Google Scholar]
- Charron F, Stein E, Jeong J, McMahon AP, Tessier-Lavigne M. The morphogen sonic hedgehog is an axonal chemoattractant that collaborates with netrin-1 in midline axon guidance. Cell. 2003;113:11–23. doi: 10.1016/s0092-8674(03)00199-5. [DOI] [PubMed] [Google Scholar]
- Chesnutt C, Burrus LW, Brown AM, Niswander L. Coordinate regulation of neural tube patterning and proliferation by TGFbeta and WNT activity. Dev Biol. 2004;274:334–347. doi: 10.1016/j.ydbio.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Chizhikov VV, Millen KJ. Roof plate-dependent patterning of the vertebrate dorsal central nervous system. Dev Biol. 2005;277:287–295. doi: 10.1016/j.ydbio.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Dewulf N, Verschueren K, Lonnoy O, Moren A, Grimsby S, Vande Spiegle K, Miyazono K, Huylebroeck D, Ten Dijke P. Distinct spatial and temporal expression patterns of two type I receptors for bone morphogenetic proteins during mouse embryogenesis. Endocrinology. 1995;136:2652–2663. doi: 10.1210/endo.136.6.7750489. [DOI] [PubMed] [Google Scholar]
- Ebisawa T, Fukuchi M, Murakami G, Chiba T, Tanaka K, Imamura T, Miyazono K. Smurf1 interacts with transforming growth factor-beta type I receptor through Smad7 and induces receptor degradation. J Biol Chem. 2001;276:12477–12480. doi: 10.1074/jbc.C100008200. [DOI] [PubMed] [Google Scholar]
- Eichmann A, Grapin-Botton A, Kelly L, Graf T, Le Douarin NM, Sieweke M. The expression pattern of the mafB/kr gene in birds and mice reveals that the kreisler phenotype does not represent a null mutant. Mech Dev. 1997;65:111–122. doi: 10.1016/s0925-4773(97)00063-4. [DOI] [PubMed] [Google Scholar]
- Ericson J, Rashbass P, Schedl A, Brenner-Morton S, Kawakami A, van Heyningen V, Jessell TM, Briscoe J. Pax6 controls progenitor cell identity and neuronal fate in response to graded Shh signaling. Cell. 1997;90:169–180. doi: 10.1016/s0092-8674(00)80323-2. [DOI] [PubMed] [Google Scholar]
- Faure S, de Santa Barbara P, Roberts DJ, Whitman M. Endogenous patterns of BMP signaling during early chick development. Dev Biol. 2002;244:44–65. doi: 10.1006/dbio.2002.0579. [DOI] [PubMed] [Google Scholar]
- Feng XH, Derynck R. Specificity and versatility in tgf-beta signaling through Smads. Annu Rev Cell Dev Biol. 2005;21:659–693. doi: 10.1146/annurev.cellbio.21.022404.142018. [DOI] [PubMed] [Google Scholar]
- Flanders KC, Kim ES, Roberts AB. Immunohistochemical expression of Smads 1–6 in the 15-day gestation mouse embryo: signaling by BMPs and TGF-betas. Dev Dyn. 2001;220:141–154. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1096>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Glasgow SM, Henke RM, Macdonald RJ, Wright CV, Johnson JE. Ptf1a determines GABAergic over glutamatergic neuronal cell fate in the spinal cord dorsal horn. Development. 2005;132:5461–5469. doi: 10.1242/dev.02167. [DOI] [PubMed] [Google Scholar]
- Goold CP, Davis GW. The BMP ligand Gbb gates the expression of synaptic homeostasis independent of synaptic growth control. Neuron. 2007;56:109–123. doi: 10.1016/j.neuron.2007.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goto K, Kamiya Y, Imamura T, Miyazono K, Miyazawa K. Selective inhibitory effects of Smad6 on bone morphogenetic protein type I receptors. J Biol Chem. 2007;282:20603–20611. doi: 10.1074/jbc.M702100200. [DOI] [PubMed] [Google Scholar]
- Goulding MD, Chalepakis G, Deutsch U, Erselius JR, Gruss P. Pax-3, a novel murine DNA binding protein expressed during early neurogenesis. EMBO J. 1991;10:1135–1147. doi: 10.1002/j.1460-2075.1991.tb08054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowan K, Helms AW, Hunsaker TL, Collisson T, Ebert PJ, Odom R, Johnson JE. Crossinhibitory activities of Ngn1 and Math1 allow specification of distinct dorsal interneurons. Neuron. 2001;31:219–232. doi: 10.1016/s0896-6273(01)00367-1. [DOI] [PubMed] [Google Scholar]
