Abstract
Background
In the present study, we examined the pattern of metastatic spread in patients with advanced non-small-cell lung cancer (nsclc) and the effect of EGFR mutations.
Methods
Patients were identified from a provincial cancer registry, and individual medical records were reviewed. Patients were included if they had stage iv nsclc and underwent diagnostic EGFR mutation testing. Patients were divided into EGFR mutation-positive (EGFR+) and EGFR wild type (wt) cohorts. The primary endpoint was the cumulative incidence for each metastatic site: lung, bone, brain, liver, adrenal glands, distant nodes, and other. Cumulative incidence curves were estimated using a competing-risks method. The secondary outcome was survival.
Results
Of the 543 identified patients, 121 (22.3%) tested as EGFR+, and 422 (77.7%) tested as EGFR wt. The incidence of brain (39.2% vs. 28.2%, p = 0.038) and lung (61.2% vs. 51.0%, p = 0.048) metastasis was higher in the EGFR+ cohort than in the EGFR wt cohort. In the EGFR+ cohort, a higher incidence of liver metastasis was associated with the exon 21 mutation subtype than with the exon 19 deletion subtype [23% vs. 7%, p < 0.01; hazard ratio (hr): 3.47]. Median survival was significantly longer for the EGFR+ cohort than for the EGFR wt cohort (22.4 months vs. 7.9 months, p < 0.001). In multivariable analysis, brain (hr: 1.73), liver (hr: 1.69), and bone (hr: 1.89) metastases were associated with worse survival.
Conclusions
Rates of lung and brain metastases are higher in EGFR mutation carriers, even when adjusted for differences in survival. Brain, liver, and bone metastases are independent negative prognostic factors for survival.
Keywords: EGFR, lung cancer, metastasis behaviour, population studies
INTRODUCTION
The pattern of disease spread in non-small-cell lung cancer (nsclc) has been described in several historic series1,2. Lung, bone, liver, and brain are frequent sites of metastatic involvement in cancer, and various hypotheses have attempted to explain this particular disposition. Paget3 proposed that a hospitable environment at the metastatic site is most important for disease spread. Ewing4 theorized that metastatic distribution relates to lymphatic or vascular flow patterns. Hellman and Weichselbaum5 suggested that tumours gradually acquire the properties necessary for efficient and widespread metastatic spread, and that the likelihood, number, and sites of metastases might reflect the state of tumour development. Other factors are likely inherent to the biology of primary tumour. With the discovery of driver mutations, such as those affecting the epidermal growth factor receptor (egfr), tumour genetics could play a significant role in metastatic behaviour.
The egfr transmembrane receptor tyrosine kinase is involved in signal transduction, regulation of dna synthesis, and cell proliferation. Mutations in the EGFR gene can result in constitutive activation of the tyrosine kinase that can lead to tumourigenesis6. Exon 19 deletions and exon 21 mutations account for 90% of the identified EGFR driver mutations. In nsclc, overexpression of egfr has an impact on the biologic behaviour of the disease, affecting survival and treatment response with egfr tyrosine kinase inhibitors (tkis)7–9.
The presence of an EGFR mutation could have a significant effect on the pattern of metastatic disease spread. Further, differences in metastatic disposition could have a differential effect on morbidity and mortality. The aim of the present study was to examine the patterns of metastatic spread in nsclc and whether it varies by EGFR mutation status. The resulting information could help to anticipate disease behaviour and to direct investigations or tailor therapy.
METHODS
Patient Selection
Patients for the years 2010–2012 were identified from a provincial (British Columbia) cancer registry. Patients were included if they had nsclc that was stage iv at initial diagnosis (American Joint Committee on Cancer staging manual, 7th edition) and had undergone conclusive EGFR mutation testing. Patients were excluded if their histology was squamous or neuroendocrine. The study start date (2010) was set for the point at which EGFR mutation testing was available at our institution. Individual medical records were reviewed to obtain patient characteristics, disease characteristics, and locations of metastasis from the time of initial diagnosis to the time of death. Diagnostic imaging reports were used to identify the locations of metastatic sites and the time at which they were first detected. Diagnostic imaging was performed at the discretion of the treating physician and as clinically indicated. The study was approved by the institutional ethics review board of the BC Cancer Agency.
