Abstract
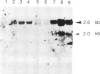
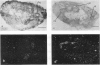
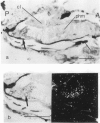
We have isolated several cDNA clones of the white locus which are derived from embryonic and pupal transcripts of Drosophila melanogaster. The cDNA sequences map within ˜7.5 kb (coordinates −3.0 to +4.6) of the genomic DNA and correspond mainly to sequences within the distal region of the gene (coordinates −0.2 to −3.0). A major RNA species of 2.6 kb was detected on Northerns of poly(A)+ RNA isolated from all developmental stages. The total accumulation of this transcript peaks in the mature third instar larva to a level of 0.003% which is about ten times higher than that observed in embryos. The spatial distribution of white locus transcripts was determined by in situ hybridization to tissue sections. In embryos, hybridization signals are restricted to the cells of the developing Malpighian tubules and the signal strength corresponds with ˜50 transcripts per cell. Before the termination of the third instar stage, hybridization signals are also detected at a comparable level in the eye antennal disks. At the same stage, a third site of labeling is observed over a small cluster of cells which seems to be associated with the larval photoreceptor organs. Thus, white locus expression is largely restricted to tissues which are known to be involved in the biosynthesis of eye pigments and these different cell types act in a temporally autonomous manner with respect to the induction of the white gene during development.
