Abstract
BACKGROUND/OBJECTIVES
Roux-en-Y gastric bypass (RYGB) surgery improves insulin sensitivity (SI) and β-cell function in obese non-diabetic subjects. Exercise also improves SI and may be an effective adjunct therapy to RYGB surgery. However, the mechanisms by which exercise or weight loss improve peripheral SI after RYGB surgery are unclear. We hypothesized that microRNAs (miRNAs) mediate at least some of the regulatory processes driving such mechanisms. Consequently, this work aimed at profiling plasma miRNAs in participants of the Physical Activity Following Surgery Induced Weight Loss study (clinicaltrials.gov identifier: NCT00692367), to assess whether miRNA levels track with improvements in SI and cardiometabolic risk factors.
SUBJECTS/METHODS
Ninety-four miRNAs implicated in metabolism were profiled in plasma samples from 22 severely obese subjects who were recruited 1–3 months after RYGB surgery and followed for 6 months of RYGB surgery-induced weight loss, with (exercise program (EX), N = 11) or without (CON, N = 11) an exercise training intervention. The subjects were selected, considering a priori sample size calculations, among the participants in the parent study. Mixed-effect modeling for repeated measures and partial correlation analysis was implemented in the R environment for statistical analysis.
RESULTS
Mirroring results in the parent trial, both groups experienced significant weight loss and improvements in cardiometabolic risk. In the CON group, weight loss significantly altered the pattern of circulating miR-7, miR-15a, miR-34a, miR-106a, miR-122 and miR-221. In the EX group, a distinct miRNA signature was altered: miR-15a, miR-34a, miR-122, miR-135b, miR-144, miR-149 and miR-206. Several miRNAs were significantly associated with improvements in acute insulin response, SI, and other cardiometabolic risk factors.
CONCLUSIONS
These findings present novel insights into the RYGB surgery-induced molecular changes and the effects of mild exercise to facilitate and/or maintain the benefits of a ‘comprehensive’ weight-loss intervention with concomitant improvements in cardiometabolic functions. Notably, we show a predictive value for miR-7, miR-15a, miR-106b and miR-135b.
INTRODUCTION
Roux-en-Y gastric bypass (RYGB) surgery-induced weight loss improves insulin sensitivity (SI) and intrinsic β-cell function (acute insulin response to glucose (AIRg) and disposition index (DI)) in obese non-diabetic patients.1 We have recently demonstrated that a 6-month exercise program following surgery elicits an additional improvement in SI and glucose effectiveness (SG) compared with surgery-induced weight loss alone.2 These data strongly advocate for the inclusion of an exercise program to optimize health benefits during active weight loss following RYGB surgery. However, the mechanisms underlying these health benefits are not clear, and not all individuals have similar improvements in metabolism. There is a wide variation in the degree of improvement in β-cell function after RYGB, most evident among obese non-diabetic individuals.3 Therefore, identifying minimally invasive biomarkers to identify and track metabolic improvements following RYGB surgery could represent a valuable strategy to gain insights into the physiological effects of the therapy and to improve decision making for patient care.
MicroRNAs (miRNAs) are naturally occurring noncoding RNAs that are abundant in many cell types and tissues of multicellular eukaryotes4 and have key roles in the regulation of a broad spectrum of physiological and pathological processes.5 It is estimated that miRNAs regulate the expression of more than 60% of protein-coding genes.6 Altered levels of circulating miRNAs have been reported in a variety of disease states including aging, obesity, metabolic dysfunction and diabetes,5,7–9 and may reflect tissue-specific activation or injury in response to disease states. Thus, miRNAs have many properties of ideal biomarkers,5 including correlation with the physiological or pathological state of an organism and stability in vivo and in vitro.
These molecular entities can also be found in a variety of body fluids including blood, breast milk, cerebrospinal fluid and urine, among others.10,11 Relevant to our study and supporting our hypothesis, changes in miR-122 abundance were recently reported after RYGB surgery in rat models and in humans.12–14 In addition, circulating miRNAs are highly stable in vivo and in vitro,15 and are consequently becoming increasingly recognized as powerful biomarkers for human disease.16–19 Furthermore, extracellular/circulating miRNAs has been found to have active roles in cell–cell communication, intercellular transport, targeting of gene expression in recipient cells and regulation of the immune system, among others.10,20,21 While evidence for a functional, endocrine/paracrine-like role for extracellular miRNAs in metabolically involved tissues is just emerging,22 the potential for endurance exercise-derived exosomes (which contain miRNAs among other ‘exerkines’) to treat metabolic diseases such as obesity and type 2 diabetes has been recently highlighted.23
There is, however, no evidence for how the circulating miRNA profile is altered by RYGB surgery-induced weight loss with adjunct exercise intervention. We hypothesized that weight loss following RYGB surgery modifies circulating miRNA signatures in humans and that these alterations are associated with improved β-cell function, muscle insulin-dependent and independent glucose uptake, and with changes in cardiorespiratory fitness and body composition. To test this hypothesis, we evaluated the changes in plasma levels of 94 metabolically involved miRNAs in morbidly obese subjects that underwent RYGB surgery-induced weight loss with or without an exercise program. Our data provide novel insights into the role of circulating miRNAs in weight loss and improved cardiometabolic risk.
MATERIALS AND METHODS
Patient recruitment
Taking into consideration a priori calculations of sample size as described below, 22 severely obese subjects (all with mixed European ancestry) that represented a subset of the RYGB-surgery patients enrolled in a larger randomized controlled exercise trial (parent trial: Physical Activity Following Surgery Induced Weight Loss; clinicaltrials.gov identifier: NCT00692367)2 were selected for this study. We chose to match groups based on the primary outcome of the parent trial (SI) as well as other clinically relevant phenotypic measurements (weight, body mass index (BMI), VO2 peak). The subgroups had similar baseline characteristics compared to parent groups (P>0.05), including age, weight, and BMI. The subgroups also had similar improvements in intravenous glucose tolerance test measurements (SI and SG), weight loss and VO2 peak compared to the parent groups. The study was conducted in a medical academic unit at the University of Pittsburgh, PA, USA. The study protocol was approved by the Human Research Protection Office of the University of Pittsburgh and all experiments were performed in accordance with relevant guidelines and regulations. All participants provided signed informed consent. Eligibility criteria included patients that underwent RGYB surgery in the 1–3 months previous to randomization, were between 21 and 60 years-of-age, had a BMI below 55 kg m−2, and did not have diabetes. Additional aspects of the parent study design, patient recruitment, and inclusion/exclusion criteria were previously described.2,24
Intervention groups
Participants were randomized to a 6-month exercise program (EX, n = 11) or a control health education intervention (CON, n = 11). The study measurements were made before and after the 6-month interventions. All participants completed an initial baseline assessment of metabolic and body composition measures before starting the interventions. Additional details of the exercise and control education programs of the parent trial are described elsewhere.2,24 For this subgroup analysis, the investigator conducting the miRNA isolation and high throughput profiling was blinded to the group allocation. Only after the experiments were conducted and data were collected and subjected to preliminary data quality control, was the investigator conducting the data analysis unblinded to the group allocation to complete the data analysis.
Intravenous glucose tolerance test
As described for the parent trial,2 insulin action was assessed using the Bergman minimal model method.25 The 3-h insulin-modified intravenous glucose tolerance test was conducted during morning hours following an overnight fast and at least 48hrs removal from the last exercise session.
Body composition and cardiorespiratory fitness
As previously described,2 anthropometric measurements were performed according to standardized protocols. Fat and lean mass were determined by dual-energy X-ray absorptiometry using a GE Lunar system (GE Healthcare, Madison, WI, USA). VO2 peak, a measure of cardiorespiratory fitness, was determined by indirect calorimetry (Moxus, AEI Technologies, Pittsburgh, PA, USA) during a 5–12 minute graded exercise test on a cycle ergometer (Lode BV, Groningen, The Netherlands). As a safety measure, 12-lead ECG recordings were monitored by the study physician and interpreted for contraindications to exercise. In addition, body weight, waist circumference, blood pressure and plasma lipids were measured by standard clinical protocols.
