SUMMARY
Caloric restriction (CR) extends lifespan in mammals, yet the mechanisms underlying its beneficial effects remain unknown. The manner in which CR has been implemented in longevity experiments is variable, with both timing and frequency of meals constrained by work schedules. It is commonplace to find that nocturnal rodents are fed during the daytime, and meals are spaced out, introducing prolonged fasting intervals. Since implementation of feeding paradigms over the lifetime is logistically difficult, automation is critical, but existing systems are expensive and not amenable to scale. We have developed a system that controls duration, amount and timing of food availability, and records feeding and voluntary wheel-running activity in mice. Using this system, mice were exposed to temporal or caloric restriction protocols. Mice under CR self-imposed a temporal component by consolidating food intake and unexpectedly increasing wheel-running activity during the rest phase, revealing previously unrecognized relationships among feeding, metabolism and behavior.
Keywords: automated feeder system, caloric restriction, temporal restriction, feeding pattern, wheel-running activity
eTOC blurb
XXX et al developed an automated feeding system that controls amount, duration and timing of food availability and also records feeding and voluntary wheel-running activity in mice. They discover that calorie restricted mice self-imposed a temporal component by consolidating food intake and unexpectedly increasing wheel-running activity during the rest phase.
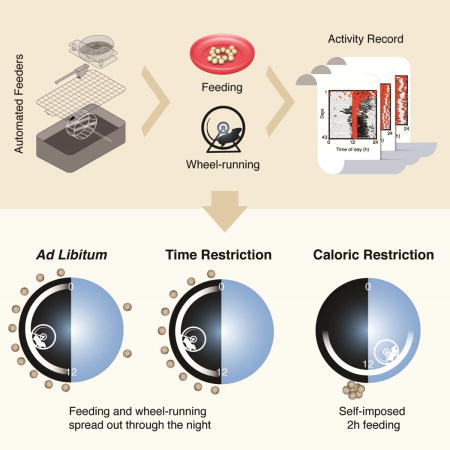
INTRODUCTION
Dietary restriction is the most robust non-genetic, non-surgical and non-pharmacologic intervention known to decrease body weight, improve health and extend lifespan in various species, including nonhuman primates (Fontana et al., 2010; Mattison et al., 2017). It has been used successfully for centuries, yet the critical factor(s) for its beneficial effects remain elusive. Dietary restriction is accomplished by restricting either how much (amount, caloric restriction (CR)), or when (timing, temporal restriction (TR)) food is provided (Table 1; Table S1). Caloric restriction studies have uncovered roles for molecules involved in various nutrient sensing pathways such as insulin/IGF-1, SIRT1, NAMPT, AMPK, PGC-1α, mTOR, GSK3β and FGF21 in regulating aging and lifespan and those pathways are also strongly affected by the timing of food availability (Lopez-Otin et al., 2016). Although dietary restriction protocols are widely used, they can be logistically difficult to implement for extended periods of time. Currently, feeding manipulations are often performed manually, which is labor intensive and can be imprecise. There are three major confounds affecting most of these studies (Table S1): 1) the amount of food provided varies greatly from 34% to 95% of ad libitum (AL) food intake levels; 2) food is usually provided in the morning when nocturnal rodents would not normally eat, although in many cases the time of food access is not even stated; and 3) animals tend to eat all of their food as soon as it is made available, which adds a temporal restriction component to the reduction in calories (Froy and Miskin, 2007). In the last decade, several laboratories have demonstrated that the timing of feeding greatly influences health parameters (Longo and Panda, 2016). Despite the importance of these factors, manipulating when and how much food is available for extended periods of time has proven difficult.
Table 1.
Description of various feeding conditions used in long-term experiments of dietary restriction in mice.
| FEEDING CONDITION | DESCRIPTION |
|---|---|
| Ad Libitum (AL) | Food is available in excess of normal consumption at all times. |
| Alternate Day (AD) | Food is provided ad libitum for 24h (feeding day) and is then withdrawn for 24h (fasting day). Also referred to as EOD – every other day. |
| Caloric Restriction (CR) | A percentage of ad libitum intake is provided once daily or in portions throughout the week. |
| Intermittent Fasting (IF) | Food is withheld for one or more days per week. |
| Temporal Restriction (TR) | Food is provided ad libitum for a limited amount of time each day (e.g. only during the night or only during the day), and is removed thereafter. |
In addition to the amount and timing of food access, other parameters such as duration and frequency of meals, as well as inter-meal fasting periods, can also influence critical health status outcomes (Mattson et al., 2014). To distinguish among the contributions of each of these variables, it is essential to both control and accurately monitor food intake. Food intake measurement is often imprecise, lacks temporal resolution, and rarely allows for long-term recording (Ellacott et al., 2010; Tschop et al., 2012). There are a few labs that have implemented automation to reduce the work load with some success, but these systems are limited in their scope and capacity to both control and measure intake (Flurkey et al., 2010; Hirao et al., 2010; Nelson et al., 1982; Nguyen et al., 2016). Although commercial automated feeders are available, these systems have various limitations (Table S2). The method by which food consumption is quantified varies among them, as does their amenability to be used with standard mouse cages or within isolation cabinets. Only a subset of these systems allows for the precise control of both timing and amount of food availability, and these are expensive and require a significant allocation of space for the large-scale experiments required to examine long-term effects of altered feeding.
In order to address some of these complexities, we have designed an automated feeder system that accurately controls duration, amount and timing of food access. These feeders record individual feeding and wheel-running patterns in mice exposed to virtually any experimenter-defined feeding schedule. Using this versatile system, we have examined how mice modify their behavior in response to changes in either amount or timing of food availability. The feeding conditions we have tested are commonly described in the literature (Table 1), and include ad libitum food access as well as temporal or caloric restriction.
RESULTS AND DISCUSSION
Automated Feeder System Reveals Individual Differences in Ingestive Behavior
The feeder system is fully programmable and is integrated with wheel-running cages (Figure 1A). It dispenses a single 300 mg precision sized food pellet (Figure S1A) and measurement of food consumption can be achieved by counting the number of pellets taken. Feeders hold food in a hopper that dispenses one pellet into a chute for presentation to the mouse (Figure 1A). A successful delivery is confirmed by the consecutive activation of two sensors located underneath the hopper (drop sensor) and on the cage top (chute sensor). Once the mouse takes the pellet, the absence of food is sensed, and the removal time is recorded and displayed by the software in real time (Figure 1B). A new pellet is then dispensed into the cage top food bin. There is a programmable 10-minute delay imposed between the last pellet taken and the next drop, which we have determined from empirical experiments to discourage mice from rapidly removing pellets and hoarding them. Feeder status can be checked remotely and error alerts facilitate the identification of any problems should they occur. In addition to feeding behavior, the automated feeder system is compatible with wheel-running activity recording and both behaviors can be analyzed together for each mouse (Figures 1C and 1D).
Figure 1. Automated Feeder System Reveals Individual Differences in Ingestive Behavior Patterns.

(A) Automated feeder unit that simultaneously monitors wheel-running and feeding behavior aided by sensors.
(B) Real-time pellet consumption recordings for C57BL/6J mice (n=6) fed ad libitum over 6 days (upper panel) or 2 days (bottom panel). White squares indicate a single pellet taken (0.315g/pellet, 3.35Kcal/g of diet). Note: mouse #5 is also shown in (C and E); whereas mouse #4 is also shown in (D and F).
(C–D) Double plotted actograms overlaying wheel-running (black histograms) and feeding (pink dots) behaviors. Isogenic C57BL/6J mice consume less (C) or more (D) pellets during the rest phase even under ad libitum food access. See more examples in Figure S3A.
(E–F) Total food intake (left panel) and day/night feeding distribution (right panel). Despite similarities in the total intake, one mouse consumes ~7% (E), whereas the other consumes ~23% (F) during the daytime.
Bar across the top of the graph indicates the light/dark cycle.