Mutation Analysis
The study population was divided into those who were EGFR mutation–positive (EGFR+), and those who were EGFR wild type (wt). The EGFR+ cohort was further divided into exon 19 deletion and exon 21 mutation subgroups. Analysis of EGFR exon 19 in-frame deletions and exon 21 point mutations were performed with a minimum of 400 ng genomic dna using polymerase chain reaction testing (the technique previously reported by Pan et al.10).
Study Endpoints
The primary endpoint was the cumulative incidence for each metastatic site. The secondary endpoints were overall survival (os) and the prognostic implication of the site of metastasis.
Statistical Analysis
The Fisher exact and Kruskal–Wallis rank-sum tests were used to compare patient and disease characteristics between the EGFR+ and EGFR wt cohorts (categorical and continuous variables respectively). The time to metastasis was measured from the date of pathology diagnosis to the date of diagnostic imaging demonstrating metastasis at a given site. Cumulative incidence curves were estimated for each metastatic site by the competing-risks method. In the estimation of incidence, patient death before the development of metastasis at a given site was considered a competing-risk event. Patients who had not developed metastasis at a given site and had not died were censored at the time of last follow-up. Differences in the cumulative incidence curves between the two cohorts were assessed using the Gray test.
The associations between metastasis at a given site, EGFR mutation status, and patient characteristics were assessed using a proportional sub-distribution hazards model (Fine–Gray model). Overall survival was estimated by the Kaplan–Meier method and was compared using the log-rank test. The associations between os, EGFR mutation status, and metastatic sites were assessed using a Cox proportional hazards model. Metastasis was treated as a time-dependent variable in the Cox regression analyses, in which the metastatic site was taken into account only after the date of diagnostic imaging demonstrating metastasis at that site. The model was tested for multicollinearity by estimating the variance inflation factor of the various variables. All reported p values are two-sided, with a p value less than 0.05 being set as the level of significance. Cox regression analysis was used to compute hrs with associated confidence intervals (cis). All cis are reported at 95%. Analyses were performed in the R software environment (version 2.1.5: The R Foundation, Vienna, Austria).
RESULTS
Patients
The initial search of the provincial cancer registry identified 1373 patients with stage iv nsclc. After 746 patients without EGFR mutation testing and 84 with non-diagnostic results had been excluded, 543 patients were eligible for study. In the study group, 121 patients (22.3%) had EGFR+ cancers, and 422 (77.7%) had EGFR wt disease. In the EGFR+ cohort, 73 cancers (60%) had exon 19 deletions, and 48 (40%) had exon 21 mutations. Median follow-up duration for living patients was 34.9 months.
Table i presents baseline patient characteristics. Median age and sex were not different between the EGFR cohorts. A greater proportion of patients of Asian ethnicity and non-smokers had EGFR+ cancers. The proportions of patients who received any systemic therapy (chemotherapy or egfr tki, or both) were different in the EGFR+ and EGFR wt cohorts (90% vs. 50%, p < 0.001). The use of an egfr tki was significantly associated with EGFR mutation status, which aligns with the approved indication for an egfr tki as first-line treatment for metastatic EGFR+ nsclc at our institution. However, only moderate collinearity was observed between EGFR+ status and use of an egfr tki (variance inflation factor: 1.8) because some patients who tested as EGFR wt received treatment with an egfr tki, and some patients who were EGFR+ did not receive an egfr tki. The first-line egfr tkis used were erlotinib (56.8% of patients) and gefitinib (40.5% of patients). Chemotherapy use was not different between the EGFR cohorts. Standard chemotherapy regimens were platinum doublets, most frequently cisplatin–gemcitabine (29.3%), carboplatin–gemcitabine (14.2%), and carboplatin–pemetrexed (12.1%).
TABLE I.