Measurement of plasma miRNAs
Arterialized blood was sampled (hepanarized plasma) from a catheter placed in an antecubital vein in the morning hours from 0700 to 0900 hours following a 12-h fast and before the intravenous glucose tolerance test. miRNAs from 200μl plasma were extracted using the miRNeasy Serum/Plasma Kit (Qiagen, Hilden, Germany) following the manufacturer’s instructions. Reverse transcription, preamplification, and real-time PCR of the plasma miRNAs were conducted using custom RT/PreAmp primer pools and TaqMan reagents from ThermoFisher Scientific (Waltham, MA, USA), following the manufacturer’s instructions. Custom TaqMan Array MicroRNA Cards (TAMC) were prepared as described in the TaqMan Array User Bulletin. Real-time PCR was performed on ViiA7 instrument (ThermoFisher) following the manufacturer’s instructions. An in-house-developed metabolism-related miRNA panel including 96 features (Supplementary Table S1) was used. Array features generating undetermined cycle threshold (Ct) values in more than 75% of samples were eliminated from further analysis. Using the HTqPCR package in the R environment,26 the raw Ct data were first normalized to the respective levels of recovered spike-in cel-miR-39 and multiplied by negative one (that is, − ΔCt = −1 * (Ctfeature.of.interest − Ctcel.miR.39)), generating − ΔCt data equivalent to the log2 of the fold change respective to cel-miR-39 (denoted herein logFC data). The logFC data were then subjected to quantile normalization using the normalizeCtData function. Features with absolute fold changes (post- and pre-intervention) lower than or equal to 1.5 (that is, abs(ΔlogFCPost-Pre) ≤ log2(1.5)) were filtered out and not included in the reported differential abundance analysis.
Statistical analysis
Group differences in baseline measures were determined using the Welch two sample t-test (two-tailed) for continuous variables and the Fisher exact test for sex ratios. Normality was tested with the Shapiro–Wilk test. Variables with high skew were log-transformed to approximate a normal distribution. Standard correlations between baseline anthropometric and metabolic measures and the longitudinal change in SI, SG, DI and AIRg were calculated to identify potential additional confounding covariates. For comparison of longitudinal changes in clinical and metabolic measures and for differential abundance analysis of circulating miRNA levels, mixed effect models for repeated measures were implemented using the nlme package in the R environment. Each variable was modeled as a function of fixed effects for Group, Time and the interaction Group*Time with Subjects as a random effect. Age and the time from bariatric surgery to pre-treatment were included as covariates in the model to adjust for potential confounding effects. Partial correlations adjusting for age and time from bariatric surgery to pre-treatment were calculated using the ppcor package. False discovery rates correcting for multiple testing were calculated using the Benjamini-Hochberg correction. Mean differences and correlations with P<0.05 were considered significant. Analyses were performed in the R 3.3.1 statistical computing environment.26
Sample size estimation
A minimum sample size of N = 11 subjects per group to detect a minimum (by design) 1.5-fold change in the levels of circulating miRNAs, with 80% power at a two-sided significance level α = 0.05 (5% type I error), was calculated in advance of subgroup selection using the formula contributed by Diggle et al.27 to detect differences between two groups of repeated measures:
where μ1–μ2 represents the mean difference of the two groups [in our case: μ1 − μ2 = log2(1.5)|; σ2 is the common variance in the two groups; n is the number of time points or repeated measures (n = 2 in a pre–post comparison); and ρ is the correlation of the repeated measures. The critical value zα is equal to 1.96 for a significance level α = 0.05 and zβ is equal to 0.842 for an 80% power of the hypothesis test. The values for σ and ρ were estimated using our preliminary data for five circulating miRNAs that were assayed using the same real-time PCR protocol and displayed an average 1.5-fold change in pre–post comparisons between two groups (unrelated study that evaluated the response to a 4-month drug treatment in subjects with prediabetes). In specific, the pooled standard deviation of the circulating miRNA levels (expressed in arbitrary normalized logFC data units) was σ = 0.65 and the intraclass correlation of the repeated measures ρ estimated at 0.1 by extracting the correlation structure in a general linear model. A minimum fold change of 1.5 is our choice of threshold for hypothesis testing that involves quantitative real-time PCR data.
Code availability
Code used to conduct the analyses in this study is based on open source and freely available packages developed for the R language and environment for statistical computing, version 3.3.1,26 as described in relevant previous sections. R packages and respective documentation can be downloaded and/or accessed at the Comprehensive R Archive Network (https://cran.r-project.org/) and Bioconductor (Open Source Software for Bioinformatics: https://www.bioconductor.org/).
RESULTS
Study participants
A schematic of the study design and sample collection is presented in Figure 1. Additional detailed description parent study design can be found in reference.2 As reported for the parent trial, both groups had similar body weight pre-surgery and pre-interventions. Both groups reported similar medication use at baseline, and there was no difference in medication use following the intervention (data not shown). The baseline characteristics of the study cohort are shown in Table 1. There were no significant baseline imbalances in any of the characteristics assessed. However, we identified a significant correlation between the baseline level of the variable ‘time from bariatric surgery to pre-treatment’ and the change in AIRg (standard Pearson’s correlation r = 0.49, P = 0.021, false discovery rate = 0.12). For this reason, we included this variable as a covariate in the mixed effect models and partial correlation calculations to account for potential confounding effects.
Figure 1.
Schematic representation of the study design and sample collection.
Table 1.
Baseline characteristics of the study cohort
| Antropometric | CON (N = 11) | EX (N = 11) | P-value | Method |
|---|---|---|---|---|
| Sex ratio (female/male) | 9F/2M | 9F/2M | 1 | Fisher’s Exact Test for Count Data |
| Days from surgery to pre-intervention | 72.0 (58.1–85.9) | 76.7 (59.1–94.4) | 0.644 | Welch two sample t-test |
| Age | 38.5 (30.3–46.6) | 43.0 (36.7–49.3) | 0.338 | Welch two sample t-test |
| BMI (kg m−2) | 40.8 (36.8–44.7) | 39.5 (36.1–42.8) | 0.572 | Welch two sample t-test |
| Weight (kg) | 111.1 (100.4–121.7) | 106.9 (97.0–116.9) | 0.534 | Welch two sample t-test |
| Waist circumference (cm) | 119.7 (109.6–129.9) | 119.8 (110.3–129.3) | 0.995 | Welch two sample t-test |
| Total fat (cm2) | 703.4 (591.8–815.1) | 729.1 (634.9–823.4) | 0.697 | Welch two sample t-test |
| Fat mass (kg) | 51.8 (42.9–60.7) | 50.2 (44.0–56.3) | 0.740 | Welch two sample t-test |
| Lean mass (kg) | 53.4 (46.6–60.1) | 51.9 (46.9–56.9) | 0.703 | Welch two sample t-test |
| Fat-free mass (FFM) (kg) | 56.2 (49.3–63.2) | 54.7 (49.4–60.0) | 0.703 | Welch two sample t-test |
| Bone mass (kg) | 2.9 (2.7–3.1) | 2.8 (2.4–3.2) | 0.795 | Welch two sample t-test |
Abbreviations: BMI, body mass index; FFM, fat-free mass. Data for continuous variables presented as mean and 95% confidence interval.
Weight, body composition and cardiorespiratory fitness
As shown in Table 2, both groups experienced similar reductions in mass, fat mass, BMI and waist circumference following the interventions. Similar to results reported for the larger parent randomized controlled trial,2 high-density lipids significantly increased while triglycerides decreased in both EX and CON subgroups. However, no significant reduction was observed in the levels of low-density lipids for either of our subgroups. EX, but not CON, significantly improved VO2 peak (Table 2), an index of cardiorespiratory fitness and an effective predictor of future morbidity and mortality. When VO2 peak is normalized against fat-free mass, both groups showed significant increases in this magnitude; however, the EX group displayed a 57% larger increase. Alanine aminotransferase (ALT), an enzyme used to detect liver injury, significantly decreased only in the EX group, whereas no change was observed in the CON group.
Table 2.