We first assessed the food intake pattern of mice under ad libitum (AL) food. Under a regular light-dark (LD) cycle, food intake and wheel-running behaviors are correlated and occur mostly during the night (Figures 1C and 1D). While feeding is predominantly nocturnal, wild-type C57BL/6J mice exhibited individual differences in feeding pattern (Figures 1C–F). Although most mice had similar total daily intake, some mice consolidated their intake during nighttime (Figures 1C and 1E), whereas others systematically consumed ~25% of food during the day (Figures 1D and 1F). There is substantial variation in body weight among C57BL/6J individuals that increases with age, despite their genetic homogeneity (see https://www.jax.org/strain/0000664), raising the interesting possibility that individual differences in daily ingestion pattern may contribute to this variation. In mice with free access to food, increased daytime feeding correlated with higher body weight (Figure S2), reinforcing the idea that the timing of feeding influences body-weight regulation (Arble et al., 2009; Zarrinpar et al., 2016). Overall, this automated feeder system allows for the simultaneous recording of wheel running and food consumption with high resolution, revealing significant differences in behavior among individuals (Figure S3A).
Caloric and Temporal Restriction Paradigms Differentially Affect Mouse Behavior
We assessed the behavior of mice exposed to feeding conditions commonly used to study the effects of dietary restriction on health and longevity (Table S1). We tested 5 different feeding paradigms involving TR and CR (Figure S1B). We fed mice ad libitum for 1 week and then randomly assigned them to one of the following conditions: 24h ad libitum food access (AL); temporal restriction for 12h during either the night (TR-night) or the day (TR-day); or, 30% caloric restriction with 24h access starting either at the beginning of the night (CR-night) or the day (CR-day).
As stated above, mice under AL food access are mainly active during the night (Figures 2A–D, S3A and S4A). Similarly, mice allowed to eat only for 12h during the night (TR-night) showed locomotor and feeding patterns that closely resemble those of mice in the AL condition (Figures 2E–H, S3B and S4B), with stable 24h behavioral profiles throughout the experiment (Figures 2B, 2C, 2F and 2G). Despite the similarities, this high-resolution feeder system allowed us to detect a subtle difference in the pattern of food ingestion, with TR-night fed mice showing a sharp peak of food intake at the beginning of mealtime (Figures 2C and 2G, and for individual mice see Figures S3A, S3B, S4A and S4B).
Figure 2. Imposed Feeding Paradigms Alter Wheel-Running Activity, Food Intake Patterns and Total Food Consumption.
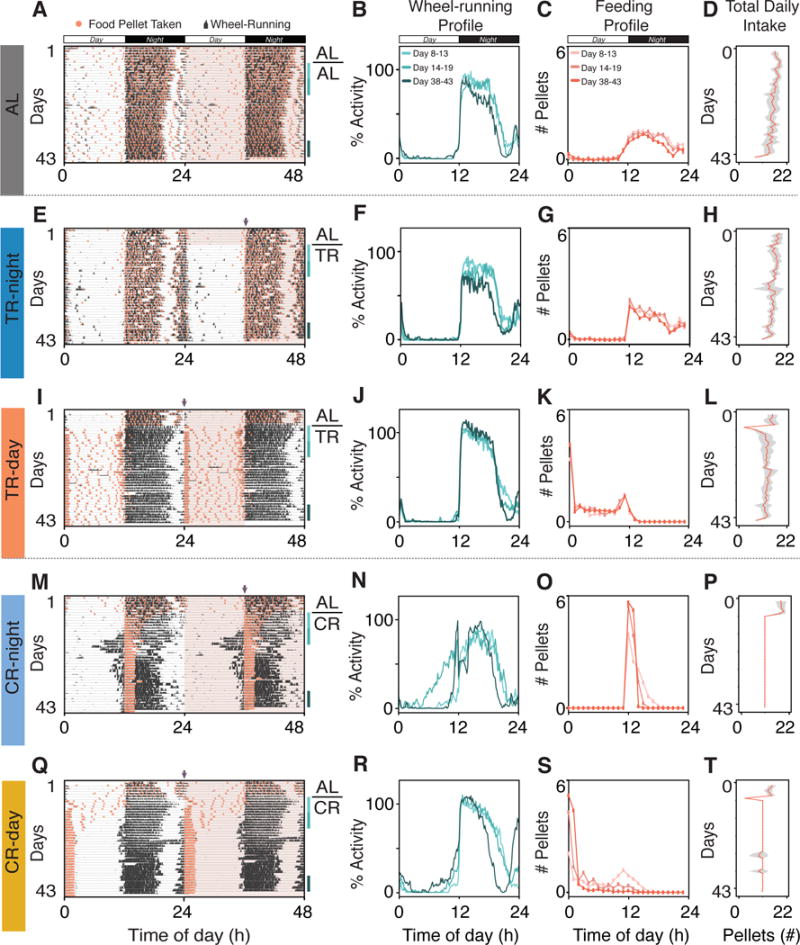
Behavioral recording and quantification of food intake and wheel running for C57BL/6J mice. Each row represents one feeding condition programmed after one week of baseline recording under ad libitum feeding: (A–D) AL: ad libitum. (E–H) TR-night: 12h food access during the night. (I–L) TR-day: 12h food access during the day. (M–P) CR-night: 30% caloric restriction with 24h food access starting at the beginning of the night. (Q–T) CR-day: 30% caloric restriction with 24h food access starting at the beginning of the day.
From left to right, each column shows the following mouse behavior parameters:
(First column) Representative double-plotted actograms. Pink shading represents time of food availability and purple arrows indicate food onset. Bars on right side show recording days used for analysis; from 8 to 13 (light blue), 14 to 19 (aqua) and 38 to 43 (dark green), respectively. The LD cycle is shown in the bar across the top.
(Second column) Average 24h wheel-running activity profiles, normalized to maximum wheel counts recorded during baseline (n=6). CR increases daytime activity. The LD cycle is shown in the bar across the top.
(Third column) Average 24h pattern of food intake (n=6). TR-night resembles AL intake pattern, whereas TR-day condition shows bimodal distribution of food intake during the daytime. CR condition results in consolidation of food intake to within 2h regardless of time of day food is presented. The LD cycle is shown in the bar across the top. Each pellet weighs 0.315g with energy intake equal to 3.35Kcal/g of diet.
(Fourth column) Total daily intake per group (n=6; Shading represents ± SD).
See also data for individual mice plotted in Figures S3A–S3E and S4A–S4E.
Notably, even when eating exclusively during the daytime, TR-day fed mice remained active during the night (Figures 2I–L), resulting in wheel-running and feeding behaviors in complete antiphase to one another (Figures 2I, 2J and 2K). In addition to the misalignment in mouse behavior, the TR-day condition also altered the food intake pattern: mice rapidly ate 5 pellets (~1.5g, 5 Kcal) within the first hour of food access at lights-on, with a secondary peak at the end of the day (Figures 2I and 2K, Figures S3C and S4C). Temporal restriction of food intake to the active phase has been shown to be beneficial; whereas eating at the “wrong time of the day” increased body-weight gain, and susceptibility to obesity, diabetes and cardiovascular diseases (Adamovich et al., 2014; Arble et al., 2009; Chaix et al., 2014; Hatori et al., 2012; Johnston et al., 2016; Shamsi et al., 2014; Sherman et al., 2012; Tsai et al., 2013). Mice were housed with access to a running wheel in only one of these studies (Shamsi et al., 2014). Because access to a running wheel impacts both total food consumption and circadian rhythmicity (Yasumoto et al., 2015), the effects of access to a running wheel on feeding behavior and daily distribution warrant further investigation. Additionally, the impact of timed feeding and its metabolic effects on longevity remains to be determined, likely because TR paradigms are difficult to maintain for long periods of time (Froy and Miskin, 2010). Indeed, timed feeding effects on lifespan have been described only for mice with compromised life expectancy (Table S1 bottom panel). The TR protocols that have been used are fraught with confounding variables related to: 1) the variation in duration of food access and therefore the length of fasting time between food ingestion; and 2) the variation in the amount of food actually consumed during food access time and whether or not this is reduced compared to mice with free access to food (Anson et al., 2005).