- Gross MK, Dottori M, Goulding M. Lbx1 specifies somatosensory association interneurons in the dorsal spinal cord. Neuron. 2002;34:535–549. doi: 10.1016/s0896-6273(02)00690-6. [DOI] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo 1951. Dev Dyn. 1992;195:231–272. doi: 10.1002/aja.1001950404. [DOI] [PubMed] [Google Scholar]
- Han G, Li AG, Liang YY, Owens P, He W, Lu S, Yoshimatsu Y, Wang D, Ten Dijke P, Lin X, Wang XJ. Smad7-induced beta-catenin degradation alters epidermal appendage development. Dev Cell. 2006;11:301–312. doi: 10.1016/j.devcel.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Hata A, Lagna G, Massague J, Hemmati-Brivanlou A. Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev. 1998;12:186–197. doi: 10.1101/gad.12.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, Abdollah S, Qiu Y, Cai J, Xu YY, Grinnell BW, Richardson MA, Topper JN, Gimbrone MA, Jr, Wrana JL, Falb D. The MAD-related protein Smad7 associates with the TGFbeta receptor and functions as an antagonist of TGFbeta signaling. Cell. 1997;89:1165–1173. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Miyazono K, ten Dijke P. TGF-beta signalling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Helms AW, Johnson JE. Progenitors of dorsal commissural interneurons are defined by MATH1 expression. Development. 1998;125:919–928. doi: 10.1242/dev.125.5.919. [DOI] [PubMed] [Google Scholar]
- Helms AW, Johnson JE. Specification of dorsal spinal cord interneurons. Curr Opin Neurobiol. 2003;13:42–49. doi: 10.1016/s0959-4388(03)00010-2. [DOI] [PubMed] [Google Scholar]
- Helms AW, Abney AL, Ben-Arie N, Zoghbi HY, Johnson JE. Autoregulation and multiple enhancers control Math1 expression in the developing nervous system. Development. 2000;127:1185–1196. doi: 10.1242/dev.127.6.1185. [DOI] [PubMed] [Google Scholar]
- Helms AW, Battiste J, Henke RM, Nakada Y, Simplicio N, Guillemot F, Johnson JE. Sequential roles for Mash1 and Ngn2 in the generation of dorsal spinal cord interneurons. Development. 2005;132:2709–2719. doi: 10.1242/dev.01859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley JA, Nornes HO, Morita M. Guidance of neuritic growth in the transverse plane of embryonic mouse spinal cord. J Comp Neurol. 1982;205:360–370. doi: 10.1002/cne.902050405. [DOI] [PubMed] [Google Scholar]
- Imamura T, Takase M, Nishihara A, Oeda E, Hanai J, Kawabata M, Miyazono K. Smad6 inhibits signalling by the TGF-beta superfamily. Nature. 1997;389:622–626. doi: 10.1038/39355. [DOI] [PubMed] [Google Scholar]
- Irving C, Malhas A, Guthrie S, Mason I. Establishing the trochlear motor axon trajectory: role of the isthmic organiser and Fgf8. Development. 2002;129:5389–5398. doi: 10.1242/dev.00117. [DOI] [PubMed] [Google Scholar]
- Jasoni CL, Walker MB, Morris MD, Reh TA. A chicken achaete-scute homolog (CASH-1) is expressed in a temporally and spatially discrete manner in the developing nervous system. Development. 1994;120:769–783. doi: 10.1242/dev.120.4.769. [DOI] [PubMed] [Google Scholar]
- Kamiya Y, Miyazono K, Miyazawa K. Smad7 inhibits transforming growth factor-beta family type I receptors through two distinct modes of interaction. The Journal of biological chemistry. 2010;285:30804–30813. doi: 10.1074/jbc.M110.166140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavsak P, Rasmussen RK, Causing CG, Bonni S, Zhu H, Thomsen GH, Wrana JL. Smad7 binds to Smurf2 to form an E3 ubiquitin ligase that targets the TGF beta receptor for degradation. Mol Cell. 2000;6:1365–1375. doi: 10.1016/s1097-2765(00)00134-9. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Jessell TM. The specification of dorsal cell fates in the vertebrate central nervous system. Annu Rev Neurosci. 1999;22:261–294. doi: 10.1146/annurev.neuro.22.1.261. [DOI] [PubMed] [Google Scholar]
