Abstract
Background
Various tyrosine kinase signalling pathways affect the development and progression of colorectal cancer (crc). In clinical trials, regorafenib has been associated with a survival benefit in metastatic crc (mcrc). We assessed the safety and efficacy of regorafenib in real-world patients.
Methods
In a retrospective review of patients with mcrc treated with regorafenib at our institution from 2013 to 2015, patient demographics, treatment, and survival data were collected. Progression-free survival (pfs) and overall survival (os) were estimated using the Kaplan–Meier method.
Results
In total, 48 patients were offered regorafenib, and 35 (73%) started treatment. Of the patients who started regorafenib, 57% were men. Median age in the cohort was 61 years, and all patients had a performance status in the range 0–2. Time from diagnosis of mcrc to regorafenib treatment was more than 18 months in 71% of patients. Starting dose was 160 mg in 54% of the patients, 120 mg in 40%, and 80 mg in 6%. Dose reductions occurred in 34% of the patients, and interruptions, in 29%. Best response was progressive disease (60%) and stable disease (17%); response in the rest of the patients was unknown. The most common adverse events on regorafenib (any grade) were fatigue (57%), hyperbilirubinemia (43%), thrombocytopenia (37%), anorexia (31%), and hypertension (31%). The most common grade 3 or 4 adverse events were fatigue (29%), hypophosphatemia (17%), weight loss (11%), and hyperbilirubinemia (9%). Common reasons for discontinuing regorafenib included progressive disease (51%) and toxicity (26%). In patients treated with regorafenib, pfs was 2.4 months (95% confidence interval: 1.8 to 3.3 months) and os was 5.6 months (95% confidence interval: 3.7 to 8.9 months). No factors were associated with survival in univariate or multivariate analysis.
Conclusions
In a real-world setting, regorafenib is associated with survival similar to that reported in the randomized controlled trials, but at the expense of toxicity leading to discontinuation in many patients. Future studies of regorafenib should focus on identifying the patients most likely to benefit and on minimizing toxicity.
Keywords: Metastatic colorectal cancer, regorafenib, systemic therapy, real-world settings
INTRODUCTION
In Canada, colorectal cancer (crc) represents the 2nd most common cancer in men and the 3rd most common cancer in women. In their lifetime, 1 in 14 men and 1 in 16 women will develop crc1. Many patients present with or develop metastatic disease.
Various tyrosine kinase signalling pathways affect the development and progression of crc. Regorafenib is a small-molecule multikinase inhibitor that blocks the activity of several protein kinases, including those involved in tumour pathogenesis (such as vascular endothelial growth factor receptors 1, 2, and 3; angiopoietin 1 receptor; stem-cell growth factor receptor; ret and braf; beta-type platelet-derived growth factor receptor; and fibroblast growth factor receptor)2.
In two large randomized placebo-controlled trials, correct3 and concur4, regorafenib was shown to be associated with a survival benefit in patients with metastatic crc (mcrc) who progressed on standard therapies. In that regard, regorafenib represents a potential further line of therapy in this otherwise treatment-refractory population.
Because the correct and concur studies were randomized trials, the criteria for enrolment were, by definition, stringent and might not have represented real-world patients and outcomes. To address that situation, the large European rebacca5 cohort study evaluated the efficacy and toxicity profile of regorafenib in a real-world setting, showing an overall survival (os) and progression-free survival (pfs) similar to those reported in the randomized studies. The toxicity profile was also similar.
The real-world analysis accomplished by rebacca was based in Europe, and therefore outcomes for patients in North America, and specifically in Canada, are unknown. We conducted a single-centre retrospective analysis to assess the real-world efficacy and toxicity of regorafenib in mcrc patients at our institution in Canada. Our institution, The Ottawa Hospital Cancer Centre, is a centralized tertiary care cancer centre serving a wide geographic region and offering comprehensive medical, radiation, and surgical oncology services.
METHODS
With local research ethics board approval, we performed a retrospective single-centre chart review of all patients with mcrc at our institution who started regorafenib from May 2013 to October 2015. Only patients with histologically-confirmed mcrc were included in the analysis.
Baseline data on patient demographics, laboratory values, performance status (ps), disease characteristics, treatment with systemic therapy, and toxicities were recorded. Data points were selected based on those included in the rebacca5 cohort. The Eastern Cooperative Oncology Group (ecog) ps6, when not recorded directly in the clinical notes, was estimated from the clinical assessment at the time of initiation of regorafenib. When the ecog ps was inferred from the clinical assessment, it was based on the physician’s documented description of patient symptomatology and functional status, using standard definitions.