In contrast to mice in the TR condition which had unlimited amount of food for only 12h each day, the CR mice had a restricted amount of food that was available for the full 24h. Compared to TR, CR had a stronger impact on mouse behavior (Figures 2M–T). The CR mice shorten their feeding time, regardless of when (day or night) the food is made available (Figures 2M, 2O, 2Q 2S, S4D and S4E), thereby self-imposing a temporal restriction of food intake to 2h daily even though they have 24h of food access. Note that the pellets could not be consumed in a shorter amount of time because of the 10 min delay between pellet drops programmed to prevent hoarding. In agreement with previous anecdotal observations, this provides a clear picture of how calorically restricted mice consolidate their food intake (Challet et al., 1998; Dhahbi et al., 2001; Dhahbi et al., 1999; Feillet et al., 2008; Gonzalez et al., 2004; Hargraves and Hentall, 2005; Harris et al., 1994; Koizumi et al., 1990; Koizumi et al., 1987; Nelson et al., 1982; Patel et al., 2016).
As part of behavioral adaptation, a dramatic increase in daytime wheel-running activity occurred concomitant with the 2h consolidation in feeding (Figures 2M, 2N, 2Q and 2R). Surprisingly, this effect was most pronounced in the CR-night fed mice (Figures 2M, 2N, S3D and S4D), whose nocturnal activity transiently advanced (day 14–19) into the middle of the rest phase. It has been previously shown that CR affects the suprachiasmatic nucleus (SCN) in the hypothalamus that controls circadian locomotor activity (Challet, 2010). Hunger – as a result of negative energy balance – can also induce diurnality in otherwise nocturnal mice (van der Vinne et al., 2014). In addition, it is likely that this shift in activity towards the daytime also reflects food anticipatory activity (FAA, independent of the SCN) that occurs prior to mealtime when food is available only for a few hours per day (Mistlberger, 1994). We found that total wheel-running activity fluctuated over the course of the experiment (Figure 3B) highlighting the importance of tracking activity levels when studying the effects of restriction on health outcomes, since both food intake and level of exercise can influence deprivation state and health outcomes (Mattson et al., 2014). Overall, our results reveal the adaptation to imposed CR feeding conditions is manifest in changes in both feeding and wheel-running activities. Although the mechanisms by which food availability modifies these behaviors are still unknown, this automated feeding system provides the means to address these open questions.
Figure 3. The Timing and Amount of Food Intake and Wheel-Running Activity is Influenced by Imposed Feeding Paradigm.
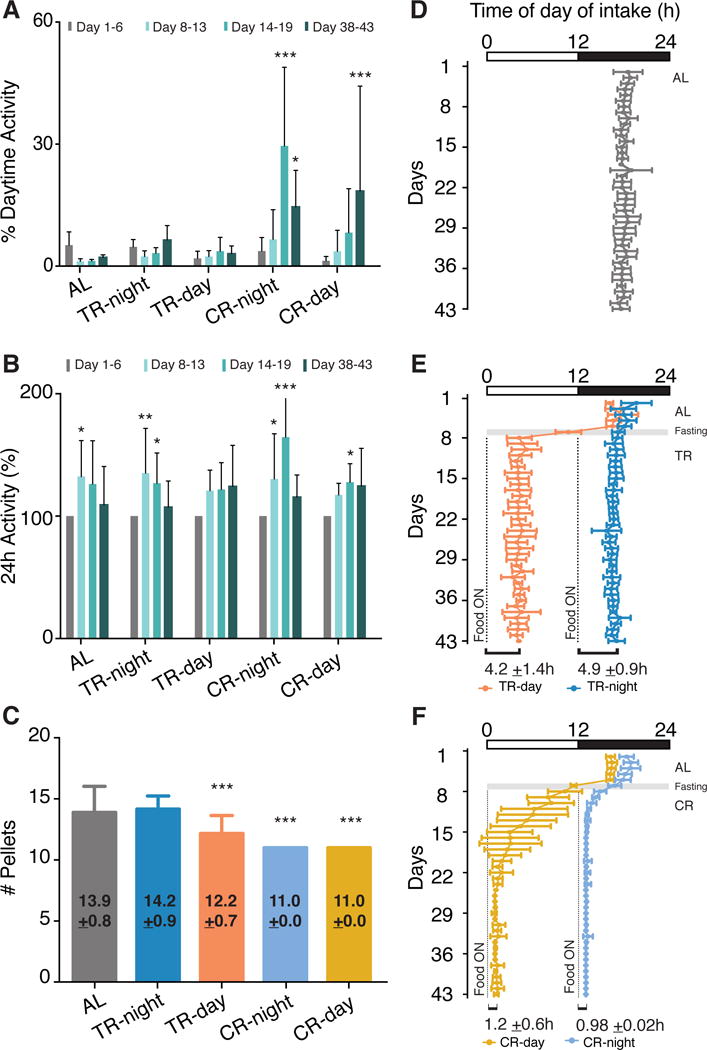
(A) Percentage of wheel-running activity occurring in the light is increased in both CR conditions. Significant difference from baseline condition (gray bar) is determined by ANOVA with Bonferroni’s correction where * p < 0.05, ** p < 0.01, and *** p < 0.001. Data are represented as mean ± SD (n=6).
(B) Total wheel-running activity increases transiently over the course of the experiment for all mice except those in TR-day. Activity is normalized to total activity recorded during baseline (gray) for each feeding condition. Significant difference from AL condition (gray bar) is determined by ANOVA with Bonferroni’s correction where * p < 0.05, ** p < 0.01, and *** p < 0.001. Data are represented as mean ± SD (n=6).
(C) Total amount of pellets consumed per day for mice in each of the feeding conditions during the last week of recording. TR leads to a decrease in total pellet consumption only when restricted to the day. Significant difference from AL condition (gray bar) is determined by ANOVA with Bonferroni’s correction where *** p < 0.001. Data are represented as mean ± SD (n=6).
(D–F) Time required to consume 50% of total daily food intake for mice with free food availability for 1 week followed by: (D) AL; (E) TR; (F) CR. TR-day mice rapidly shift ingestive behavior according to the imposed feeding schedule. CR mice shorten their food intake to within the first hour after food on. Notably, CR-day (light orange) fed mice take longer to consolidate their intake than CR-night (light blue). Gray shading indicates 12h fasting prior to the temporal restriction paradigm. Dotted line denotes the time at which the food is made available. Bar across top indicates LD cycle. Data are represented as mean ± SD (n=6).
Unlike CR groups that ate exactly 11 pellets per day (i.e., maximum allowed; 3.3g/day, equivalent to ~11kcal/day); total intake in TR fed mice was dependent on the time of food availability (Figure 3C). TR-day fed mice consumed ~15% less than those in the AL and TR-night groups (12 vs. 14 pellets per day, 12 vs. 14 kcal/day, respectively; Figure 3C). This indicates that experimenter-imposed daytime feeding schedules involve a certain degree of CR, and that the timing of food availability also influences total daily consumption. Although TR-day mice ate less, both TR groups consumed food until satisfied during the 12h feeding window, since they both delayed consumption of the last pellet for ~2h (Figures 2E, 2I, S3B and S3C). Other laboratories have reported that mice with 8–12h of food access during the daytime consume equivalent amounts of calories to those fed AL (Moran-Ramos et al., 2016). While the reasons for the difference between our results and those previously reported remain unclear, it may reflect differences in diet composition, e.g., high fat diet vs. regular chow and/or the improved temporal resolution (accuracy, frequency and duration) in measuring food intake continuously over longer time periods with this system.