- Lee KJ, Mendelsohn M, Jessell TM. Neuronal patterning by BMPs: a requirement for GDF7 in the generation of a discrete class of commissural interneurons in the mouse spinal cord. Genes Dev. 1998;12:3394–3407. doi: 10.1101/gad.12.21.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KJ, Dietrich P, Jessell TM. Genetic ablation reveals that the roof plate is essential for dorsal interneuron specification. Nature. 2000;403:734–740. doi: 10.1038/35001507. [DOI] [PubMed] [Google Scholar]
- Liem KF, Jr, Tremml G, Jessell TM. A role for theroof plate andits resident TGFbeta-related proteins in neuronal patterning in the dorsal spinal cord. Cell. 1997;91:127–138. doi: 10.1016/s0092-8674(01)80015-5. [DOI] [PubMed] [Google Scholar]
- Lyuksyutova AI, Lu CC, Milanesio N, King LA, Guo N, Wang Y, Nathans J, Tessier-Lavigne M, Zou Y. Anterior–posterior guidance of commissural axons by Wnt-frizzled signaling. Science. 2003;302:1984–1988. doi: 10.1126/science.1089610. [DOI] [PubMed] [Google Scholar]
- McCabe BD, Marques G, Haghighi AP, Fetter RD, Crotty ML, Haerry TE, Goodman CS, O’Connor MB. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron. 2003;39:241–254. doi: 10.1016/s0896-6273(03)00426-4. [DOI] [PubMed] [Google Scholar]
- Millonig JH, Millen KJ, Hatten ME. The mouse Dreher gene Lmx1a controls formation of the roof plate in the vertebrate CNS. Nature. 2000;403:764–769. doi: 10.1038/35001573. [DOI] [PubMed] [Google Scholar]
- Mochizuki T, Miyazaki H, Hara T, Furuya T, Imamura T, Watabe T, Miyazono K. Roles for the MH2 domain of Smad7 in the specific inhibition of transforming growth factor-beta superfamily signaling. J Biol Chem. 2004;279:31568–31574. doi: 10.1074/jbc.M313977200. [DOI] [PubMed] [Google Scholar]
- Moustakas A, Heldin CH. The regulation of TGFbeta signal transduction. Development. 2009;136:3699–3714. doi: 10.1242/dev.030338. [DOI] [PubMed] [Google Scholar]
- Muller T, Brohmann H, Pierani A, Heppenstall PA, Lewin GR, Jessell TM, Birchmeier C. The homeodomain factor lbx1 distinguishes two major programs of neuronal differentiation in the dorsal spinal cord. Neuron. 2002;34:551–562. doi: 10.1016/s0896-6273(02)00689-x. [DOI] [PubMed] [Google Scholar]
- Murakami G, Watabe T, Takaoka K, Miyazono K, Imamura T. Cooperative inhibition of bone morphogenetic protein signaling by Smurf1 and inhibitory Smads. Mol Biol Cell. 2003;14:2809–2817. doi: 10.1091/mbc.E02-07-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao A, Afrakhte M, Moren A, Nakayama T, Christian JL, Heuchel R, Itoh S, Kawabata M, Heldin NE, Heldin CH, ten Dijke P. Identification of Smad7, a TGFbeta-inducible antagonist of TGF-beta signalling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- Novitch BG, Chen AI, Jessell TM. Coordinate regulation of motor neuron subtype identity and pan-neuronal properties by the bHLH repressor Olig2. Neuron. 2001;31:773–789. doi: 10.1016/s0896-6273(01)00407-x. [DOI] [PubMed] [Google Scholar]
- Parikh P, Hao Y, Hosseinkhani M, Patil SB, Huntley GW, Tessier-Lavigne M, Zou H. PNAS Plus: regeneration of axons in injured spinal cord by activation of bone morphogenetic protein/Smad1 signaling pathway in adult neurons. Proc Natl Acad Sci U S A. 2011;108:E99–E107. doi: 10.1073/pnas.1100426108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KD, Hazen VM, Frendo M, Jia Z, Butler SJ. The bone morphogenetic protein roof plate chemorepellent regulates the rate of commissural axonal growth. J Neurosci. 2010;30:15430–15440. doi: 10.1523/JNEUROSCI.4117-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierani A, Moran-Rivard L, Sunshine MJ, Littman DR, Goulding M, Jessell TM. Control of interneuron fate in the developing spinal cord by the progenitor homeodomain protein Dbx1. Neuron. 2001;29:367–384. doi: 10.1016/s0896-6273(01)00212-4. [DOI] [PubMed] [Google Scholar]