Patient characteristics by EGFR mutation status
| Characteristic | Overall | EGFR WT | EGFR-positive | p Value |
|---|---|---|---|---|
| Patients (n) | 543 | 422 | 121 | |
| Age at diagnosis (years) | ||||
| Median | 66 | 67 | 66 | 0.75a |
| IQR | 58–74 | 58–73 | 55–77 | |
| ECOG PS [n (%)] | ||||
| 0 | 23 (4) | 14 (3) | 9 (7) | 0.008b |
| 1 | 229 (42) | 166 (39) | 63 (52) | |
| 2 | 160 (29) | 129 (31) | 31 (26) | |
| 3 | 116 (21) | 100 (24) | 16 (13) | |
| 4 | 15 (3) | 13 (3) | 2 (2) | |
| Asian ethnicity [n (%)] | ||||
| Yes | 123 (23) | 64 (15) | 59 (49) | <0.001b |
| No | 420 (77) | 358 (85) | 62 (51) | |
| Sex | ||||
| Women | 327 (60) | 247 (59) | 80 (66) | 0.14b |
| Men | 216 (40) | 175 (41) | 41 (34) | |
| Smoking (pack–years) | ||||
| Median | 21 | 30 | 0 | <0.001a |
| IQR | 0–40 | 10–40 | 0–5 | |
| Chemotherapy | ||||
| Yes | 267 (49) | 208 (49) | 59 (49) | 1b |
| No | 276 (51) | 214 (51) | 62 (51) | |
| EGFR TKI | ||||
| Yes | 222 (41) | 117 (28) | 105 (87) | <0.001b |
| No | 321 (59) | 305 (72) | 16 (13) |
Calculated using the Kruskal–Wallis rank-sum test.
Calculated using the Fisher exact test.
WT = wild type; IQR = interquartile range; ECOG PS = Eastern Cooperative Oncology Group performance status; EGFR TKI = epidermal growth factor receptor tyrosine kinase inhibitor.
Incidence of Metastases
Table ii presents the 10 most frequent sites of metastasis at initial diagnosis. The metastatic sites were lung (including pleura), bone (including vertebral spine), brain (including intracranial leptomeninges), liver, adrenal glands, distant (extrathoracic) lymph nodes, soft tissue, pericardium, spleen, and kidney. At presentation, no difference in metastatic involvement for any organ site was observed for the EGFR+ and EGFR wt cohorts. Of the patients overall, 323 (59.5%) presented with a single-organ site of metastasis, and 220 (40.5%), with multiple organ metastases.
TABLE II.
Metastatic sites: frequency at initial diagnosis and three-year cumulative incidence ratea by EGFR mutation status
| Site | Frequency | Cumulative incidence (%) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| Overall | EGFR WT | EGFR-positive | p Valueb | Overall | EGFRWT | EGFR-positive | p Valuec | |||||||
|
|
|
|
|
|
|
|||||||||
| (n) | (%) | (n) | (%) | (n) | (%) | Rate | 95% CI | Rate | 95% CI | Rate | 95% CI | |||
| Lung | 287 | 52.9 | 215 | 50.9 | 72 | 59.5 | 0.10 | 53.2 | 48.9 to 57.3 | 51.0 | 46.1 to 55.6 | 61.2 | 51.8 to 69.2 | 0.048 |
| Bone | 211 | 38.9 | 167 | 39.6 | 44 | 36.4 | 0.60 | 40.4 | 36.2 to 44.5 | 40.8 | 36.1 to 45.4 | 38.9 | 30.2 to 47.6 | 0.65 |
| Brain | 118 | 21.7 | 89 | 21.1 | 29 | 24.0 | 0.53 | 30.7 | 26.8 to 34.6 | 28.2 | 23.9 to 32.6 | 39.2 | 30.4 to 47.9 | 0.038 |
| Liver | 62 | 11.4 | 48 | 11.4 | 14 | 11.6 | 1.00 | 12.4 | 9.8 to 15.3 | 12.1 | 9.2 to 15.4 | 13.2 | 7.9 to 20 | 0.74 |