To characterize further how mice adapt to externally imposed feeding schedules, we examined the total intake dynamics over the course of the experiment. Mice consumed 50% of their daily food intake within 4–5h after the food is presented under TR or AL conditions (Figures 3D–E); whereas, if only a limited amount of food was provided, mice rapidly ate half of their meal within 1h despite having 24h in which to consume their food allotment (Figure 3F). We expected that CR-day fed mice would adapt faster to mealtime since mice were fasted for 12h before starting the restriction and, therefore, they skipped the biggest meal prior the restriction (Figures 2H, 2L, 2P and 2T). Surprisingly, despite this initial food deficit, CR-day mice took longer to consolidate food consumption compared to CR-night mice (Figures 2M, 2Q and 3F). This difference likely reflects an interaction between circadian and homeostatic processes exerting opposing influences on the adaptation to the imposed feeding schedule, since the majority of the wheel-running activity these mice exhibited remained constrained to the dark phase while feeding occurred mainly during the day (Figures 2Q and S3E). A more surprising finding is that despite only a single pellet difference in total food intake, TR-day and CR-day fed mice exhibited completely different feeding patterns (Figures 3C, 3E and 3F). This suggests that there is threshold energy deficit required to trigger a behavioral change.
In summary, this feeder system enabled us to identify behavioral adaptation to the various imposed feeding schedules. The comparison of these five feeding conditions highlights how several feeding parameters (duration, timing and amount of food) strongly and differentially impact both locomotor and ingestive behaviors and emphasizes the importance of monitoring food intake throughout the course of food restriction experiments.
Alternate Day Feeding Induces a Distinct Pattern of Ingestion
It has been suggested that the beneficial health outcomes of dietary restriction may be due to the fasting duration between meals (Mattson et al., 2014). Commonly used feeding protocols involve fasting periods that vary greatly from hours to days (Table S1), with the alternate day (AD) feeding paradigm [also known as every other day (EOD) feeding] being one of the more prominent paradigms used. To examine the behavioral adaptation of mice to AD feeding, mice were given unlimited food access for 24h starting 3h after lights-on followed by 24h of fasting (Figures 4A and S1). Initially, AD fed mice showed longer bouts of nocturnal activity on the fasting days compared to feeding days that corresponded to elevated total activity levels, but this pattern was not maintained throughout the experiment (Figures 4A–C, S3F and S4F). Despite food onset occurring during the rest phase, mice did not increase their daytime activity or show food anticipatory activity (FAA) in the hours immediately preceding mealtime (Figure 4D). This is as expected since the AD feeding schedule is outside the circadian limits of entrainment (Mistlberger, 1994; Stephan, 2002).
Figure 4. Food Intake and Wheel-Running Activity in Mice Provided with an Alternate Day (AD) Feeding.
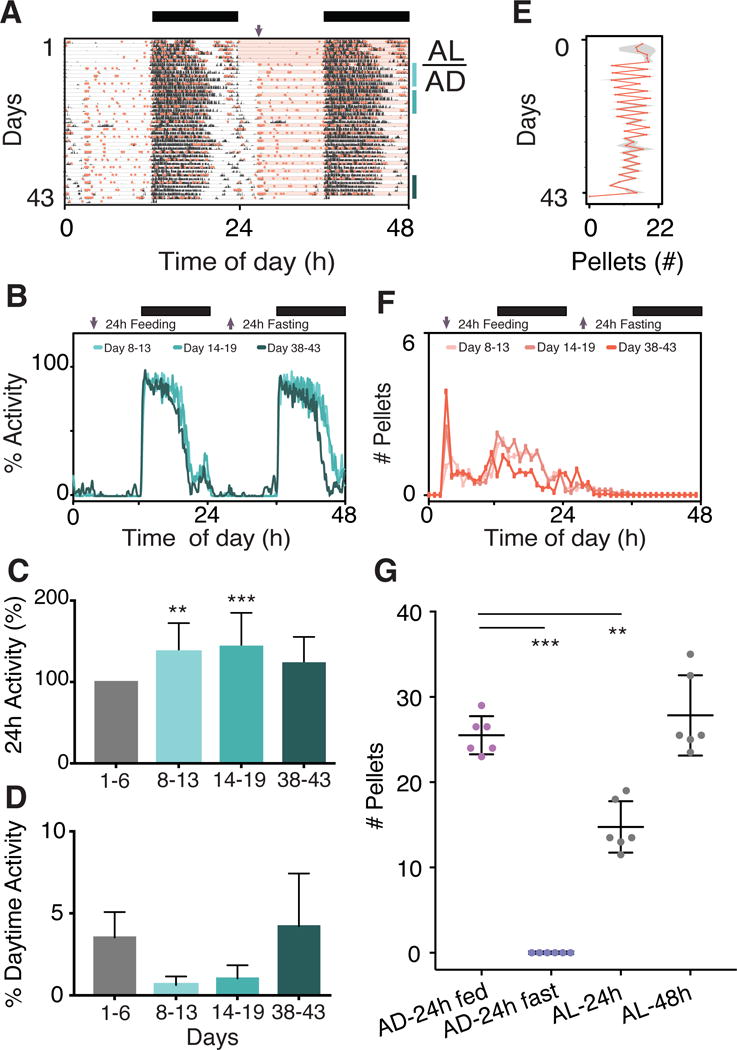
(A) Representative double-plotted actogram of a mouse in the AD condition. Pink shading represents time of food availability and purple arrows indicate food onset. Bars on right side show recording days used for analysis; from 8 to 13 (light-blue), 14 to 19 (aqua) and 38 to 43 (dark green), respectively. The LD cycle is shown in the bar across the top.
(B) Average 48h wheel-running activity profiles, normalized to maximum wheel counts recorded during baseline (n=6). The graph is plotted over 48 hours showing both the feeding (left) and fasting (right) days, with increased activity duration on fasting days. Arrows indicate food on and food off. The LD cycle is shown in the bar across the top.
(C) Total activity increases transiently over the course of the experiment for AD fed mice. Activity is normalized to total activity recorded during baseline (gray). Significant difference from baseline (gray bar) is determined by ANOVA with Bonferroni’s correction where ** p < 0.01, and *** p < 0.001. Data are represented as mean ± SD (n=6).
(D) AD condition does not alter percentage of wheel-running activity occurring in the light. Significant difference from baseline condition (gray bar) is determined by ANOVA with Bonferroni’s correction where * p < 0.05, ** p < 0.01, and *** p < 0.001. Data are represented as mean ± SD (n=6).
(E) The mean total pellets (± SD) consumed per day by mice in the AD condition (n=6). Fluctuations in number of pellets taken per day reflect fasting versus feeding days, although feeding windows span two 24h periods. Each pellet weighs 0.315g with energy intake equal to 3.35Kcal/g of diet.
(F) Average 48h pattern of food intake for AD mice (n=6) shows a sharp peak of food intake once food becomes available on feeding day. The graph is plotted over 48 hours showing both the feeding (left) and fasting (right) days. Arrows indicate food on and food off. The LD cycle is shown in the bar across the top.
(G) In 24h of food access, AD mice consume as much food as AL mice do over 48h. Significant difference determined by ANOVA with Bonferroni’s correction with *** p < 0.001. Data are represented as mean ± SD (n=6).
See also data for individual mice plotted in Figures S3F and S4F.
AD feeding significantly alters total daily intake and 24h ingestive profiles in mice (Figures 4E–F; see more examples in Figure S3F). Ingestive profiles consisted of acute increased pellet consumption as soon as the food was presented, followed by sporadic intake during the rest of the daytime (like TR-day in Figure 2K). The pattern then followed a more normal nocturnal intake until the food availability is turned off (Figures 4F, S3F and S4F). The dispersed pattern of food intake is most evident in the day/night feeding plots of Figure S3F. Strikingly, mice in the AD condition consume, in only 24h, the same amount that AL fed mice would have normally eaten in 48h (Figure 4G). This shows that AD fed mice under these conditions fully compensate for the loss of intake on fasting days, as was previously shown by Anson and colleagues (Anson et al., 2003).