Our objectives were to describe the clinical characteristics of the mcrc population taking regorafenib and to determine the efficacy and toxicity of regorafenib in the real-world setting. Patients were offered regorafenib at the discretion of their treating physician. Patients treated with regorafenib were identified through the special access funding program. All patients treated with regorafenib applied through that program regardless of the funding method ultimately selected.
The primary outcomes of interest were os and pfs. Overall survival was defined as time from the mcrc diagnosis to death or last known follow-up; pfs was defined as time from the mcrc diagnosis to progression of disease or last known follow-up. Progressive disease was defined radiographically, based on the radiologist’s interpretation. Disease control was defined radiographically as stable disease or partial response, based on the radiologist’s interpretation. The Kaplan–Meier method was used to estimate os and pfs, and Cox proportional hazards modelling was used to evaluate predictors of those outcomes. The covariates included in the multivariate analysis were chosen based on significant factors identified in the prior literature5. Covariates included in the pfs multivariate analysis were a ps greater than 0, liver metastasis, and time from the mcrc diagnosis of 18 months or less. Covariates included in the os multivariate analysis were a ps greater than 0, liver metastasis, and a starting dose of regorafenib of 120 mg or less. The number of covariates analyzed was selected based on the sample size. Toxicities were recorded as explicitly stated in the documentation by the treating physicians or in the laboratory values obtained while on treatment with regorafenib. All statistical analyses were performed using the SAS software application (version 9.4: SAS Institute, Cary, NC, U.S.A.).
RESULTS
Of 48 patients who were offered regorafenib, 35 (73%) started treatment. Reasons that patients did not start regorafenib included patient choice (n = 5, 38%), deterioration (n = 2, 15%), no funding (n = 1, 8%), and unknown (n = 5, 38%). Patients who did not start regorafenib are not further reported.
Table i presents baseline demographics for the 35 patients who started regorafenib. Of those patients, 57% were men. Median age in the cohort was 61 years, and all patients had a ps of 0–2. With respect to metastasis, 78% had more than 1 metastatic site, and the most common sites of metastasis were liver (80%), lung (71%), and lymph nodes (35%).
TABLE I.
Baseline characteristics of patients treated with regorafenib
| Characteristic | Value |
|---|---|
| Patients (n) | 35 |
| Sex [n (%) men] | 20 (57) |
| Age (years) | |
| Median | 61 |
| Range | 37–84 |
| Performance status [n (%)] | |
| 0 | 14 (40) |
| 1 | 20 (57) |
| 2 | 1 (3) |
| 3–4 | 0 |
| Mutation status [n (%)] | |
| KRAS | |
| Positive | 16 (46) |
| Negative | 18 (51) |
| Unknown | 1 (3) |
| NRAS | |
| Positive | 1 (3) |
| Negative | 5 (14) |
| Unknown | 29 (83) |
| BRAF | |
| Positive | 0 |
| Negative | 2 (6) |
| Unknown | 33 (94) |
| Metastasis [n (%)] | |
| Number of sites | |
| 1 | 8 (23) |
| 2 | 12 (34) |
| 3 | 11 (31) |
| ≥4 | 4 (11) |
| Location | |
| Liver | 28 (80) |
| Lung | 24 (69) |
| Lymph nodes | 12 (34) |
| Bone | 8 (23) |
| Peritoneum | 6 (17) |
| Other | 4 (11) |
Patients had received a median of 3 lines of systemic therapy before regorafenib initiation. All patients had previously been treated with a thymidylate synthase inhibitor and irinotecan. Most patients had previously been treated with bevacizumab (89%), oxaliplatin (80%), and an epidermal growth factor inhibitor (51%). Most patients (71%) had been diagnosed with metastatic disease more than 18 months before the initiation of regorafenib.
A dose reduction was needed in 34% of the patients, and treatment interruption was needed in 29%. The best response to therapy was progressive disease in 60% of patients; 17% experienced stable disease. The best response to therapy in the rest of the patients was unknown. Common reasons for discontinuing regorafenib included progressive disease (51%) and toxicity (26%). Table ii sets out the treatment characteristics for patients receiving regorafenib.
TABLE II.