| Adrenal gland | 59 | 10.9 | 49 | 11.6 | 10 | 8.3 | 0.41 | 11.1 | 8.6 to 13.9 | 11.9 | 9 to 15.1 | 8.3 | 4.2 to 14 | 0.27 |
| Distal nodes | 56 | 10.3 | 44 | 10.4 | 12 | 9.9 | 1.00 | 10.7 | 8.3 to 13.5 | 10.9 | 8.2 to 14.2 | 9.9 | 5.4 to 16 | 0.75 |
| Soft tissue | 22 | 4.1 | 17 | 4.0 | 5 | 4.1 | 1.00 | 4.8 | 3.2 to 6.8 | 4.8 | 3 to 7.1 | 5.0 | 2 to 9.9 | 0.93 |
| Pericardium | 15 | 2.8 | 10 | 2.4 | 5 | 4.1 | 0.34 | 3.1 | 1.9 to 4.9 | 2.6 | 1.4 to 4.5 | 5.0 | 2 to 9.9 | 0.19 |
| Spleen | 6 | 1.1 | 4 | 0.9 | 2 | 1.7 | 0.62 | 1.1 | 0.5 to 2.3 | 0.9 | 0.3 to 2.3 | 1.7 | 0.3 to 5.3 | 0.51 |
| Kidney | 2 | 0.4 | 1 | 0.2 | 1 | 0.8 | 0.40 | 0.4 | 0.1 to 1.3 | 0.2 | 0 to 1.3 | 0.8 | 0.1 to 4.1 | 0.35 |
Estimated using a competing-risks method.
Calculated using the Fisher exact test.
Calculated using the Gray test.
WT = wild type; CI = confidence interval.
Table ii also presents the 3-year cumulative incidence rates for each metastatic site. The most common sites of metastasis were lung, bone, and brain (in 53%, 40%, and 31% of patients respectively). The cumulative incidences for lung (61.2% vs. 51.0%, p = 0.048) and brain (39.2% vs. 28.2%, p = 0.038) metastasis were significantly higher in the EGFR+ cohort than in the EGFR wt cohort. With respect to the other metastatic sites, the incidence of metastatic involvement was not significantly different between the cohorts.
In an analysis of the 6-month incidence rates for each metastatic site (full data not shown), the findings were the same, with lung (p = 0.048) and brain (p = 0.038) metastasis being higher in the EGFR+ cohort. Within the EGFR+ cohort, the incidence of liver metastasis was significantly higher in patients with an exon 21 mutation than in those with an exon 19 deletion (23% vs. 7%, p < 0.01; hr: 3.47). No other differences in metastatic spread were observed between the exon subtypes.
Survival
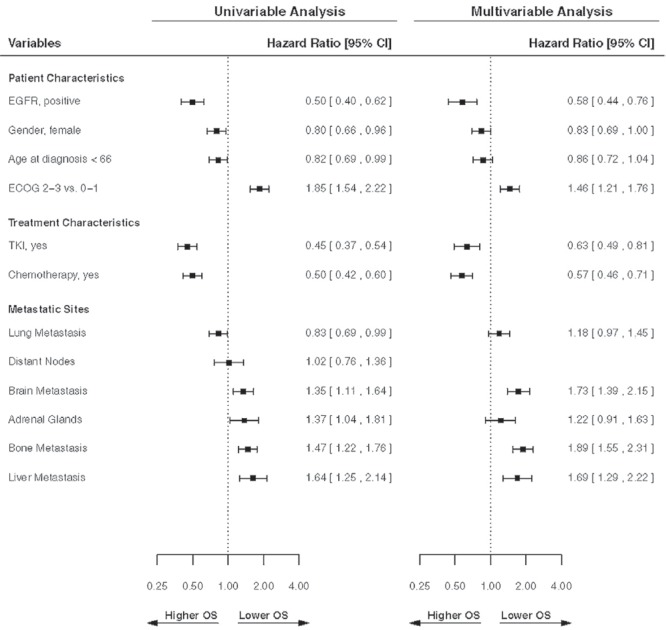
Median os duration was 22.4 months for the EGFR+ cohort and 7.9 months for the EGFR wt cohort (p < 0.001). Figure 1 presents a forest plot of the univariable and multivariable analyses for os. In the multivariable analysis, EGFR+ status (p < 0.001; hr for death: 0.58), younger age (p = 0.05; hr: 0.86), chemotherapy use (p < 0.001; hr: 0.57), and use of egfr tki (p < 0.001; hr: 0.63) were significant factors for longer survival. Poor functional status was significant for worse survival (p < 0.001). Female sex was significant for survival in the univariable analysis, but not in the multivariable analysis (p = 0.13). In the multivariable analysis, the development of brain (p < 0.001; hr: 1.73), bone (p < 0.001; hr: 1.89), and liver (p < 0.001; hr: 1.69) metastasis was significant for worse survival.