One paradigm commonly used for caloric restriction involves providing a limited amount of food 3 times per week, instead of providing food daily (Pugh et al., 1999). In these studies, feeding is usually done early in the light phase when mice do not normally eat. Based on our CR and AD results, we infer that those mice likely undergo >24h fasting until the next refill; and thereby, this protocol would comprise a combination of both CR and regular intermittent fasting.
In our experiment, the fasting period of each group is ~3h for AL; ~10h for TR; ~21.5h for CR and ~23.5h for the AD condition. Note that pellets were dropped within a 12h window for the TR groups, however mice tended to eat their last pellet ~2h later, and thereby mice are indeed fasted for 10h instead of 12h. If the fasting period is the critical component of all dietary restriction paradigms, then the CR and the AD conditions should produce indistinguishable results, whereas the TR condition may not prove beneficial. Since TR effects on life span have only been tested in two studies of mice with severely compromised life expectancy (Skillings et al., 2014; Wu et al., 2004), it remains to be determined whether TR is as effective as CR or AD paradigms.
Restricted Feeding Regimes Differentially Impact Body-Weight Regulation, Acute Food Intake, and Blood Glucose Homeostasis
To investigate the physiological consequences of each feeding condition, we assessed body weight, blood glucose levels and acute food intake followed by tissue weight measurement. Mice were weighed at the start of the experiment, and again at the end, before the onset of mealtime (Figure 5A). Nocturnal rodents eating during the light phase (TR-day and CR-day) had similar body-weight gain when compared with the AL and TR-night groups (Figure 5A), despite consuming less food (Figure 3C). Only mice in the CR-night condition lost weight (86.8% in CR-night vs 106.1% in AL, p<0001) (Figure 5A). Other laboratories have reported that feeding time significantly impacts body-weight regulation (Arble et al., 2009; Hatori et al., 2012; Tsai et al., 2013). The difference in body-weight maintenance over the course of the experiment in the two CR conditions (-night and -day) reinforces the idea that the time of food consumption is critical, since these mice consumed the same amount of food each day.
Figure 5. Effects of Imposed Feeding Condition on Body Weight, Blood Glucose Level, Acute Food Intake and Peripheral Tissues.
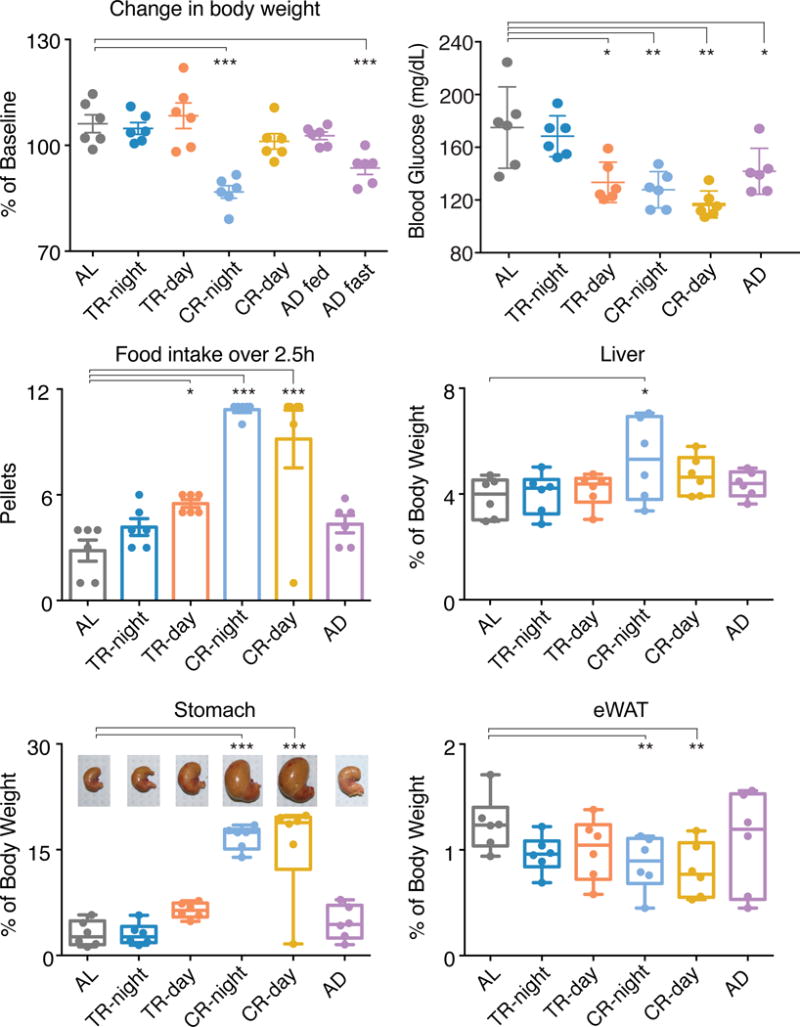
(A) CR during the night decreases body weight, whereas CR during the day does not. Mice in the AD conditions were weighed on two consecutive days – fed and fasted, showing alternate days of weight gain and loss.
(B) Blood glucose levels are lower for mice with reduced food intake. An average of glucose levels measured at 4 time points for each mouse was used to determine the mean (± SD) per feeding condition (n=6).
(C) Mice in the CR condition consume significantly more food within the first 2.5h of food availability on the final day of the experiment. This correlates with the pattern of food consumption shown in Figure 2 -third column. With 0.315g/pellet and 3.35Kcal/g of diet, 11 pellets are equivalent to 11 Kcal. See also Figure S5D.
(D–F) Liver (D), stomach (E) and eWAT (F) weights as a percentage of total body weight measured for mice in each of the feeding conditions. Pictures of stomachs from representative mice are shown. See also Figure S5D for stomach size at the time of tissue collection.
Significant difference from AL condition (gray) is determined by ANOVA with Bonferroni’s correction where * p < 0.05, ** p < 0.01, and *** p < 0.001. Data are represented as mean ± SD (n=6).
Notably, mice in the AD condition undergo a recurring fluctuation in body weight every second day. Mice lost weight during the fasting period (94.84% vs 103.7% in AL; p<0.01) and then recovered during the feeding period (104.4% vs 103.7% in AL; p>0.05) (Figure 5A). The long-term effect of this weight oscillation remains to be determined.
We measured blood glucose at four time points throughout the day for mice in all feeding conditions (Figure S5A). Blood glucose homeostasis is tightly regulated by both feeding/fasting and the circadian system (Gatfield and Schibler, 2008). Here we show that even in the case of misalignment, when feeding and activity occur in opposite phases of the day, blood glucose increases when the mice are eating (Figures S5B and S5C). This suggests that the glucose profile is mainly driven by the feeding/fasting cycles as opposed to time of day, and because of this, the altered feeding pattern adopted by mice fed during the light phase may affect the capacity to maintain normal daily patterns of blood glucose homeostasis. The overall levels of blood glucose, on the other hand, were reduced in the groups of mice that consumed less food (TR-day and both CR conditions, Figures 3D and 5B). This is in agreement with previous studies reporting that hypocaloric feeding leads to a hypoglycemic state (Mendoza et al., 2005; Speakman and Mitchell, 2011), as the daily amount of food intake influences the level of circulating glucose. Overall, we show that the levels of blood glucose correlate with food intake and since TR-day mice consume less food they cluster with the caloric restricted groups. Several hormones – such as insulin, glucagon and glucocorticoids – play a key role in regulating blood glucose homeostasis. The levels of these hormones oscillate throughout the day, and are susceptible to feeding/fasting cycles (Kalsbeek et al., 2014). Thus, it is possible that the self-imposed feeding pattern adopted by each group of mice induces hormonal profile changes that ultimately impact blood glucose levels.