Characteristics of regorafenib treatment in the study population
| Characteristic | Value |
|---|---|
| Therapy before regorafenib [n (%)] | |
| Agent | |
| Thymidylate synthase inhibitor | 35 (100) |
| Irinotecan | 35 (100) |
| Bevacizumab | 31 (89) |
| Oxaliplatin | 28 (80) |
| EGFR inhibitor | 18 (51) |
| Othera | 5 (14) |
| Lines received [n (%)] | |
| 1 | 4 (11) |
| 2 | 13 (37) |
| 3 | 18 (51) |
| Median lines received (n) | 3 |
| Time from diagnosis of metastatic disease | |
| ≤18 Months | 9 (26) |
| >18 Months | 25 (71) |
| Unknown | 1 (3) |
| Regorafenib therapy | |
| Starting dose [n (%)] | |
| 160 mg | 19 (54) |
| 120 mg | 14 (40) |
| 80 mg | 2 (6) |
| Dose modification [n (%)] | |
| Interruption | 10 (29) |
| Reduction | 12 (34) |
| Increase | 5 (14) |
| Interruption and reduction | 10 (29) |
| No modificationb | 18 (51) |
| Best response [n (%)] | |
| Partial response | 0 |
| Stable disease | 6 (17) |
| Progressive disease | 21 (60) |
| Unknown | 8 (23)) |
| Cystic changes on imaging [n (%)] | 5 (14) |
| Worst biochemical level recorded [median (IQR)] | |
| AST | 62 (66) |
| ALT | 33 (33) |
| Bilirubin | 21 (16) |
| Phosphate | 0.77 (0.33) |
| Reason for regorafenib discontinuation [n (%)] | |
| Progressive disease | 18 (51) |
| Toxicity | 9 (26) |
| Still on therapy at study close | 6 (17) |
| Otherc | 1 (3) |
| Unknown | 1 (3) |
Other systemic therapies received (alone or in combinations) included aflibercept, BBI503, buparlisib, Reolysin (Oncolytics Biotech Inc., Calgary, AB), and MG1MA3.
No modification indicates no interruption, reduction, or increase.
Died while on regorafenib without known progressive disease or regorafenib toxicity.
IQR = interquartile range; AST = aspartate aminotransferase; ALT = alanine aminotransferase.
The most common toxicities of any grade (Table iii) were fatigue (57%), hyperbilirubinemia (43%), thrombocytopenia (37%), anorexia (31%), and hypertension (31%). The most common grade 3 or 4 toxicities were fatigue (29%), hypophosphatemia (17%), hand–foot syndrome (14%), and weight loss (11%).
TABLE III.
Toxicities while on regorafenib
| Adverse event | Grade [n (%)] | |
|---|---|---|
|
| ||
| Any | 3 or 4 | |
| Fatigue | 20 (57) | 10 (29) |
| Hand–foot syndrome | 8 (23) | 5 (14) |
| Diarrhea | 5 (14) | 2 (6) |
| Anorexia | 11 (31) | 2 (6) |
| Hypertension | 11 (31) | 2 (6) |
| Mucositis | 1 (3) | 0 |
| Weight loss | 4 (11) | 4 (11) |
| Rash | 2 (6) | 0 |
| Muscle pain | 5 (14) | 0 |
| Thrombocytopenia | 13 (37) | 1 (3) |
| Hyperbilirubinemia | 15 (43) | 3 (9) |
| Proteinuria | 2 (6) | 1 (3) |
| Hypophosphatemia | 8 (23) | 6 (17) |
| Hyponatremia | 7 (20) | 2 (6) |
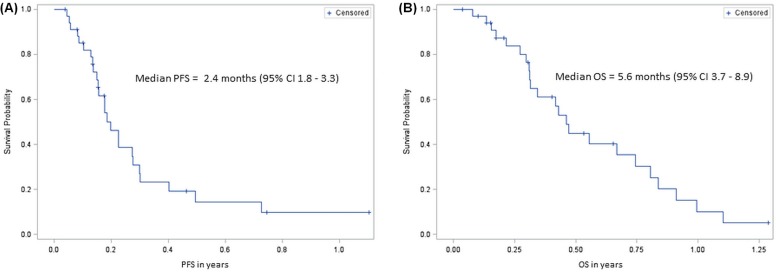
Median pfs in this cohort was 2.4 months (95% ci: 1.8 to 3.3 months). Median os was 5.6 months (95% ci: 3.7 to 8.9 months). Figure 1 shows the Kaplan–Meier survival curves for pfs and os in patients taking regorafenib.
FIGURE 1.
Kaplan–Meier survival curves for the study population. PFS = progression-free survival; CI = confidence interval; OS = overall survival.