FIGURE 1.
Forest plots showing the univariable and multivariable analyses for overall survival (OS). Hazard ratios were estimated in a Cox proportional hazards regression model. The multivariable analysis was limited to variables that showed significance in the univariate analysis. All metastatic sites were analyzed as time-dependent variables. CI = confidence interval; EGFR = epidermal growth factor receptor; ECOG = Eastern Cooperative Oncology Group; TKI = tyrosine kinase inhibitor.
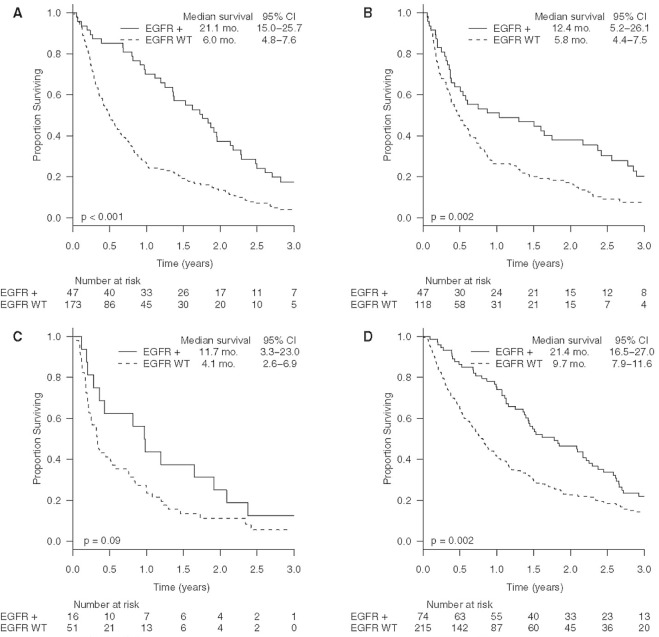
In a multivariable analysis including only EGFR+ patients, the only metastatic site significant for worse survival was liver (p < 0.04; hr: 1.83). For the study population, the median survival durations after a diagnosis of brain, bone, and liver metastasis were 6.5 months (ci: 5.1 to 8.6 months), 7.9 months (ci: 6.1 to 9.8 months), and 4.3 months (ci: 3.1 to 9.8 months) respectively. Figure 2 shows survival after a diagnosis of brain, bone, liver, and lung metastasis by EGFR mutation status. In the figure, patients represented on each Kaplan–Meier curve might have had more than 1 site of metastasis, and patients with multiple metastases might be represented on more than one curve. In a time-dependent Cox regression analysis, no difference in survival was observed for single compared with multiple organ sites of metastasis.
FIGURE 2.
Kaplan–Meier curves showing survival after the diagnosis of (A) bone, (B) brain, (C) liver, and (D) lung metastases by EGFR mutation status. CI = confidence interval; WT= wild-type.
DISCUSSION
To our knowledge, the present study is the largest to use a cohort of patients of mixed Asian and non-Asian ethnicity to examine the metastatic behaviour of nsclc and the influence of EGFR mutations. Our study also examined patient subgroups by metastasis site and treatment type to identify factors associated with survival. Based on clinical evaluation, the metastatic sites of highest incidence for both EGFR cohorts were lung, brain, and bone. Those sites are likely to have the greatest clinical impact in terms of management resources during the course of a patient’s advanced disease.