At the end of the experiment, we determined the total food intake and weighed the stomachs, livers and unilateral epididymal white adipose tissue (eWAT) after 2.5h of feeding (Figures 5C–F). Mice in the TR-day, CR-night and CR-day conditions consumed significantly more food than AL mice within these initial hours of food access (Figure 5C). With the exception of one mouse that did not consolidate its daily food intake (Figure S3E), both CR groups of mice consumed all 11 pellets (i.e., 11 Kcal) within the 2.5 h period and accordingly; those mice had significantly larger stomachs than mice in the other groups (Figure 5E and Figure S5D). The liver size of mice in the CR-night condition was significantly larger than those of AL mice (Figure 5D), but this is due to their lower body weight (Figure 5A), since the non-normalized liver mass did not differ between groups. Mice with reduced amount of food intake (CR groups) had the lowest eWAT mass (Figure 5F). This is consistent with the fact that hypocaloric feeding reduces adiposity, thus leading to a decrease in body weight; however, the CR-day group of mice maintained their body weight throughout the experiment. One possibility is that these mice retain or increase fat stores in other tissues (Moran-Ramos et al., 2016). On the contrary, TR-day mice did not show reduced eWAT mass when compared to AL mice (Figure 5F) even though they did consume less food than AL mice, and only 1 pellet more per day than the CR-day mice (Figure 3D). The effects of these different types of restricted feeding paradigms on body composition remain to be fully characterized.
Altogether, these results suggest that eating at the “right” time of the day might play a more substantial role than CR in physiological outcomes. This automated feeding system will be invaluable for examining other physiological and metabolic parameters to uncover the mechanisms behind the effects of various feeding paradigms.
CONCLUSION
We developed a system that allows us to uncover detailed individual behavioral changes that mice undergo when exposed to conflicting environmental signals (food and light/dark cycles). Importantly, we have found that CR leads to a profound temporal restriction in feeding behavior. Since TR alone, without caloric deficit, has been shown to have beneficial metabolic effects, classic CR experiments on longevity need to be re-evaluated to determine whether caloric or time restriction is the critical factor. Understanding these feeding-induced behavioral changes will also help elucidate the as yet unknown mechanism underlying the beneficial effects of temporally restricted feeding.
Overall, our results show that CR affected the temporal patterns of wheel running more than TR does. Surprisingly, mice in the CR-night condition appear to be most affected by the imposed feeding regimen, despite food availability coinciding with the normal peak of food intake under AL conditions. We also show that these effects on behavior change over the course of the experiment. This is important to consider when interpreting the results of studies of both short and long durations. In short experiments with CR imposed for only a matter of weeks and food provided during the active phase, the resulting behavioral alterations with increased activity during the rest phase could impact the outcome measures of these studies. These feeders provide the opportunity to determine how circadian organization and expression of behavioral patterns change over that length of time.
CR also affected food intake patterns more severely than TR did, resulting in a drastic shortening of food intake duration per day. TR of food availability to 12h per day affected intake patterns most when food was provided during the day. Both TR-day and CR-day conditions resulted in food intake and wheel-running activity occurring in anti-phase to one another. This suggests that these mice may be subjected to desynchrony and chronic sleep deprivation. Given that the majority of studies involving CR likely provide food during the daytime, this may be a significant confounding variable.
Timing of food intake can influence body-weight regulation and numerous other health parameters (Johnston et al., 2016). It serves as a powerful synchronizer of circadian rhythms in peripheral metabolic tissues such as the liver (Damiola et al., 2000; Stokkan et al., 2001; Vollmers et al., 2009). TR to the daytime in nocturnal species is able to uncouple peripheral circadian oscillators from the central pacemaker in the SCN, altering body temperature, and greatly influencing the expression and phase of numerous circadian rhythms (Damiola et al., 2000). Conversely, restricting food only to the night can increase the consolidation and the amplitude of these rhythms, and this may counteract the decrease in consolidation and amplitude seen in aging (Froy, 2011). The effects of dietary restriction on circadian rhythmicity may be the route by which longevity affects are manifest.
These automated feeders allow the implement of long-term experiments with specific feeding regimens, recording with a very high resolution both feeding and wheel-running activity, which would open a previously unexplored avenue of investigation into the means to improve circadian rhythmic parameters and, thereby, health and lifespan, independent of dietary restriction.
STAR METHODS
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Bacterial and Virus Strains | ||
| Biological Samples | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| Critical Commercial Assays | ||
| Automated Feeder System | Phenome Technologies and Actimetrics | http://actimetrics.com/products/clocklab/clocklab-feeder-control/ |
| Dustless Precision Pellets®, Rodent, Grain-Based | BioServ | F0175 |
| Precision Xtra Blood Glucose & Ketone Monitoring System | Abbott Diabetes Care | 98814-65 |
| Precision Xtra Blood Glucose test strips | Abbott Diabetes Care | 99878-BX |
| Standard polycarbonate mouse cages with running wheels | Actimetrics | http://actimetrics.com/products/clocklab/running-wheel-cages/ |
| Deposited Data | ||
| Raw data files for feeding and wheel-running activity | This paper | http://dx.doi.org/10.17632/hxwwyycjy7.1 |
| Raw mouse body weight data & blood glucose levels | This paper | http://dx.doi.org/10.17632/hxwwyycjy7.1 |
| Experimental Models: Cell Lines | ||
| Experimental Models: Organisms/Strains | ||
| C57BL/6J, male | Mouse Breeding Core, Wakeland lab, UT Southwestern Medical Center | |
| Oligonucleotides | ||
| Recombinant DNA | ||
| Software and Algorithms | ||
| ggplot2 R package | CRAN | https://cran.r-project.org/web/packages/ggplot2/index.html |
| Prism (version 7.0a) | GraphPad Software | https://www.graphpad.com/scientific-software/prism/ |
| ClockLab USB Data Collection Program (version 3.15) | Actimetrics | http://actimetrics.com/downloads/clocklab/ |
| ClockLab Analysis (version 6.0.34 –stand alone and version 2.72 – Matlab based) | Actimetrics | http://actimetrics.com/downloads/clocklab/ |
| ClockLab Chamber Control | Actimetrics | http://actimetrics.com/downloads/clocklab/ |
| Other | ||
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources and reagents should be directed and will be fulfilled by the Lead Contact, Dr. Joseph S. Takahashi (joseph.takahashi@utsouthwestern.edu)
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Mice
C57BL/6J male mice (8 week-old, n=36) were obtained from our in-house breeding colony (Mouse Breeding Core, Wakeland lab, UT Southwestern Medical Center, Dallas, TX, USA). They were allowed to acclimatize for 2 weeks and were then individually housed in standard polycarbonate mouse cages (Fischer Scientific, Cat. Nos. 01-288-1B and 01-288-21) containing a 4.75″ diameter stainless steel running wheel inside isolation cabinets containing 12 cages each (Siepka and Takahashi, 2005) (http://actimetrics.com/products/clocklab/running-wheel-cages/). Temperature and humidity were monitored, and the mice were housed under a 12:12 LD cycle (green LEDs, ~100 lux at the level of the cage floor). Water was provided ad libitum throughout the study. All animal protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Texas Southwestern Medical Center (APN 2015-100925).
METHOD DETAILS
Feeding and Behavioral Recording
Mice were fed with round pellets of 315 ± 4 mg each containing 3.35 Kcal/g (Dustless Precision Pellets®, Rodent, Grain-Based, F0170, BioServ, Flemington, NJ, USA) (Figure S1A). Pellet composition is equivalent to regular chow, with 10% Kcal from fat, 25% Kcal from protein and 65% Kcal from carbohydrates. Food access was controlled by the automated feeder system designed in our lab with Phenome Technologies, Skokie, IL, USA (Figure 1A), that precisely controls the duration, amount and timing of food access. We used ClockLab Chamber Control Software (Actimetrics Inc., Wilmette, IL, USA) to program feeding schedules and record ingestive events. The feeders can be monitored via remote access in real time, allowing for detection of any problems that may occur. Error messages that indicate problems such as cage or feeder chute misalignments, motor errors, and sensor failures can be detected and rectified quickly. A 10 min delay was programmed after each pellet was taken before the next pellet was dropped to prevent hoarding behavior. Wheel-running behavior was recorded throughout the study using ClockLab Data Acquisition System (Actimetrics Inc., Wilmette, IL, USA).