The responses detected with the study subgroups mirror those reported in the parent study
| Control (N = 11) | Exercise (N = 11) | Effects (P-value) | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Pre | Post | Pre | Post | Group | Time | Group * time | |
| Anthropometry and fitness | |||||||
| BMI (kg m −2) | 40.8 (36.8–44.7) | 30.6 (27.8–33.4)a | 39.5 (36.1–42.8) | 30.2 (27.5–33.0)a | 0.744 | <0.001 | 0.432 |
| Weight (kg) | 111.1 (100.4–121.7) | 83.4 (75.0–91.8)a | 106.9 (97.0–116.9) | 81.9 (73.9–89.9)a | 0.599 | <0.001 | 0.443 |
| Waist circumference (cm) | 119.7 (109.6–129.9) | 98.1 (91.0–105.3)a | 119.8 (110.3–129.3) | 98.8 (91.0–106.5)a | 0.927 | <0.001 | 0.764 |
| Total fat (cm2) | 703.4 (591.8–815.1) | 422.9 (357.5–488.3)a | 729.1 (634.9–823.4) | 432.4 (349.3–515.5)a | 0.483 | <0.001 | 0.722 |
| Fat mass (kg) | 51.8 (42.9–60.7) | 29.5 (25.1–33.9)a | 50.2 (44.0–56.3) | 28.9 (24.6–33.3)a | 0.859 | <0.001 | 0.758 |
| Lean mass (kg) | 53.4 (46.6–60.1) | 51.9 (44.5–59.2)d | 51.9 (46.9–56.9) | 50.6 (45.3–55.8) | 0.504 | 0.058 | 0.87 |
| FFM (kg) | 56.2 (49.3–63.2) | 54.7 (47.1–62.2)d | 54.7 (49.4–60.0) | 53.3 (47.7–58.9) | 0.504 | 0.045 | 0.878 |
| Bone mass (kg) | 2.9 (2.7–3.1) | 2.8 (2.6–3.0)a | 2.8 (2.4–3.2) | 2.7 (2.3–3.1)a | 0.618 | 0.001 | 0.701 |
| VO2 max (l min−1) | 2.1 (1.7–2.4) | 2.1 (1.7–2.4) | 2.2 (2.0–2.4) | 2.4 (2.0–2.8)c | 0.611 | 0.979 | 0.104 |
| VO2 max (ml · min · kgFFM) | 18.5 (16.7–20.3) | 24.4 (21.6–27.2)a | 20.5 (18.5–22.6) | 29.8 (25.6–34.0)a | 0.321 | <0.001 | 0.063 |
| IVGTT-derived | |||||||
| Fasting glucose (mg dl−1) | 85.1 (77.5–92.8) | 85.0 (78.4–91.6) | 85.0 (80.1–89.9) | 84.7 (81.8–87.6) | 0.466 | 0.956 | 0.945 |
| Fasting insulin (μIU ml−1) | 5.3 (3.8–6.8) | 3.7 (2.6–4.8)b | 5.0 (3.5–6.5) | 3.9 (2.5–5.2)c | 0.735 | 0.005 | 0.526 |
| Sg (min−1) | 0.015 (0.012–0.017) | 0.017 (0.014–0.019) | 0.014 (0.008–0.020) | 0.020 (0.015–0.025)b | 0.736 | 0.312 | 0.142 |
| SI (min−1 · μU ml−1) | 1.9 (1.2–2.6) | 3.5 (2.8–4.3)a | 1.9 (1.3–2.5) | 5.3 (4.0–6.6)a | 0.926 | 0.001 | 0.011 |
| DI (‘ 10-5 · min−1) | 725.5 (431.2–1019.7) | 1047.3 (650.7–1443.9) | 772.5 (405.1–1140.0) | 1545.3 (929.2–2161.4)a | 0.814 | 0.147 | 0.151 |
| AIRg (pmol l−1) | 475.5 (232.0–719.1) | 280.0 (187.1–372.9)d | 428.8 (252.9–604.7) | 298.0 (204.4–391.5) | 0.937 | 0.063 | 0.650 |
| HOMA-IR (mg dl−1 · μIU ml−1) | 1.1 (0.8–1.4) | 0.8 (0.5–1.0)c | 1.1 (0.7–1.5) | 0.8 (0.5–1.0)c | 0.936 | 0.020 | 0.999 |
| Plasma lipids | |||||||
| Total cholesterol (mg dl−1) | 133.6 (107.6–159.7) | 140.6 (121.1–160.2) | 142.5 (124.2–160.9) | 145.8 (130.9–160.7) | 0.714 | 0.335 | 0.714 |
| HDL cholesterol (mg dl−1) | 29.5 (24.3–34.6) | 44.5 (37.1–51.8)a | 33.1 (29.3–36.9) | 47.7 (41.0–54.5)a | 0.546 | <0.001 | 0.926 |
| LDL cholesterol (mg dl−1) | 79.0 (59.0–99.0) | 77.8 (64.2–91.4) | 87.0 (70.6–103.4) | 81.6 (70.3–92.9) | 0.594 | 0.835 | 0.602 |
| Triglycerides (mg dl−1) | 126.1 (106.3–145.9) | 91.8 (66.7–116.9)b | 112.3 (83.0–141.6) | 81.9 (56.1–107.7)b | 0.376 | 0.004 | 0.798 |
| VLDL cholesterol (mg dl−1) | 25.2 (21.1–29.2) | 18.4 (13.3–23.4)b | 22.5 (16.5–28.4) | 16.5 (11.2–21.7)b | 0.385 | 0.005 | 0.790 |
Abbreviations: BMI, body mass index; FDR, false discovery rate; FFM, fat-free mass; HDL, high-density lipid; HOMA-IR, homeostatic model assessment for insulin resistance; IVGTT, intravenous glucose tolerance test; LDL, low-density lipid; VLDL, very low-density lipid. Pre–post comparisons for clinical variables using mixed effect models for repeated measures (nlme R package) and post hoc interaction analysis (phia R package). Data presented as mean and 95% confidence interval.
Significant difference within group, P<0.001 and FDR <0.001.
Significant difference within group, P<0.01 and FDR <0.01.
Significant difference within group, P<0.05 and FDR <0.05.
Significant difference within group, P<0.05 and FDR <0.10.
Values in bold denote significant effects (P<0.05) as estimated by mixed effect models for repeated measures.
Intravenous glucose tolerance test
Baseline measurement for SI, fasting insulin, fasting glucose and homeostatic model assessment for insulin resistance (HOMA-IR) were similar for both groups (Table 2). Following the interventions, SI, fasting insulin, and HOMA-IR were improved in both groups. However, the EX group had a significantly greater improvement in SI compared to the CON group (Group*Time interaction effect with P = 0.011). In accordance with the design of the subgroup analysis, the intervention effects on weight, body composition, cardiorespiratory fitness and SI reported here for these subgroups, were similar to those reported for the parent randomized controlled trial.2 Specifically, both groups experimented a significant decrease in BMI, body weight, waist circumference and fat mass, among others, whereas the normalized VO2 max and SI significantly increased (all comparisons with Time effect P ≤ 0.001, Table 2).
Alterations in circulating miRNA signatures
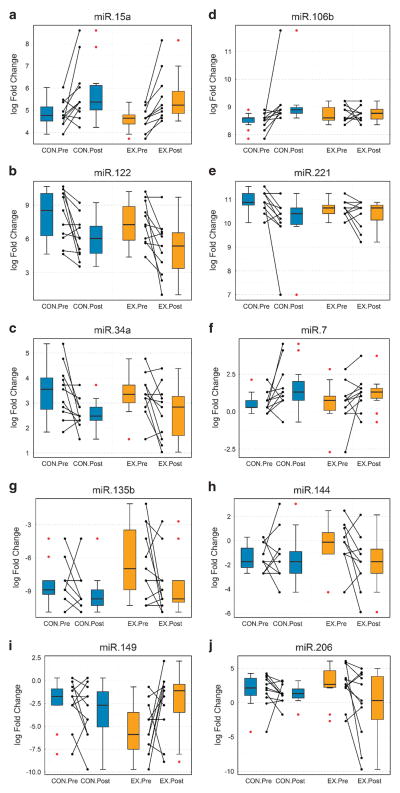
We measured a 96-feature TaqMan MicroRNA panel including 94 miRNAs involved in a number of metabolically active tissues and organs (such as muscle, adipose, liver, kidney, brain and pancreas; refer to Supplementary Table S1 for details). By evaluating the significance of the circulating miRNA abundance differences per group (post vs pre-intervention comparison), we observed three different patterns of change in the circulating miRNA levels in our study cohort (Table 3, PFigure 2). On one hand, three miRNAs significantly changed (>1.5-fold and <0.05) in the same direction in both EX and CON groups (miR-15a increased, miR-34a decreased and miR-122 decreased; Table 3-pattern A, Figures 2a–c). On the other hand, three miRNAs significantly changed only in the CON group (miR-7 increased, miR-106 increased and miR-221 decreased; Table 3-pattern B, Figures 2d–f), whereas four other miRNAs significantly changed only in the EX group (miR-135b decreased, miR-144 decreased, miR-149 increased and miR-206 decreased; Table 3-pattern C, Figures 2g–j). By additionally evaluating the baseline-adjusted comparison between the groups, all pattern A and Pattern B miRNAs displayed significant Time effects (P<0.05, Table 3), whereas pattern C miR-149 displayed significant group-by-time interaction effect (P = 0.043, Table 3). Results from the differential abundance analysis for all miRNA features, including those that did not reach the fold change threshold (greater than 1.5-fold) for statistical inference analysis, are presented in Supplementary Table S2.