Differences in the pattern of metastatic spread are observed depending on EGFR mutation status. We observed a higher cumulative incidence of lung and brain metastasis in the EGFR+ cohort compared with the EGFR wt cohort. That observation supports emerging evidence suggesting characteristic differences in metastases from EGFR+ cancers compared with those from EGFR wt cancers. For instance, Laack et al.11 described a miliary pattern of pulmonary metastasis strongly associated with EGFR exon 19 deletion in 5 patients. Sekine et al.12 reported that patients with exon 19 deletion were more likely to have multiple and smaller brain metastases. Our study found a significant difference in the incidence of liver metastasis between the EGFR exon 19 deletion and exon 21 mutation subtypes. One explanation is a predisposition for liver spread in exon 21–mutant disease. An alternative explanation comes from case reports associating exon 19 deletion with a particular pattern of tiny and innumerable metastases in lung and brain resembling a miliary pattern11–13. A presentation of that kind in the liver would make the metastases more difficult to detect on computed tomography or magnetic resonance imaging14 and could explain our finding of fewer liver metastases in our exon 19 subgroup. Future studies using detailed and dedicated liver imaging will be needed to investigate that hypothesis. The results from our study could affect surveillance strategies for brain or liver depending on EGFR mutation status.
Compared with their EGFR wt counterparts, patients with EGFR+ nsclc experience longer survival and therefore a longer period at risk for metastatic spread. We used the competing-risks method to adjust for the difference in survival duration between the cohorts, with death as a competing-risk event. Compared with the Kaplan–Meier method, the competing-risks method provides a better estimation of incidence when death rates are high15,16. Our findings using that analysis methodology make it likely that the underlying biology of EGFR mutation is responsible for the differences in incidence. Further, the differences in incidence for lung and brain metastasis were seen at 6 months, indicating that the competing-risks curves separate early for the EGFR+ and EGFR wt patients during the course of their disease. Additionally, if the higher incidence for lung and brain metastasis came solely from longer survival, it would be expected that the incidences for most other metastatic sites would be higher as well, and heightened metastasis at those sites was not observed.
We also looked at the differential effect of metastasis on outcomes. Lung metastasis was most frequent in our population, but our analysis did not find that patients with lung metastasis did significantly worse than patients without metastasis at that site. That observation could be the result of a better systemic therapy response for malignant lung disease or available options for focused treatment with radiotherapy. In contrast, the incidence of liver metastasis was notably lower, and patients with liver involvement experienced significantly worse survival. In fact, patients with liver metastasis were seen to have the shortest survival duration after diagnosis. That finding is congruent with other nsclc series reporting that liver metastasis predicts for poor survival17,18.
Brain and bone metastasis were both common and independent negative prognostic factors. Other literature about brain and bone metastasis in nsclc support that finding19–21. As an adverse prognostic event, bone metastasis could relate to secondary complications such as pathologic fracture, spinal cord compression, and morbidity from other skeletal-related events. Another possibility is less sensitivity to cytotoxic therapy than is seen with visceral organ metastasis22.
We also investigated how survival relates to the number of metastatic sites. In our analysis, the specific organ of metastatic involvement and not the number of sites involved is significantly associated with survival.
Our findings complement results from prior autopsy series23,24. However, a clinically-based study such as ours has advantages over post-mortem data. Our analyses were able to examine metastasis at various sites as time-dependent variables, allowing for a better evaluation of metastasis as a prognostic factor. Furthermore, autopsy studies are more likely to capture micrometastatic disease that might not be clinically significant.
As in all retrospective analyses, interpretation of the results is limited by bias. Many of the patients in our population were excluded from the study because they had not undergone EGFR mutation testing. It is possible that exclusions could have affected the accounting of metastatic spread. However, the large size of the two EGFR cohorts makes it less likely that our findings are a result of chance. We examined only EGFR mutations because of the available data at our institution. It is possible that other driver mutations also affect the pattern of metastatic spread. However, the frequencies of other driver mutations are comparatively low, and multiple driver mutations are rarely found concurrently in the same tumour.