After one week of recording under ad libitum food access, mice were randomly assigned to one of 6 feeding conditions (n=6 per condition): 24h access ad libitum (AL); unlimited amount but temporally restricted to 12h during the dark (TR-night), or light (TR-day) phase; 24h access but calorically restricted (11 pellets corresponding to 70% of baseline ad libitum levels) fed at the start of the dark (CR-night) or light (CR-day) phase; and fed on alternate days (AD) starting 3h after the onset of light (Figure S1B). Cages were changed on day 21, and bedding was checked for any evidence of food spillage on this day and on the final day of the experiment. No food was found in any of the cages on either day.
Blood Glucose and Tissue Collection
At the end of the experiment, blood glucose levels were measured at various time points throughout the day for the 6 mice in each of the feeding conditions (Figure S5A). Blood was collected from a small cut in the tail and was measured using the Precision Xtra blood glucose meters and glucose test strips (Abbott Diabetes Care Inc., Almeda, CA). Four days later, all mice were removed from wheels and tissues were collected 2.5h after the onset of food availability for each condition (Figures S5B–C). Tissue collection for mice in the AL condition was performed 2.5h after the onset of darkness (Figure S5D). Stomachs, livers and unilateral epididymal white adipose tissue (eWAT) were rapidly dissected and weighed.
QUANTIFICATION AND STATISTICAL ANALYSIS
Statistical Analysis
All behavioral and food intake data were collected and analyzed using Clocklab and Feeder software from Actimetrics, Inc. Additional plots were generated and analyzed using Prism 6 (GraphPad Software, Inc.) and R (ggplot2 package). Pairwise comparisons were made using ANOVA with post hoc analyses to compare experimental conditions to control. Tests with p < 0.05 were deemed statistically significant. Unless otherwise stated, all values are presented as mean ± SD, with statistical results presented as: * p < 0.05, ** p < 0.01, and *** p < 0.001.
Supplementary Material
Highlights.
Automated feeder system enables long-term control and measurement of food access.
Calorically restricted mice rapidly eat within 2h, despite 24h food availability.
Calorically restricted mice increase their locomotor activity during the rest phase.
Daytime feeding prevents body weight loss under 30% caloric restriction in mice.
Acknowledgments
Research was supported by the Howard Hughes Medical Institute (J.S.T.) and NIH/NIA grant R01 AG045795 (J.S.T. and C.B.G.). We would like to thank Delali Bassowou for assistance with animal care and maintenance, Dr. Shin Yamazaki and Dr. Jeremy Stubblefield for helpful discussions, and Fernando Augusto for the generation of the feeder diagram. We thank Michael Wellems (Phenome Technologies, Inc.) and Dr. David Ferster (Actimetrics, Inc.) for their contribution in developing the automated feeder system. J.S.T. is a co-founder of, a Scientific Advisory Board member of, and a paid consultant for Reset Therapeutics, Inc., a biotechnology company aimed at discovering small-molecule therapies that modulate circadian activity for a variety of disease indications. C.B.G. is a paid consultant for Reset Therapeutics, Inc. J.S.T. is an Investigator, M.H.M.dG. is a Research Specialist, and F.R-F. is a Research Associate in the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
Automated feeder system design, development and quality control, V.A.A-R.; experimental design, V.A.A-R., M.H.M.dG., C.B.G., J.S.T.; animal care and maintenance, V.A.A-R., M.H.M.dG.; performing experiments and data acquisition, V.A.A-R., M.H.M.dG., F.R-F.; data analysis, interpretation and generation of figures, V.A.A-R., M.H.M.dG., F.R-F., C.B.G., J.S.T.; writing, reviewing and editing the manuscript, V.A.A-R., M.H.M.dG., F.R-F., C.B.G., J.S.T.; conducting scientific direction and funding acquisition, C.B.G., J.S.T.
References
- Adamovich Y, Rousso-Noori L, Zwighaft Z, Neufeld-Cohen A, Golik M, Kraut-Cohen J, Wang M, Han X, Asher G. Circadian clocks and feeding time regulate the oscillations and levels of hepatic triglycerides. Cell Metab. 2014;19:319–330. doi: 10.1016/j.cmet.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anson RM, Guo Z, de Cabo R, Iyun T, Rios M, Hagepanos A, Ingram DK, Lane MA, Mattson MP. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci U S A. 2003;100:6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anson RM, Jones B, de Cabod R. The diet restriction paradigm: a brief review of the effects of every-other-day feeding. Age (Dordr) 2005;27:17–25. doi: 10.1007/s11357-005-3286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009;17:2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab. 2014;20:991–1005. doi: 10.1016/j.cmet.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Challet E. Interactions between light, mealtime and calorie restriction to control daily timing in mammals. J Comp Physiol B. 2010;180:631–644. doi: 10.1007/s00360-010-0451-4. [DOI] [PubMed] [Google Scholar]
- Challet E, Solberg LC, Turek FW. Entrainment in calorie-restricted mice: conflicting zeitgebers and free-running conditions. Am J Physiol. 1998;274:R1751–1761. doi: 10.1152/ajpregu.1998.274.6.R1751. [DOI] [PubMed] [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhahbi JM, Mote PL, Wingo J, Rowley BC, Cao SX, Walford RL, Spindler SR. Caloric restriction alters the feeding response of key metabolic enzyme genes. Mech Ageing Dev. 2001;122:1033–1048. doi: 10.1016/s0047-6374(01)00230-5. [DOI] [PubMed] [Google Scholar]
- Dhahbi JM, Mote PL, Wingo J, Tillman JB, Walford RL, Spindler SR. Calories and aging alter gene expression for gluconeogenic, glycolytic, and nitrogen-metabolizing enzymes. Am J Physiol. 1999;277:E352–360. doi: 10.1152/ajpendo.1999.277.2.E352. [DOI] [PubMed] [Google Scholar]
- Ellacott KL, Morton GJ, Woods SC, Tso P, Schwartz MW. Assessment of feeding behavior in laboratory mice. Cell Metab. 2010;12:10–17. doi: 10.1016/j.cmet.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feillet CA, Mendoza J, Albrecht U, Pevet P, Challet E. Forebrain oscillators ticking with different clock hands. Mol Cell Neurosci. 2008;37:209–221. doi: 10.1016/j.mcn.2007.09.010. [DOI] [PubMed] [Google Scholar]
- Flurkey K, Astle CM, Harrison DE. Life Extension by Diet Restriction and N-Acetyl-l-Cysteine in Genetically Heterogeneous Mice. J Gerontol A Biol Sci Med Sci. 2010;65:1275–1284. doi: 10.1093/gerona/glq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Partridge L, Longo VD. Extending healthy life span–from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froy O. Circadian rhythms, aging, and life span in mammals. Physiology (Bethesda) 2011;26:225–235. doi: 10.1152/physiol.00012.2011. [DOI] [PubMed] [Google Scholar]
- Froy O, Miskin R. The interrelations among feeding, circadian rhythms and ageing. Prog Neurobiol. 2007;82:142–150. doi: 10.1016/j.pneurobio.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Froy O, Miskin R. Effect of feeding regimens on circadian rhythms: implications for aging and longevity. Aging (Albany NY) 2010;2:7–27. doi: 10.18632/aging.100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatfield D, Schibler U. Circadian glucose homeostasis requires compensatory interference between brain and liver clocks. Proc Natl Acad Sci U S A. 2008;105:14753–14754. doi: 10.1073/pnas.0807861105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez AA, Kumar R, Mulligan JD, Davis AJ, Weindruch R, Saupe KW. Metabolic adaptations to fasting and chronic caloric restriction in heart, muscle, and liver do not include changes in AMPK activity. Am J Physiol Endocrinol Metab. 2004;287:E1032–1037. doi: 10.1152/ajpendo.00172.2004. [DOI] [PubMed] [Google Scholar]