Table 3.
Several miRNAs are differentially abundant in the circulation of trial participants
| Control (N = 11) | Exercise (N = 11) | Effects (P-value) | |||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| Pre | Post | Pre | Post | Group | Time | Group * time | |
| Patern A | |||||||
| miR-15a-5p | 4.9 (4.5–5.3) | 5.8 (4.9–6.7)a | 4.6 (4.3–4.9) | 5.6 (4.8–6.3)a | 0.681 | 0.007 | 0.917 |
| miR-122-5p | 8.1 (6.5–9.6) | 6.0 (4.8–7.2)a | 7.4 (6.2–8.7) | 5.1 (3.4–6.8)a | 0.442 | 0.012 | 0.796 |
| miR-34a-5p | 3.5 (2.8–4.2) | 2.6 (2.2–2.9)a | 3.3 (2.8–3.9) | 2.6 (1.9–3.4)b | 0.523 | 0.013 | 0.655 |
| Patern B | |||||||
| miR-221-3p | 10.9 (10.6–11.2) | 10.1 (9.3–10.8)a | 10.6 (10.3–10.8) | 10.4 (10.0–10.8) | 0.207 | 0.007 | 0.126 |
| miR-106b-5p | 8.5 (8.3–8.7) | 9.1 (8.5–9.7)a | 8.8 (8.6–9.0) | 8.8 (8.6–8.9) | 0.327 | 0.009 | 0.055 |
| miR-7-5p | 0.6 (0.2–1.0) | 1.6 (0.6–2.7)b | 0.6 (−0.3–1.5) | 1.2 (0.5–2.0) | 0.96 | 0.041 | 0.558 |
| Patern C | |||||||
| miR-149-5p | − 2.5 (−4.1 to − 0.8) | − 3.3 (−5.4 to − 1.3) | − 5.3 (−7.2 to − 3.4) | − 2.4 (−4.7 to − 0.1)b | 0.057 | 0.489 | 0.043 |
| miR-144-3p | − 1.5 (−2.2 to − 0.7) | − 1.4 (−2.8 to 0.0) | − 0.3 (−1.5 to 0.8) | − 1.7 (−3.2 to − 0.3)b | 0.291 | 0.92 | 0.142 |
| miR-206 | 1.8 (0.1–3.4) | 1.2 (0.3–2.2) | 2.7 (0.8–4.6) | − 0.3 (−3.7 to 3.1)b | 0.76 | 0.673 | 0.18 |
| miR-135b-5p | − 8.3 (−9.5 to − 7.1) | − 9.2 (−10.5 to − 8.0) | − 6.4 (−8.6 to − 4.2) | − 8.5 (−10.3 to − 6.7)b | 0.072 | 0.336 | 0.399 |
| With nonsignificant differences | |||||||
| miR-193b-3p | 5.3 (4.0–6.6) | 4.3 (3.6–5.0) | 5.2 (4.0–6.3) | 4.4 (3.8–5.0) | 0.812 | 0.078 | 0.771 |
| miR-499a-5p | − 4.7 (−7.2 to − 2.2) | − 2.0 (−4.5 to 0.4) | − 4.7 (−7.2 to − 2.2) | − 4.0 (−6.6 to − 1.4) | 0.944 | 0.097 | 0.369 |
| miR-375 | 6.1 (5.1–7.2) | 5.3 (4.2–6.3) | 6.2 (5.3–7.1) | 5.9 (4.6–7.1) | 0.894 | 0.176 | 0.561 |
| miR-184 | − 3.1 (−5.8 to − 0.4) | − 1.5 (−3.3 to 0.4) | − 1.9 (−4.2 to 0.3) | − 0.7 (−2.5 to 1.0) | 0.365 | 0.23 | 0.817 |
| miR-9-5p | 2.5 (1.8–3.1) | 2.1 (0.8–3.4) | 1.6 (0.7–2.5) | 2.2 (1.5–3.0) | 0.261 | 0.477 | 0.161 |
| miR-138-5p | − 0.8 (−3.0 to 1.3) | − 1.5 (−3.1 to 0.1) | − 1.6 (−3.3 to 0.1) | − 2.2 (−3.7 to − 0.8) | 0.613 | 0.506 | 0.951 |
| miR-193a-3p | − 4.9 (−6.7 to − 3.0) | − 5.4 (−7.5 to − 3.4) | − 6.4 (−8.1 to − 4.6) | − 5.1 (−8.0 to − 2.2) | 0.323 | 0.679 | 0.351 |
| miR-208a-3p | − 8.7 (−10.1 to − 7.3) | − 8.2 (−9.8 to − 6.6) | − 7.6 (−9.7 to − 5.4) | − 6.8 (−8.8 to −4.9) | 0.355 | 0.684 | 0.869 |
| miR-183-5p | 0.9 (0.2–1.6) | 0.9 (−1.0 to 2.8) | 1.5 (0.8–2.2) | 0.4 (−2.1 to 2.9) | 0.731 | 0.995 | 0.428 |
Abbreviation: FDR, false discovery rate. Data presented as mean of the quantile-normalized log fold change (logFC) relative to spiked-in cel-miR-39 and 95% confident interval between parentheses. Data show miRNA features with |ΔlogFCPOST-PRE| >1.5, either for CON and/or EX group. Pre–post comparisons for clinical variables using mixed effect models for repeated measures (nlme R package) and post hoc interaction analysis (phia R package). Data presented as mean and 95% confidence interval.
Significant difference within group, P<0.01 and FDR <0.05.
Significant difference within group, P<0.05 and FDR <0.10.
Values in bold denote significant effects (P<0.05) as estimated by mixed effect models for repeated measures.
Figure 2.
Dual boxplot/dotplot for miRNAs that significantly changed in the exercise (EX) and/or the control (CON) groups. (a–c), Pattern A miRNAs; (d–f), Pattern B miRNAs; (g–j), Pattern C miRNAs. Data presented are quantile-normalized log fold changes respective to spiked-in cel-miR-39. Dual plots include (pre–post) connected dotplots for each group (CON and EX), where connected dots represent corresponding pre-intervention–post-intervention measurements for each individual participant (n = 11 per group). Boxplots (boxes delineating first and third quartile; whiskers delineating the smallest and largest values inside a 1.5 box-length (IQR) from the end of the box) summarize the data presented in the corresponding (side-by-side) dotplot. Red dots represent potential outliers located at greater that 1.5 IQR from the end of the box. Blue filling for CON group and orange filling for EX group. IQR, interquartile range.