Given the retrospective nature of our study, diagnostic imaging was obtained at the discretion of the treating physician; no routine interval imaging was performed. It is possible that asymptomatic or small-volume metastasis during the patient’s disease trajectory was not detected. Nonetheless, it is unlikely that any future prospective study examining metastatic behaviour in a time-dependent model with routine imaging protocols would be undertaken in this palliative population. Treatment with chemotherapy or egfr tki could affect patterns of spread such that they become different from the natural history of the disease. With that caveat, our study reports real-world findings with contemporary chemotherapy regimens and molecularly-targeted agents.
Into the future, the information gained from our study could be used to help develop tools for estimating survival that consider EGFR mutation status and sites of metastatic spread. Current survival nomograms, such as the diagnosis-specific graded prognostic assessment for brain metastases25, will have to be updated to account for survival differences for patients with driver mutations. Differences in the characteristics of metastasis might provide clues to the presence of driver mutations or disease behaviour.
CONCLUSIONS
The most frequent sites of metastatic spread from nsclc are lung, brain, and bone. Mutations in EGFR affect the metastatic behaviour of disease, resulting in a higher incidence of lung and brain metastasis, even when adjusted for differences in survival. The incidence of detected liver metastasis is significantly different between the EGFR exon 19 deletion and exon 21 mutation subtypes. The effect on survival duration varies depending on the site of metastatic involvement, with the prominent negative prognostic factors being brain, liver, and bone metastasis.
ACKNOWLEDGMENTS
Financial support for this work came from the Abbotsford Centre Radiation Therapy Academic Fund and the Eleni Skalbania Endowment for Lung Cancer Research, BC Cancer Foundation.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: FH has received a research grant from Varian Medical Systems; AN has received a research grant and speaker honorarium from Varian Medical Systems; CH has received grants and honoraria from Boehringer Ingelheim, Eli Lilly, Roche, AstraZeneca, Bayer, and Pfizer outside the submitted work; and ADC, DA, and TT have no conflicts to disclose.
REFERENCES
- 1.Hess KR, Varadhachary GR, Taylor SH, et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106:1624–33. doi: 10.1002/cncr.21778. [DOI] [PubMed] [Google Scholar]
- 2.Viadana E, Au KL. Patterns of metastases in adenocarcinoma of man. J Med. 1975;6:1–14. [PubMed] [Google Scholar]
- 3.Paget S. The distribution of secondary growths in cancer of the breast. Cancer Metastasis Rev. 1989;8:98–101. [PubMed] [Google Scholar]
- 4.Ewing J. Metastasis. In: Ewing J, editor. Neoplastic Diseases: A Treatise on Tumours. 3rd ed. Philadelphia: W.B. Saunders; 1928. pp. 76–88. [Google Scholar]
- 5.Hellman S, Weichselbaum RR. Oligometastases. J Clin Oncol. 1995;13:8–10. doi: 10.1200/JCO.1995.13.1.8. [DOI] [PubMed] [Google Scholar]
- 6.Fujino S, Enokibori T, Tezuka N, et al. A comparison of epidermal growth factor receptor levels and other prognostic parameters in non–small cell lung cancer. Eur J Cancer. 1996;32A:2070–4. doi: 10.1016/S0959-8049(96)00243-2. [DOI] [PubMed] [Google Scholar]
- 7.Douillard JY, Shepherd FA, Hirsh V, et al. Molecular predictors of outcome with gefitinib and docetaxel in previously treated nonsmall-cell lung cancer: data from the randomized phase iii interest trial. J Clin Oncol. 2010;28:744–52. doi: 10.1200/JCO.2009.24.3030. [DOI] [PubMed] [Google Scholar]