- Roelen BA, Goumans MJ, van Rooijen MA, Mummery CL. Differential expression of BMP receptors in early mouse development. Int J Dev Biol. 1997;41:541–549. [PubMed] [Google Scholar]
- Shi Y, Liu JP. Gdf11 facilitates temporal progression of neurogenesis in the developing spinal cord. J Neurosci. 2011;31:883–893. doi: 10.1523/JNEUROSCI.2394-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs K, Martin DM, Novitch BG. Regulation of spinal interneuron development by the Olig-related protein Bhlhb5 and notch signaling. Development. 2011;138(15):3199–3211. doi: 10.1242/dev.057281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souchelnytskyi S, Nakayama T, Nakao A, Moren A, Heldin CH, Christian JL, ten Dijke P. Physical and functional interaction of murine and Xenopus Smad7 with bone morphogenetic protein receptors and transforming growth factor-beta receptors. J Biol Chem. 1998;273:25364–25370. doi: 10.1074/jbc.273.39.25364. [DOI] [PubMed] [Google Scholar]
- Tang Y, Liu Z, Zhao L, Clemens TL, Cao X. Smad7 stabilizes beta-catenin binding to E-cadherin complex and promotes cell–cell adhesion. J Biol Chem. 2008;283:23956–23963. doi: 10.1074/jbc.M800351200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmer JR, Wang C, Niswander L. BMP signaling patterns the dorsal and intermediate neural tube via regulation of homeobox and helix–loop–helix transcription factors. Development. 2002;129:2459–2472. doi: 10.1242/dev.129.10.2459. [DOI] [PubMed] [Google Scholar]
- Timmer J, Chesnutt C, Niswander L. The activin signaling pathway promotes differentiation of dI3 interneurons in the spinal neural tube. Dev Biol. 2005;285:1–10. doi: 10.1016/j.ydbio.2005.05.039. [DOI] [PubMed] [Google Scholar]
- Tsuchida T, Ensini M, Morton SB, Baldassare M, Edlund T, Jessell TM, Pfaff SL. Topographic organization of embryonic motor neurons defined by expression of LIM homeobox genes. Cell. 1994;79:957–970. doi: 10.1016/0092-8674(94)90027-2. [DOI] [PubMed] [Google Scholar]
- Ulloa F, Briscoe J. Morphogens and the control of cell proliferation and patterning in the spinal cord. Cell Cycle. 2007;6:2640–2649. doi: 10.4161/cc.6.21.4822. [DOI] [PubMed] [Google Scholar]
- Vargesson N, Laufer E. Negative Smad expression and regulation in the developing chick limb. PLoS One. 2009;4:e5173. doi: 10.1371/journal.pone.0005173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wine-Lee L, Ahn KJ, Richardson RD, Mishina Y, Lyons KM, Crenshaw EB., III Signaling through BMP type 1 receptors is required for development of interneuron cell types in the dorsal spinal cord. Development. 2004;131:5393–5403. doi: 10.1242/dev.01379. [DOI] [PubMed] [Google Scholar]
- Yamauchi K, Phan KD, Butler SJ. BMP type I receptor complexes have distinct activities mediating cell fate and axon guidance decisions. Development. 2008;135:1119–1128. doi: 10.1242/dev.012989. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Nakashima K, Takeda K, Ochiai W, Takizawa T, Ueno M, Takizawa M, Shibuya H, Taga T. Inhibition of BMP2-induced, TAK1 kinase-mediated neurite outgrowth by Smad6 and Smad7. Genes Cells. 2001;6:1091–1099. doi: 10.1046/j.1365-2443.2001.00483.x. [DOI] [PubMed] [Google Scholar]
- Zhang S, Fei T, Zhang L, Zhang R, Chen F, Ning Y, Han Y, Feng XH, Meng A, Chen YG. Smad7 antagonizes transforming growth factor beta signaling in the nucleus by interfering with functional Smad–DNA complex formation. Mol Cell Biol. 2007;27:4488–4499. doi: 10.1128/MCB.01636-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang B, Sockanathan S. Dorsal–ventral patterning: a view from the top. Curr Opin Neurobiol. 2006;16:20–24. doi: 10.1016/j.conb.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Zou H, Ho C, Wong K, Tessier-Lavigne M. Axotomy-induced Smad1 activation promotes axonal growth in adult sensory neurons. J Neurosci. 2009;29:7116–7123. doi: 10.1523/JNEUROSCI.5397-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]