Correlations between changes in miRNA levels and changes in clinical indices
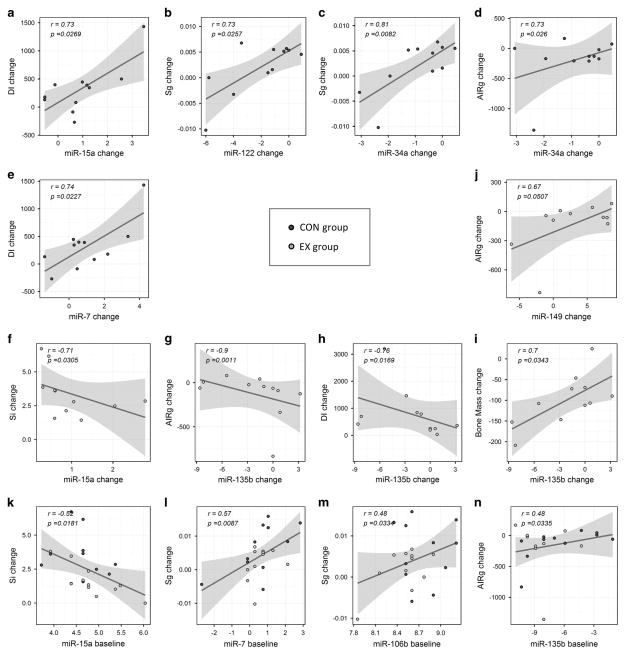
To gain insights into potential molecular mechanisms regulated by miRNAs and altered by RYGB surgery with and without an exercise intervention, we assessed the partial correlations between the changes in circulating miRNA levels and the changes (post–pre) in cardiometabolic risk factors including SI, SG, DI and AIRg, among others. As shown in Figure 3 and Supplementary Table S3, the changes in plasma levels of the 10 (above listed) differentially abundant miRNAs significantly correlated (absolute |r| >0.45, P<0.05) with changes in important indices of insulin resistance (HOMA-IR), β-cell function (fasting insulin levels, AIRg, DI, SI and SG), body composition (BMI, waist circumference and fat mass among others), plasma lipids (cholesterol and LDL), and liver function (ALT, AST and ALP). Although correlation does not necessarily imply causation, our results agree with previous preclinical and clinical reports (see Discussion section) that suggest a role in pancreatic β-cell and liver functions (among others) for several of the miRNAs reported here.
Figure 3.
Correlations between the changes in cardiometabolic risk factors and differentially abundant circulating miRNAs. (a–e) Relevant correlations with changes in patterns A and B miRNAs in the CON group; (f–j) relevant correlations with changes in patterns A and C miRNAs in the EX group; (k–n): relevant correlations with baseline miRNA levels in the complete cohort (miRNAs with predictive potential). Blue and orange dots represent individual data points for CON and EX group, respectively. Reported are partial correlations adjusted for age and time from bariatric surgery to pre-treatment. Data points ploted are unadjusted quantile-normalized log fold changes (x axis) respective to spiked-in cel-miR-39 levels. For visualization purposes, a fitted line on the unadjusted data with 95% confidence band is also drawn. A full color version of this figure is available at the International Journal of Obesity journal online.
To assess the potential predictive value of the baseline levels of circulating miRNAs, we evaluated the partial correlations between the pre-intervention (baseline) miRNA levels and the changes in measures of cardiometabolic risk factors. Notably, the baseline levels of four out of the 10 differentially abundant plasma miRNAs also significantly correlated with the observed changes in SI (baseline miR-15a, r = − 0.52, P = 0.018), SG (baseline miR-7, r = 0.57, P = 0.009 and baseline miR-106b, r = 0.48, P = 0.033), and AIRg (baseline miR-135b, r = 0.48, P = 0.034).
DISCUSSION
We provide here the first evidence, to our knowledge, of a plasma miRNA signature in patients with severe obesity who had undergone RYGB surgery with and without an exercise intervention. Relevantly, we identified ten miRNAs with altered plasma levels and significant association with measures of improved β-cell function (AIRg, DI), peripheral insulin sensitivity (SI), glucose effectiveness (SG), liver function (ALT and AST) and changes in additional cardiovascular risk factors such as plasma cholesterol, LDL, BMI, waist circumference and fat mass (Table 2, Figures 2 and 3 and Supplementary Table S3).
Pattern A miRNAs
The significantly altered levels of circulating miR-15a (increased), miR-34a (reduced), and miR-122 (reduced) in both EX and CON groups seems to indicate that these miRNAs represent a general response to RYGB surgery-induced weight loss (Figures 2a–c). In concordance with our findings, the opposite behavior (a reduction in circulating levels) was reported for miR-15a specifically in morbidly obese men13 and in patients with type 2 diabetes.28 Functional studies have reported that miR-15a positively regulates β-cell function and insulin synthesis by inhibiting expression of the uncoupling protein-2 (UCP-2)29 and negatively affect adipo-genesis by directly targeting delta-like 1 homolog (Dlk1). In addition, Dlk1 was recently found to be a key regulator of the myogenic program in skeletal muscle, with a dual role that promotes the myogenic program during embryogenesis but inhibits muscle regeneration in the adults.30 Therefore, the observed upregulation of miR-15a after bariatric surgery may contribute to an enhanced ability of adult patients to regenerate muscle tissue. Importantly, our correlation analysis detected significant association between the change in miR-15a and the change in DI in the CON group (Figure 3a) and the change in SI in the EX group (Figure 3f), which may suggest a role for this miRNA in the regulation of the β-cell sensitivity-secretion relationship. Interestingly, baseline levels of miR-15a showed a significant inverse correlation with the change in SI (Figure 3k), which suggests that morbidly obese individuals with lower baseline levels of this particular miRNA might benefit more from gastric bypass. Together, these results suggest a potential role for using baseline miR15a measurement to predict response to RYGB surgery.
Regarding miR-122, elevated levels in circulation have been positively associated with obesity and insulin resistance in young Chinese adults.31 Relevantly, changes in miR-122 were reported after bariatric surgery in rat models and in humans.12–14 As the expression of miR-122 is mostly restricted to the liver, where it regulates cholesterol production and hepatic function,32 the reduced abundance of circulating miR-122 in our study participants may indicate improved liver functions after bariatric surgery. This reasoning is supported by the significant positive correlation observed between the change in miR-122 abundance and the change in ALT and ALS levels in the EX group and by the negative correlation with circulating cholesterol and low-density lipoprotein in the CON group (Supplementary Table S3). Interestingly, elevated miR-122 and miR-34a (another RYGB-downregulated miRNA in this study) have been associated with dyslipidemia in patients with non-alcoholic fatty liver disease.33 In addition, miR-34a was reported as the most highly expressed hepatic miRNA in obese mice, where it impaired physiological postprandial responses through attenuation of FGF19 by directly targeting β-Klotho.34 Our study demonstrated significant correlations between the change in circulating levels of miR-122 and miR-34a and the change in SG (Figures 3b and c), and between the changes in miR-34a and AIRg (Figure 3d). Taken together, these results suggest a role for miR-15a, miR-34a and miR-122 during the re-establishment of improved pancreatic β-cell and liver functions after RGYB surgery in morbidly obese subjects.
Pattern B miRNAs
This pattern involved three circulating miRNAs that significantly changed only in the CON group (miR-7 (elevated), miR-106b (elevated), and miR-221 (reduced)) (Figures 2d–f). Downregulation of miR-221, one of the most well-known oncogenic miRNAs, is concordant with reports that indicate a reduction of the risk of cancer after bariatric surgery.35 Relevantly, this miRNA was increased in the livers of ob/ob mice and suggested to regulate insulin resistance via its effect on adiponectin signaling by targeting adiponectin receptor 1.36,37 It was also recently reported that metformin downregulates miR-22138 and normalizes the levels of this miRNA in the internal mammary arteries of diabetic subjects undergoing coronary artery bypass surgery.39 In concordance with our results regarding increased levels of miR-106, downregulation of this miRNA was reported in diet-induce obese mice, and this pattern switched to a significant upregulation in diet-induce obese mice that underwent a weight-loss intervention.40 Downregulation of miR-106b is also involved in the establishment of cellular senescence and dysregulation of the insulin/IGF-1/FoxO pathway implicated in aging.41,42 Supporting a beneficial role for the elevated miR-7 levels, this miRNA was found decreased in obese and diabetic mouse models and in human islets from obese and moderately diabetic subjects with compensated β-cell function.43 In addition, our study demonstrated a significant positive correlation between the change in miR-7 and DI in the CON group (Figure 3e), which also suggests a role for miR-7 in the regulation of the β-cell sensitivity-secretion relationship. Furthermore, the baseline levels of both miR-7 and miR-106 significantly correlated with the change in SG, (Figures 3l and m), which underscore the potential predictive value of these miRNAs as biomarkers of cardiometabolic improvement.