No factors were significantly associated with pfs in the univariate analysis: ps greater 0 [hazard ratio (hr): 0.98; 95% ci: 0.43 to 2.2; p = 0.97], liver metastasis (hr: 0.62; 95% ci: 0.24 to 1.6; p = 0.31), starting dose of regorafenib of 120 mg or less (hr: 0.85; 95% ci: 0.38 to 1.90; p = 0.68), and time from the initial mcrc diagnosis of 18 months or less (hr: 1.04; 95% ci: 0.45 to 2.4; p = 0.91).
No factors were significantly associated with os in the univariate analysis: ps greater 0 (hr: 1.6; 95% ci: 0.66 to 3.8; p = 0.30), liver metastasis (hr: 1.8; 95% ci: 0.59 to 5.6; p = 0.29), starting dose of regorafenib of 120 mg or less (hr: 0.45; 95% ci: 0.18 to 1.15; p = 0.09), and time from the initial mcrc diagnosis of 18 months or less (hr: 0.59; 95% ci: 0.21 to 1.7; p = 0.31).
No factors were associated with pfs or os in the multivariate analysis (Table iv).
TABLE IV.
Factors associated with survival in multivariate analysis
| Factor | Survival type | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Progression-free | Overall | |||||
|
|
|
|||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Performance status > 0 | 1.25 | 0.47 to 3.3 | 0.66 | 1.5 | 0.5 to 4.5 | 0.46 |
| Liver metastases | 0.52 | 0.16 to 1.67 | 0.27 | 1.12 | 0.27 to 4.55 | 0.88 |
| Time from diagnosis of mCRC ≤ 18 months | 0.90 | 0.36 to 2.2 | 0.81 | — | — | — |
| Initial dose ≤120 mg | — | — | — | 0.46 | 0.17 to 1.2 | 0.11 |
HR = hazard ratio; CI = confidence interval; mCRC = metastatic colorectal cancer.
DISCUSSION
We conducted a retrospective real-world analysis of patients with mcrc taking regorafenib who had progressed on prior lines of therapy at our institution. We found that median pfs was 2.4 months and median os was 5.6 months in our cohort. The median pfs and os in our cohort were similar to those for patients in the European real-world rebacca5 cohort (pfs: 2.9 months; os: 5.6 months), even though more patients in our cohort than in the rebacca cohort started at the lower dose of regorafenib (46% for our cohort vs. 18% for the rebecca cohort). Our cohort might have been more robust at baseline: only 3% of our patients, compared with 11% in the rebacca cohort, had an ecog ps greater than 1.
Our cohort experienced a shorter os than was reported in the correct3 (6.4 months) and concur4 randomized trials (8.8 months). That difference might be attributable to the more stringent enrolment criteria in the trial populations and to baseline demographics different from those in our cohort. For example, median age in our cohort was older than the median age in the concur trial by 4 years. Furthermore, a substantial proportion of patients in our cohort started at a reduced dose of regorafenib; in contrast, patients in both trials started at full dose. Interestingly, our cohort experienced a longer pfs than was reported in the correct trial (1.9 months), which might be a result of a longer restaging interval in our cohort, given that restaging was performed at the discretion of the clinician.
We found no factors predictive of pfs or os in our cohort of patients. That observation should be interpreted with caution because of our small sample size. The rebacca5 real-world analysis found several factors associated with shorter os: high ecog ps, a shorter time from the initial diagnosis of metastasis, an initial regorafenib dose of less than 160 mg, more than 3 metastatic sites, liver metastasis, and KRAS mutation. We were not able to confirm those results, likely because of our small sample size.
Most patients in our cohort required a regorafenib interruption or dose reduction. Compared with the rebacca5 cohort, our cohort required a dose interruption in similar proportion, but fewer of our patients required a regorafenib dose reduction. It appears that, compared with the rebacca cohort, our cohort better tolerated regorafenib at the starting dose. That observation might be related to the fact that a greater proportion of our cohort started at a reduced dose of regorafenib. Compared with the correct3 trial (in which 70% of patients taking regorafenib required a dose interruption, and 20% required a dose reduction) and the concur4 trial (in which 63% of patients required a dose interruption, and 40% required a dose reduction), our trial had a smaller proportion of patients who required a dose interruption and a similar proportion who required a dose reduction.