- 8.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of nonsmall-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–39. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 9.Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase iii, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (ipass) J Clin Oncol. 2011;29:2866–74. doi: 10.1200/JCO.2010.33.4235. [DOI] [PubMed] [Google Scholar]
- 10.Pan Q, Pao W, Ladanyi M. Rapid polymerase chain reaction-based detection of epidermal growth factor receptor gene mutations in lung adenocarcinomas. J Mol Diagn. 2005;7:396–403. doi: 10.1016/S1525-1578(10)60569-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laack E, Simon R, Regier M, et al. Miliary never-smoking adenocarcinoma of the lung: strong association with epidermal growth factor receptor exon 19 deletion. J Thorac Oncol. 2011;6:199–202. doi: 10.1097/JTO.0b013e3181fb7cf1. [DOI] [PubMed] [Google Scholar]
- 12.Sekine A, Kato T, Hagiwara E, et al. Metastatic brain tumors from non–small cell lung cancer with EGFR mutations: distinguishing influence of exon 19 deletion on radiographic features. Lung Cancer. 2012;77:64–9. doi: 10.1016/j.lungcan.2011.12.017. [DOI] [PubMed] [Google Scholar]
- 13.Poonia S, Berge EM, Aisner DL, Damek D, Doebele RC. EGFR exon 19 deletion mutations and systemic/central nervous system miliary metastasis: clinical correlations and response to therapy. Clin Lung Cancer. 2014;15:387–9. doi: 10.1016/j.cllc.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fazio N, Di Meglio G, Lorizzo K, de Brand F. Miliary hepatic metastases from neuroendocrine carcinoma. Dig Surg. 2008;25:330. doi: 10.1159/000158907. [DOI] [PubMed] [Google Scholar]
- 15.van Walraven C, McAlister FA. Competing risk bias was common in Kaplan–Meier risk estimates published in prominent medical journals. J Clin Epidemiol. 2016;69:170–3. doi: 10.1016/j.jclinepi.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 16.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007;26:2389–430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 17.Mordant P, Arame A, De Dominicis F, et al. Which metastasis management allows long-term survival of synchronous solitary M1b non–small cell lung cancer? Eur J Cardiothorac Surg. 2012;41:617–22. doi: 10.1093/ejcts/ezr042. [DOI] [PubMed] [Google Scholar]
- 18.Wu KL, Tsai MJ, Yang CJ, et al. Liver metastasis predicts poorer prognosis in stage iv lung adenocarcinoma patients receiving first-line gefitinib. Lung Cancer. 2015;88:187–94. doi: 10.1016/j.lungcan.2015.02.012. [DOI] [PubMed] [Google Scholar]
- 19.Kuchuk M, Kuchuk I, Sabri E, et al. The incidence and clinical impact of bone metastases in non–small cell lung cancer. Lung Cancer. 2015;89:197–202. doi: 10.1016/j.lungcan.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 20.Tsuya A, Kurata T, Tamura K, Fukuoka M. Skeletal metastases in non–small cell lung cancer: a retrospective study. Lung Cancer. 2007;57:229–32. doi: 10.1016/j.lungcan.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 21.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165–76. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 22.Langer C, Hirsh V. Skeletal morbidity in lung cancer patients with bone metastases: demonstrating the need for early diagnosis and treatment with bisphosphonates. Lung Cancer. 2010;67:4–11. doi: 10.1016/j.lungcan.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Disibio G, French SW. Metastatic patterns of cancers: results from a large autopsy study. Arch Pathol Lab Med. 2008;132:931–9. doi: 10.5858/2008-132-931-MPOCRF. [DOI] [PubMed] [Google Scholar]
- 24.Line DH, Deeley TJ. The necropsy findings in carcinoma of the bronchus. Br J Dis Chest. 1971;65:238–42. doi: 10.1016/0007-0971(71)90032-5. [DOI] [PubMed] [Google Scholar]
- 25.Sperduto PW, Kased N, Roberge D, et al. Summary report on the graded prognostic assessment: an accurate and facile diagnosis-specific tool to estimate survival for patients with brain metastases. J Clin Oncol. 2012;30:419–25. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]