Pattern C miRNAs
The abundance in the circulation of miR-135b (reduced), miR-144 (reduced), miR-149 (elevated), and miR-206 (reduced) were significantly altered only in the EX group (Figures 2g–j). This suggests that expression of these miRNAs in the respective source tissues is specifically downregulated (miR-135b/144/206) or overexpressed (miR-149) in response to the exercise intervention after RYGB surgery. The following discussion of biological processes affected by these miRNAs highlights added benefits of the adjunct exercise intervention after bariatric surgery.
Upregulation of miR-135b has been mostly reported in the context of cancer and bone disease.44–46 Now, our study suggests that reduction of miR-135b levels after RYGB with adjunct exercise intervention may have an important role in the regulation of the acute insulin response to glucose and insulin secretion and/or insulin sensitivity, as indicated by the highly significant inverse correlations between the change in miR-135b and the change in AIRg and DI among EX subjects (Figures 3g and h). Of note, the high correlation between miR-135b and bone mass among EX subjects (r = 0.70, P = 0.0343, Figure 3i) suggests that bone (possibly bone marrow) may be a source of circulating miR-135b. Supporting our reasoning, exosomal secretion of functional miR-135b during osteogenic differentiation and under conditions of hypoxic bone marrow has been reported44,45 and high levels of circulating miR-135b has been found to predict development and prognosis of myeloma bone disease.46 Mechanistically, the transcription factor FOXO1 and glycogen synthase kinase 3 beta (GSK-3β), both validated direct targets of miR-135b,47,48 could represent key mediators of miR-135b effects after the exercise intervention. Remarkably, bone-specific deletion of GSK-3β affected global metabolism and lead to metabolic disease and age-related diabetic-like complications in mice.49 On the other hand, the upregulation of FOXO1 during starvation and exercise is well described.50,51 We reason that upregulation of FOXO1 (for example, in the liver) due to reduced levels of functional circulating miR-135b might, among other things, increase systemic insulin sensitivity by improving the dynamic control of hepatic glucose production and de novo lipogenesis via its dual role over glucose-6-phosphatase and glucokinase, as proposed by Haeusler et al.51 Important to highlight here is our result demonstrating that baseline levels of miR-135b significantly correlated with the changes in AIRg (Figure 3n), suggesting potential predictive value as a biomarker of response to the exercise intervention. Taken together, these results indicate that the observed changes in miR-135b after the exercise intervention may contribute to bone health and improved systemic insulin sensitivity in humans. Given that a role for miR-135b in the metabolic benefits of exercise has not previously been described, these findings deepen our mechanistic understanding of the process.
Circulating miR-144 (significantly reduced in EX subjects) was found upregulated in human type 2 diabetes in both the circulation and muscle52 and in people with impair fasting glucose.53 This miRNA targets the insulin receptor substrate 1 (IRS1).53 Consistently, we found a significant negative correlation between the change in miR-144 plasma levels and the change in AIRg in the whole cohort (r = − 0.47, P = 0.0378; Supplementary Table S3). In addition, the Nord-Trøndelag Health (HUNT) study revealed that levels of five serum miRNAs, including elevated miR-144, predicted future fatal acute myocardial infarction in healthy individuals with 78% overall diagnostic accuracy.54 These results highlight the beneficial impact on insulin-related physiology and cardiovascular health of exercise-induced reduction of miR-144 levels after RGYB surgery with adjunct exercise intervention.
Mitochondrial miR-149 was significantly upregulated by the adjunct exercise intervention in our EX group but downregulated in the CON group and demonstrated a strong group-by-time interaction effect (Table 2). Importantly, the changes in this circulating miRNA strongly correlated with the changes in EX group AIRg (Figure 3j) and DI in the whole cohort (Supplementary Table S3). Expression of miR-149 was reported to promote mitochondrial biogenesis through inhibition of PARP-2 that consequently activates SIRT-1 and concomitantly elevates the cellular NAD+ levels.55 The same authors showed that the miR-149/PARP-2/SIRT-1 axis was dysregulated in the skeletal muscle from obese mice, with downregulation of miR-149 responsible for the weakening of the skeletal muscle metabolism.55 In addition, over a fourfold reduction in the circulating levels of miR-149 was reported in middle-aged and elderly patients with coronary artery disease.56,57 Furthermore, miR-149 was reported significantly downregulated during cardiac remodeling in response to acute myocardial infarction in mouse and human hearts.58 Taken together, our results suggest that exercise-induced miR-149 (for example, in skeletal and cardiac muscle tissue) adds onto the beneficial cardiometabolic improvements of bariatric surgery.
The significant reduction observed in the circulating levels of miR-206 in the EX group (Table 2, Figure 1j) is counterintuitive because miR-206 is a muscle-specific miRNA that promotes myogenic differentiation59 and is increased by exercise.60 However, miR-206 was recently found upregulated in peripheral blood endothelial progenitor cells from patients with coronary artery disease as compared to healthy donors,61 whereas knockdown of miR-206 in coronary artery disease endothelial progenitor cells rescued both their angiogenic and vasculogenic abilities in a mouse model of ischemia.62
To our knowledge, this is the first human study reporting miRNA signatures identified in an exercise program adjunct to bariatric surgery. A limitation of our study, as reported for the parent trial, is that participants were mostly young to middle-aged women; therefore, it is difficult to determine sex-specific effects. Because our study used smaller number of samples from the original parent trial, which was relatively short-term, our findings need to be confirmed in larger trials of a longer duration.
CONCLUSIONS
We found that the plasma levels of 10 miRNAs were significantly altered after RYGB surgery followed by an adjunct exercise or educational intervention in humans. Our findings from the assessment of circulating miRNAs associated with metabolically involved tissue and organ functions including liver, adipose, pancreatic and skeletal muscle tissues suggest that RYGB surgery ‘resets’ a variety of molecular networks that can directly lead to improvements in cardiometabolic function and can be additionally impacted by exercise. Notably, the baseline levels of several differentially abundant miRNAs correlated with the post-operative increases in insulin sensitivity, which underscores their predictive clinical value as biomarkers of response to the intervention. A key and previously unknown role for miR-135b in mediating the metabolic benefits of exercise, possibly through regulation of FOXO1 and GSK-3β, is suggested. The consistent implication of FOXO1 as a direct target of several of the differentially abundant circulating miRNAs underscore the important role of this gene in mediating beneficial effects of bariatric surgery and exercise. Consequently, these findings offer novel insights into the molecular changes induced by the RYGB procedure and the effects of mild exercise to facilitate and/or maintain the benefits of a ‘comprehensive’ weight loss intervention with concomitant improvements in cardiometabolic functions.