Disease control (stable disease or partial response) was seen in 17% of our population, which is less than the 40% seen in the correct3 trial and the 51% seen in the concur4 trial. Reasons for that discrepancy are unclear, but might again be a result of different baseline factors in our cohort. For example, compared with the correct population, our patients had a lower proportion of KRAS mutation–positive tumours, and at the time of regorafenib initiation, fewer patients had had metastatic disease for more than 18 months. Another explanation is that a substantial proportion of the patients in our cohort started at a reduced regorafenib dose. Furthermore, the time interval to the first restaging imaging could have been different between the cohorts. In our cohort, restaging was performed at the discretion of the treating physician. Per our institution’s standard of care, restaging scans were likely obtained every 2–3 months in general; however, we were unable to capture the interval to restaging in our analysis. An inappropriate restaging interval would introduce potential bias. Lastly, a substantial proportion of patients (24%) were not response-evaluable, which could certainly affect the disease control rate. Determining factors associated with disease control was beyond the scope of our analysis.
The most common toxicities of any grade in our cohort were fatigue, hyperbilirubinemia, thrombocytopenia, anorexia, and hypertension. The most common grade 3 or 4 toxicities were fatigue, hypophosphatemia, hand–foot syndrome, and weight loss. That profile is generally similar to the toxicity profile reported in the rebacca5 real-world cohort and in the correct3 and concur4 randomized trials. A similar toxicity profile was reported for the prospective single-arm consign7 study, which assessed regorafenib in previously-treated mcrc patients. The most common reasons for discontinuing regorafenib in our cohort were progressive disease and toxicity, reasons that were similar to those reported in the rebacca5 cohort.
Prior oxaliplatin use was lower than expected in our cohort (80%) compared with the rebacca5 cohort (98%). Determining the reasons for the use or lack thereof of prior systemic therapy regimens was beyond the scope of our analysis; however, we could hypothesize that oxaliplatin was omitted in some patients because of comorbidities (such as underlying neuropathy) and possibly patient preference. Oxaliplatin might also have been omitted in patients who recurred within a short interval after adjuvant treatment and who thus would have been considered refractory to oxaliplatin.
Limitations of our study include its single-centre retrospective design, which might not be generalizable to other populations. The included patients were those deemed fit enough for treatment with regorafenib, which might not necessarily represent average patients with mcrc. Furthermore, our small sample size limits the evaluation of predictors of outcome and therefore should be interpreted with caution.
Future directions include expanding the cohort to encompass multiple Canadian centres to determine factors associated with improved survival during regorafenib treatment while toxicity is minimized. Identifying patients who will tolerate full-dose regorafenib and those who should start at a reduced dose is of importance.
CONCLUSIONS
In the real-world setting, regorafenib is associated with survival durations similar to those reported in randomized controlled trials in highly selected patients. Many patients experience toxicity leading to dose modifications and discontinuation. Future studies of regorafenib should focus on the identification of patients most likely to benefit and on minimization of toxicity.
ACKNOWLEDGMENTS
This work was supported by an unrestricted educational grant received from Bayer.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare the following interests: RG has served on an advisory board for Bayer, and TH has served on advisory boards for Pfizer and Celgene. The remaining authors have no disclosures to make.
REFERENCES
- 1.Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian Cancer Statistics 2015. Toronto, ON: Canadian Cancer Society; 2015. [Google Scholar]
- 2.Sartore-Bianchi A, Zeppellini A, Amatu A, Ricotta R, Bencardino K, Siena S. Regorafenib in metastatic colorectal cancer. Expert Rev Anticancer Ther. 2014;14:255–65. doi: 10.1586/14737140.2014.894887. [DOI] [PubMed] [Google Scholar]
- 3.Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (correct): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381:303–12. doi: 10.1016/S0140-6736(12)61900-X. [DOI] [PubMed] [Google Scholar]
- 4.Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (concur): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015;16:619–29. doi: 10.1016/S1470-2045(15)70156-7. [DOI] [PubMed] [Google Scholar]
- 5.Adenis A, de la Fouchardiere C, Paule B, et al. Survival, safety, and prognostic factors for outcome with regorafenib in patients with metastatic colorectal cancer refractory to standard therapies: results from a multicenter study (rebacca) nested within a compassionate use program. BMC Cancer. 2016;16:412. doi: 10.1186/s12885-016-2440-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. doi: 10.1097/00000421-198212000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Van Cutsem E, Ciardiello F, Seitz JF, et al. Results from the large, open-label phase 3b consign study of regorafenib in patients with previously treated metastatic colorectal cancer (mcrc) [abstract] Ann Oncol. 2015;26(suppl 4):iv42. [Google Scholar]