- Hargraves WA, Hentall ID. Analgesic effects of dietary caloric restriction in adult mice. Pain. 2005;114:455–461. doi: 10.1016/j.pain.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Harris SB, Gunion MW, Rosenthal MJ, Walford RL. Serum glucose, glucose tolerance, corticosterone and free fatty acids during aging in energy restricted mice. Mech Ageing Dev. 1994;73:209–221. doi: 10.1016/0047-6374(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Hatori M, Vollmers C, Zarrinpar A, DiTacchio L, Bushong EA, Gill S, Leblanc M, Chaix A, Joens M, Fitzpatrick JA, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15:848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirao A, Nagahama H, Tsuboi T, Hirao M, Tahara Y, Shibata S. Combination of starvation interval and food volume determines the phase of liver circadian rhythm in Per2::Luc knock-in mice under two meals per day feeding. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1045–1053. doi: 10.1152/ajpgi.00330.2010. [DOI] [PubMed] [Google Scholar]
- Johnston JD, Ordovas JM, Scheer FA, Turek FW. Circadian Rhythms, Metabolism, and Chrononutrition in Rodents and Humans. Adv Nutr. 2016;7:399–406. doi: 10.3945/an.115.010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalsbeek A, La Fleur S, Fliers E. Circadian control of glucose metabolism. Mol Metab. 2014;3:372–383. doi: 10.1016/j.molmet.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koizumi A, Wada Y, Tsukada M, Kamiyama S, Weindruch R. Effects of energy restriction on mouse mammary tumor virus mRNA levels in mammary glands and uterus and on uterine endometrial hyperplasia and pituitary histology in C3H/SHN F1 mice. J Nutr. 1990;120:1401–1411. doi: 10.1093/jn/120.11.1401. [DOI] [PubMed] [Google Scholar]
- Koizumi A, Weindruch R, Walford RL. Influences of dietary restriction and age on liver enzyme activities and lipid peroxidation in mice. J Nutr. 1987;117:361–367. doi: 10.1093/jn/117.2.361. [DOI] [PubMed] [Google Scholar]
- Longo Valter D, Panda S. Fasting, Circadian Rhythms, and Time-Restricted Feeding in Healthy Lifespan. Cell Metab. 2016;23:1048–1059. doi: 10.1016/j.cmet.2016.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Galluzzi L, Freije JM, Madeo F, Kroemer G. Metabolic Control of Longevity. Cell. 2016;166:802–821. doi: 10.1016/j.cell.2016.07.031. [DOI] [PubMed] [Google Scholar]
- Mattison JA, Colman RJ, Beasley TM, Allison DB, Kemnitz JW, Roth GS, Ingram DK, Weindruch R, de Cabo R, Anderson RM. Caloric restriction improves health and survival of rhesus monkeys. Nat Commun. 2017;8:14063. doi: 10.1038/ncomms14063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Allison DB, Fontana L, Harvie M, Longo VD, Malaisse WJ, Mosley M, Notterpek L, Ravussin E, Scheer FA, et al. Meal frequency and timing in health and disease. Proc Natl Acad Sci U S A. 2014;111:16647–16653. doi: 10.1073/pnas.1413965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza J, Graff C, Dardente H, Pevet P, Challet E. Feeding cues alter clock gene oscillations and photic responses in the suprachiasmatic nuclei of mice exposed to a light/dark cycle. J Neurosci. 2005;25:1514–1522. doi: 10.1523/JNEUROSCI.4397-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE. Circadian food-anticipatory activity: Formal models and physiological mechanisms. Neurosci Biobehav Rev. 1994;18:171–195. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Moran-Ramos S, Baez-Ruiz A, Buijs RM, Escobar C. When to eat? The influence of circadian rhythms on metabolic health: are animal studies providing the evidence? Nutr Res Rev. 2016:1–14. doi: 10.1017/S095442241600010X. [DOI] [PubMed] [Google Scholar]
- Nelson W, Fundakowski R, Baer J, Cadotte L, Halberg F. An apparatus for automatically timing access to food by mice. Lab Anim Sci. 1982;32:66–69. [PubMed] [Google Scholar]
- Nguyen KP, O’Neal TJ, Bolonduro OA, White E, Kravitz AV. Feeding Experimentation Device (FED): A flexible open-source device for measuring feeding behavior. J Neurosci Methods. 2016;267:108–114. doi: 10.1016/j.jneumeth.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SA, Velingkaar N, Makwana K, Chaudhari A, Kondratov R. Calorie restriction regulates circadian clock gene expression through BMAL1 dependent and independent mechanisms. Sci Rep. 2016;6:25970. doi: 10.1038/srep25970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh TD, Klopp RG, Weindruch R. Controlling caloric consumption: protocols for rodents and rhesus monkeys. Neurobiol Aging. 1999;20:157–165. doi: 10.1016/s0197-4580(99)00043-3. [DOI] [PubMed] [Google Scholar]
- Shamsi NA, Salkeld MD, Rattanatray L, Voultsios A, Varcoe TJ, Boden MJ, Kennaway DJ. Metabolic consequences of timed feeding in mice. Physiol Behav. 2014;128:188–201. doi: 10.1016/j.physbeh.2014.02.021. [DOI] [PubMed] [Google Scholar]
- Sherman H, Genzer Y, Cohen R, Chapnik N, Madar Z, Froy O. Timed high-fat diet resets circadian metabolism and prevents obesity. FASEB J. 2012;26:3493–3502. doi: 10.1096/fj.12-208868. [DOI] [PubMed] [Google Scholar]
- Siepka SM, Takahashi JS. Methods to Record Circadian Rhythm Wheel Running Activity in Mice. Methods Enzymol. 2005;393:230–239. doi: 10.1016/S0076-6879(05)93008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skillings EA, Wood NI, Morton AJ. Beneficial effects of environmental enrichment and food entrainment in the R6/2 mouse model of Huntington’s disease. Brain Behav. 2014;4:675–686. doi: 10.1002/brb3.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speakman JR, Mitchell SE. Caloric restriction. Mol Aspects Med. 2011;32:159–221. doi: 10.1016/j.mam.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Stephan FK. The “other” circadian system: food as a Zeitgeber. J Biol Rhythms. 2002;17:284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291:490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- Tsai JY, Villegas-Montoya C, Boland BB, Blasier Z, Egbejimi O, Gonzalez R, Kueht M, McElfresh TA, Brewer RA, Chandler MP, et al. Influence of dark phase restricted high fat feeding on myocardial adaptation in mice. J Mol Cell Cardiol. 2013;55:147–155. doi: 10.1016/j.yjmcc.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschop MH, Speakman JR, Arch JR, Auwerx J, Bruning JC, Chan L, Eckel RH, Farese RV, Jr, Galgani JE, Hambly C, et al. A guide to analysis of mouse energy metabolism. Nat Methods. 2012;9:57–63. doi: 10.1038/nmeth.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vinne V, Riede SJ, Gorter JA, Eijer WG, Sellix MT, Menaker M, Daan S, Pilorz V, Hut RA. Cold and hunger induce diurnality in a nocturnal mammal. Proc Natl Acad Sci U S A. 2014;111:15256–15260. doi: 10.1073/pnas.1413135111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmers C, Gill S, DiTacchio L, Pulivarthy SR, Le HD, Panda S. Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc Natl Acad Sci U S A. 2009;106:21453–21458. doi: 10.1073/pnas.0909591106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MW, Li XM, Xian LJ, Levi F. Effects of meal timing on tumor progression in mice. Life Sci. 2004;75:1181–1193. doi: 10.1016/j.lfs.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Yasumoto Y, Nakao R, Oishi K. Free access to a running-wheel advances the phase of behavioral and physiological circadian rhythms and peripheral molecular clocks in mice. PLoS One. 2015;10:e0116476. doi: 10.1371/journal.pone.0116476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrinpar A, Chaix A, Panda S. Daily Eating Patterns and Their Impact on Health and Disease. Trends Endocrinol Metab. 2016;27:69–83. doi: 10.1016/j.tem.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