Supplementary Material
Acknowledgments
This study was supported by funding from the NIDDK (R01DK078192, R01DK078192-02S1, BHG), and the University of Pittsburgh Clinical Translational Research Center (M01RR00056) and Obesity and Nutrition Research Center (P30DK46204). We would like to thank for Florida hospital for making the funds available to AAS and BHG to conduct this study.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
AAS contributed to the conception and design of the research design, analysis plan, supervision of the analysis, study implementation, data acquisition and interpretation, writing of the manuscript and critical revision and final approval of the manuscript. YONL performed the experiments, conducted the data and statistical analysis, contributed to the data interpretation, writing of the manuscript and critical revision of the manuscript. PMC and BHG contributed to the study design, study implementation, the data interpretation, writing of the manuscript and critical revision and final approval of the manuscript. AAS is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Supplementary Information accompanies this paper on International Journal of Obesity website (http://www.nature.com/ijo)
References
- 1.Bradley D, Magkos F, Klein S. Effects of bariatric surgery on glucose homeostasis and type 2 diabetes. Gastroenterology. 2012;143:897–912. doi: 10.1053/j.gastro.2012.07.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coen PM, Tanner CJ, Helbling NL, Dubis GS, Hames KC, Xie H, et al. Clinical trial demonstrates exercise following bariatric surgery improves insulin sensitivity. J Clin Invest. 2015;125:248–257. doi: 10.1172/JCI78016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plum L, Ahmed L, Febres G, Bessler M, Inabnet W, Kunreuther E, et al. Comparison of glucostatic parameters after hypocaloric diet or bariatric surgery and equivalent weight loss. Obesity. 2011;19:2149–2157. doi: 10.1038/oby.2011.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebert MS, Sharp PA. Roles for microRNAs in conferring robustness to biological processes. Cell. 2012;149:515–524. doi: 10.1016/j.cell.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seyhan AA. microRNAs with different functions and roles in disease development and as potential biomarkers of diabetes: progress and challenges. Mol Biosyst. 2015;11:1217–1234. doi: 10.1039/c5mb00064e. [DOI] [PubMed] [Google Scholar]
- 6.Friedman RC, Farh KK, Burge CB, Bartel DP. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonafe M, Olivieri F. Circulating microRNAs in aging. Oncotarget. 2015;6:1340–1341. doi: 10.18632/oncotarget.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kirby TJ, McCarthy JJ. MicroRNAs in skeletal muscle biology and exercise adaptation. Free Radic Biol Med. 2013;64:95–105. doi: 10.1016/j.freeradbiomed.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seyhan AA, Nunez Lopez YO, Xie H, Yi F, Mathews C, Pasarica M, et al. Pancreas-enriched miRNAs are altered in the circulation of subjects with diabetes: a pilot cross-sectional study. Sci Rep. 2016;6:31479. doi: 10.1038/srep31479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cortez MA, Bueso-Ramos C, Ferdin J, Lopez-Berestein G, Sood AK, Calin GA. MicroRNAs in body fluids—the mix of hormones and biomarkers. Nat Rev Clin Oncol. 2011;8:467–477. doi: 10.1038/nrclinonc.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weber JA, Baxter DH, Zhang S, Huang DY, Huang KH, Lee MJ, et al. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56:1733–1741. doi: 10.1373/clinchem.2010.147405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kwon IG, Ha TK, Ryu SW, Ha E. Roux-en-Y gastric bypass stimulates hypothalamic miR-122 and inhibits cardiac and hepatic miR-122 expressions. J Surg Res. 2015;199:371–377. doi: 10.1016/j.jss.2015.05.055. [DOI] [PubMed] [Google Scholar]
- 13.Ortega FJ, Mercader JM, Catalan V, Moreno-Navarrete JM, Pueyo N, Sabater M, et al. Targeting the circulating microRNA signature of obesity. Clin Chem. 2013;59:781–792. doi: 10.1373/clinchem.2012.195776. [DOI] [PubMed] [Google Scholar]
- 14.Wu Q, Li JV, Seyfried F, le Roux CW, Ashrafian H, Athanasiou T, et al. Metabolic phenotype-microRNA data fusion analysis of the systemic consequences of Roux-en-Y gastric bypass surgery. Int J Obes (Lond) 2015;39:1126–1134. doi: 10.1038/ijo.2015.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Creemers EE, Tijsen AJ, Pinto YM. Circulating microRNAs: novel biomarkers and extracellular communicators in cardiovascular disease? Circ Res. 2012;110:483–495. doi: 10.1161/CIRCRESAHA.111.247452. [DOI] [PubMed] [Google Scholar]
- 16.Cho WC. Circulating microRNAs as minimally invasive biomarkers for cancer theragnosis and prognosis. Front Genet. 2011;2:7. doi: 10.3389/fgene.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ortega FJ, Mercader JM, Moreno-Navarrete JM, Rovira O, Guerra E, Esteve E, et al. Profiling of circulating microRNAs reveals common microRNAs linked to type 2 diabetes that change with insulin sensitization. Diabetes Care. 2014;37:1375–1383. doi: 10.2337/dc13-1847. [DOI] [PubMed] [Google Scholar]
- 18.Raffort J, Hinault C, Dumortier O, Van Obberghen E. Circulating microRNAs and diabetes: potential applications in medical practice. Diabetologia. 2015;58:1978–1992. doi: 10.1007/s00125-015-3680-y. [DOI] [PubMed] [Google Scholar]
- 19.Schwarzenbach H, Nishida N, Calin GA, Pantel K. Clinical relevance of circulating cell-free microRNAs in cancer. Nat Rev Clin Oncol. 2014;11:145–156. doi: 10.1038/nrclinonc.2014.5. [DOI] [PubMed] [Google Scholar]
- 20.Wu C, Arora P. MicroRNA passenger strand: orchestral symphony of paracrine signaling. Circ Cardiovasc Genet. 2014;7:567–568. doi: 10.1161/CIRCGENETICS.114.000805. [DOI] [PubMed] [Google Scholar]
- 21.Katsuda T, Ikeda S, Yoshioka Y, Kosaka N, Kawamata M, Ochiya T. Physiological and pathological relevance of secretory microRNAs and a perspective on their clinical application. Biol Chem. 2014;395:365–373. doi: 10.1515/hsz-2013-0222. [DOI] [PubMed] [Google Scholar]
- 22.Arner P, Kulyte A. MicroRNA regulatory networks in human adipose tissue and obesity. Nat Rev Endocrinol. 2015;11:276–288. doi: 10.1038/nrendo.2015.25. [DOI] [PubMed] [Google Scholar]
- 23.Safdar A, Saleem A, Tarnopolsky MA. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat Rev Endocrinol. 2016;12:504–517. doi: 10.1038/nrendo.2016.76. [DOI] [PubMed] [Google Scholar]
- 24.Coen PM, Menshikova EV, Distefano G, Zheng D, Tanner CJ, Standley RA, et al. Exercise and weight loss improve muscle mitochondrial respiration, lipid partitioning, and insulin sensitivity after gastric bypass surgery. Diabetes. 2015;64:3737–3750. doi: 10.2337/db15-0809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dirksen C, Jorgensen NB, Bojsen-Moller KN, Jacobsen SH, Hansen DL, Worm D, et al. Mechanisms of improved glycaemic control after Roux-en-Y gastric bypass. Diabetologia. 2012;55:1890–1901. doi: 10.1007/s00125-012-2556-7. [DOI] [PubMed] [Google Scholar]
- 26.R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2016. [Google Scholar]
- 27.Diggle P, Heagerty P, Liang K-Y, Zeger S. Analysis of Longitudinal Data. 2. Oxford University Press; 2002. p. 396. [Google Scholar]
- 28.Zampetaki A, Kiechl S, Drozdov I, Willeit P, Mayr U, Prokopi M, et al. Plasma microRNA profiling reveals loss of endothelial miR-126 and other microRNAs in type 2 diabetes. Circ Res. 2010;107:810–817. doi: 10.1161/CIRCRESAHA.110.226357. [DOI] [PubMed] [Google Scholar]
- 29.Sun LL, Jiang BG, Li WT, Zou JJ, Shi YQ, Liu ZM. MicroRNA-15a positively regulates insulin synthesis by inhibiting uncoupling protein-2 expression. Diabetes Res Clin Pract. 2011;91:94–100. doi: 10.1016/j.diabres.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 30.Andersen DC, Laborda J, Baladron V, Kassem M, Sheikh SP, Jensen CH. Dual role of delta-like 1 homolog (DLK1) in skeletal muscle development and adult muscle regeneration. Development (Cambridge, England) 2013;140:3743–3753. doi: 10.1242/dev.095810. [DOI] [PubMed] [Google Scholar]
- 31.Wang R, Hong J, Cao Y, Shi J, Gu W, Ning G, et al. Elevated circulating microRNA-122 is associated with obesity and insulin resistance in young adults. Eur J Endocrinol. 2015;172:291–300. doi: 10.1530/EJE-14-0867. [DOI] [PubMed] [Google Scholar]
- 32.Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 33.Salvoza NC, Klinzing DC, Gopez-Cervantes J, Baclig MO. Association of circulating serum miR-34a and miR-122 with dyslipidemia among patients with non-alcoholic fatty liver disease. PloS One. 2016;11:e0153497. doi: 10.1371/journal.pone.0153497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu T, Choi SE, Kim DH, Seok S, Suino-Powell KM, Xu HE, et al. Aberrantly elevated microRNA-34a in obesity attenuates hepatic responses to FGF19 by targeting a membrane coreceptor beta-Klotho. Proc Natl Acad Sci USA. 2012;109:16137–16142. doi: 10.1073/pnas.1205951109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christou NV, Lieberman M, Sampalis F, Sampalis JS. Bariatric surgery reduces cancer risk in morbidly obese patients. Surg Obes Relat Dis. 2008;4:691–695. doi: 10.1016/j.soard.2008.08.025. [DOI] [PubMed] [Google Scholar]
- 36.Deiuliis JA. MicroRNAs as regulators of metabolic disease: pathophysiologic significance and emerging role as biomarkers and therapeutics. Int J Obes (Lond) 2016;40:88–101. doi: 10.1038/ijo.2015.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lustig Y, Barhod E, Ashwal-Fluss R, Gordin R, Shomron N, Baruch-Umansky K, et al. RNA-binding protein PTB and microRNA-221 coregulate AdipoR1 translation and adiponectin signaling. Diabetes. 2014;63:433–445. doi: 10.2337/db13-1032. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka R, Tomosugi M, Horinaka M, Sowa Y, Sakai T. Metformin causes G1-phase arrest via down-regulation of MiR-221 and enhances TRAIL sensitivity through DR5 up-regulation in pancreatic cancer cells. PloS One. 2015;10:e0125779. doi: 10.1371/journal.pone.0125779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coleman CB, Lightell DJ, Jr, Moss SC, Bates M, Parrino PE, Woods TC. Elevation of miR-221 and -222 in the internal mammary arteries of diabetic subjects and normalization with metformin. Mol Cell Endocrinol. 2013;374:125–129. doi: 10.1016/j.mce.2013.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hsieh CH, Rau CS, Wu SC, Yang JC, Wu YC, Lu TH, et al. Weight-reduction through a low-fat diet causes differential expression of circulating microRNAs in obese C57BL/6 mice. BMC Genomics. 2015;16:699. doi: 10.1186/s12864-015-1896-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang H, Yang H, Zhang C, Jing Y, Wang C, Liu C, et al. Investigation of microRNA expression in human serum during the aging process. J Gerontol A Biol Sci Med Sci. 2015;70:102–109. doi: 10.1093/gerona/glu145. [DOI] [PubMed] [Google Scholar]
- 42.Li G, Luna C, Qiu J, Epstein DL, Gonzalez P. Alterations in microRNA expression in stress-induced cellular senescence. Mech Ageing Dev. 2009;130:731–741. doi: 10.1016/j.mad.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Latreille M, Hausser J, Stutzer I, Zhang Q, Hastoy B, Gargani S, et al. MicroRNA-7a regulates pancreatic beta cell function. J Clin Invest. 2014;124:2722–2735. doi: 10.1172/JCI73066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Xu JF, Yang GH, Pan XH, Zhang SJ, Zhao C, Qiu BS, et al. Altered microRNA expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PloS One. 2014;9:e114627. doi: 10.1371/journal.pone.0114627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Umezu T, Tadokoro H, Azuma K, Yoshizawa S, Ohyashiki K, Ohyashiki JH. Exosomal miR-135b shed from hypoxic multiple myeloma cells enhances angiogenesis by targeting factor-inhibiting HIF-1. Blood. 2014;124:3748–3757. doi: 10.1182/blood-2014-05-576116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hao M, Zang M, Zhao L, Deng S, Xu Y, Qi F, et al. Serum high expression of miR-214 and miR-135b as novel predictor for myeloma bone disease development and prognosis. Oncotarget. 2016;7:19589–600. doi: 10.18632/oncotarget.7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xiao S, Yang Z, Lv R, Zhao J, Wu M, Liao Y, et al. miR-135b contributes to the radioresistance by targeting GSK3beta in human glioblastoma multiforme cells. PloS One. 2014;9:e108810. doi: 10.1371/journal.pone.0108810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pei H, Jin Z, Chen S, Sun X, Yu J, Guo W. MiR-135b promotes proliferation and invasion of osteosarcoma cells via targeting FOXO1. Mol Cell Biochem. 2015;400:245–252. doi: 10.1007/s11010-014-2281-2. [DOI] [PubMed] [Google Scholar]
- 49.Gillespie JR, Bush JR, Bell GI, Aubrey LA, Dupuis H, Ferron M, et al. GSK-3beta function in bone regulates skeletal development, whole-body metabolism, and male life span. Endocrinology. 2013;154:3702–3718. doi: 10.1210/en.2013-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kamei Y, Mizukami J, Miura S, Suzuki M, Takahashi N, Kawada T, et al. A forkhead transcription factor FKHR up-regulates lipoprotein lipase expression in skeletal muscle. FEBS Lett. 2003;536:232–236. doi: 10.1016/s0014-5793(03)00062-0. [DOI] [PubMed] [Google Scholar]
- 51.Haeusler RA, Hartil K, Vaitheesvaran B, Arrieta-Cruz I, Knight CM, Cook JR, et al. Integrated control of hepatic lipogenesis versus glucose production requires FoxO transcription factors. Nat Commun. 2014;5:5190. doi: 10.1038/ncomms6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhu H, Leung SW. Identification of microRNA biomarkers in type 2 diabetes: a meta-analysis of controlled profiling studies. Diabetologia. 2015;58:900–911. doi: 10.1007/s00125-015-3510-2. [DOI] [PubMed] [Google Scholar]
- 53.Karolina DS, Armugam A, Tavintharan S, Wong MT, Lim SC, Sum CF, et al. MicroRNA 144 impairs insulin signaling by inhibiting the expression of insulin receptor substrate 1 in type 2 diabetes mellitus. PloS One. 2011;6:e22839. doi: 10.1371/journal.pone.0022839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bye A, Rosjo H, Nauman J, Silva GJ, Follestad T, Omland T, et al. Circulating microRNAs predict future fatal myocardial infarction in healthy individuals - The HUNT study. J Mol Cell Cardiol. 2016;97:162–168. doi: 10.1016/j.yjmcc.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 55.Mohamed JS, Hajira A, Pardo PS, Boriek AM. MicroRNA-149 inhibits PARP-2 and promotes mitochondrial biogenesis via SIRT-1/PGC-1alpha network in skeletal muscle. Diabetes. 2014;63:1546–1559. doi: 10.2337/db13-1364. [DOI] [PubMed] [Google Scholar]
- 56.Sayed AS, Xia K, Li F, Deng X, Salma U, Li T, et al. The diagnostic value of circulating microRNAs for middle-aged (40–60-year-old) coronary artery disease patients. Clinics (Sao Paulo, Brazil) 2015;70:257–263. doi: 10.6061/clinics/2015(04)07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ali Sheikh MS, Xia K, Li F, Deng X, Salma U, Deng H, et al. Circulating miR-765 and miR-149: potential noninvasive diagnostic biomarkers for geriatric coronary artery disease patients. Biomed Res Int. 2015;2015:740301. doi: 10.1155/2015/740301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci USA. 2008;105:13027–13032. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ma J, Yu S, Wang F, Bai L, Xiao J, Jiang Y, et al. MicroRNA transcriptomes relate intermuscular adipose tissue to metabolic risk. Int J Mol Sci. 2013;14:8611–8624. doi: 10.3390/ijms14048611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gomes CP, Oliveira GP, Jr, Madrid B, Almeida JA, Franco OL, Pereira RW. Circulating miR-1, miR-133a, and miR-206 levels are increased after a half-marathon run. Biomarkers. 2014;19:585–589. doi: 10.3109/1354750X.2014.952663. [DOI] [PubMed] [Google Scholar]
- 61.Tang Y, Zhang Y, Chen Y, Xiang Y, Xie Y. Role of the microRNA, miR-206, and its target PIK3C2alpha in endothelial progenitor cell function - potential link with coronary artery disease. FEBS J. 2015;282:3758–3772. doi: 10.1111/febs.13372. [DOI] [PubMed] [Google Scholar]
- 62.Tang Y, Zhang Y, Chen Y, Xiang Y, Xie Y. Role of the microRNA, miR-206, and its target PIK3C2α in endothelial progenitor cell function – potential link with coronary artery disease. FEBS J. 2015;282:3758–3772. doi: 10.1111/febs.13372. n/a–n/a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.