Keywords: Drosophila melanogaster, white locus, cDNA clones, developmental expression
Full text
PDF
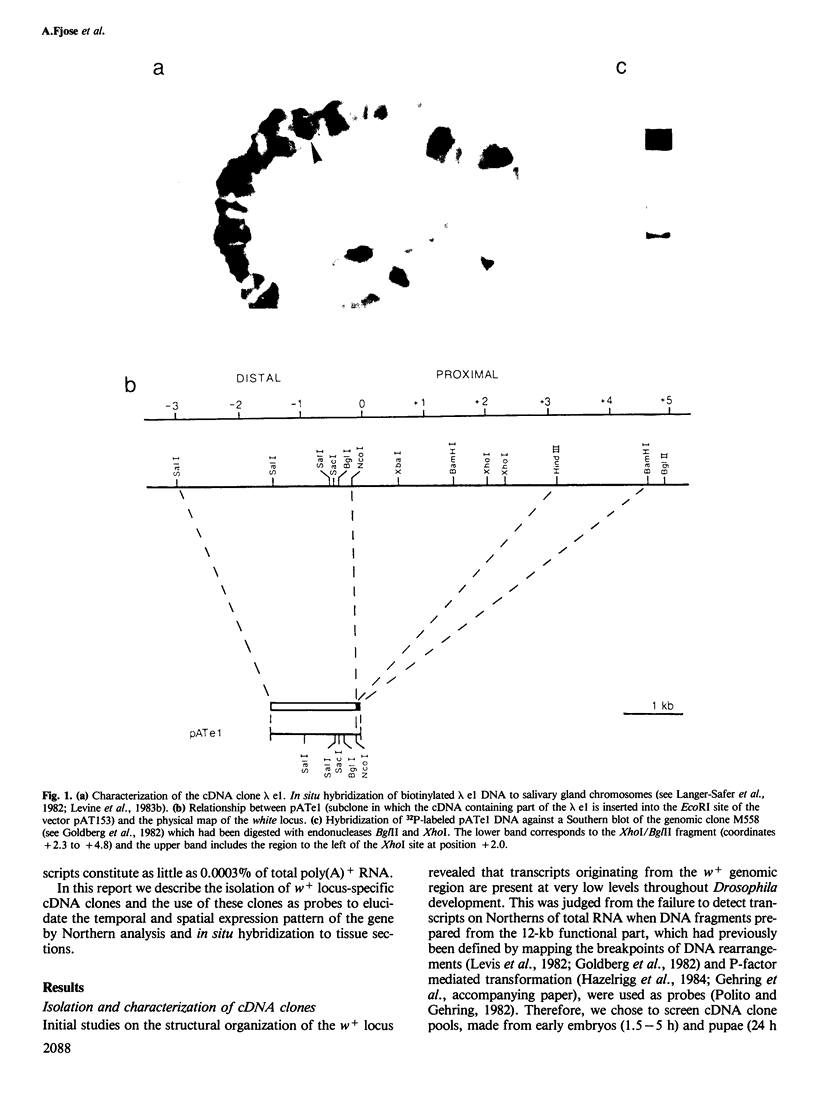

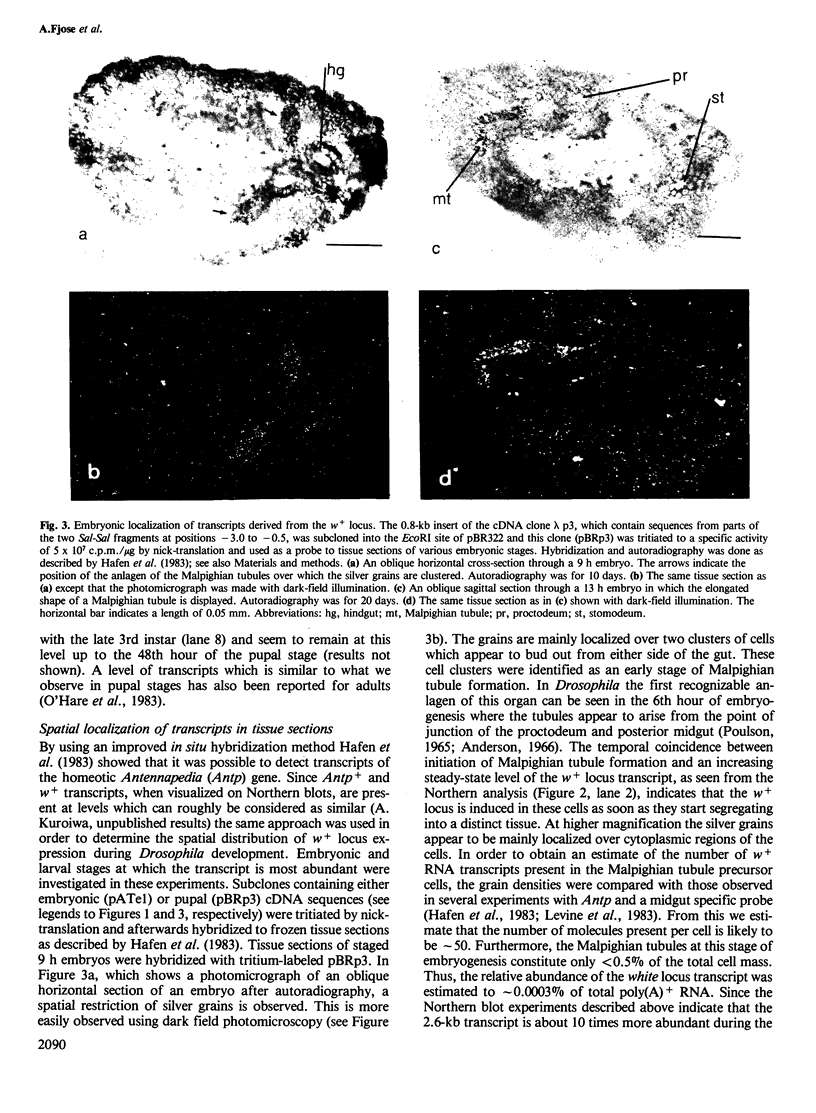




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benyajati C., Spoerel N., Haymerle H., Ashburner M. The messenger RNA for alcohol dehydrogenase in Drosophila melanogaster differs in its 5' end in different developmental stages. Cell. 1983 May;33(1):125–133. doi: 10.1016/0092-8674(83)90341-0. [DOI] [PubMed] [Google Scholar]
- Bonse A. Uber das Auftreten von Pterinen, Tryptophan and dessen Derivate in verschiedenen Organen der Mutante white von Drosophila melanogaster. Z Naturforsch B. 1969 Jan;24(1):128–131. [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Mahaffey J. W., Bond B. J., Davidson N. Transcripts of the six Drosophila actin genes accumulate in a stage- and tissue-specific manner. Cell. 1983 May;33(1):115–123. doi: 10.1016/0092-8674(83)90340-9. [DOI] [PubMed] [Google Scholar]
- Garber R. L., Kuroiwa A., Gehring W. J. Genomic and cDNA clones of the homeotic locus Antennapedia in Drosophila. EMBO J. 1983;2(11):2027–2036. doi: 10.1002/j.1460-2075.1983.tb01696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg M. L., Paro R., Gehring W. J. Molecular cloning of the white locus region of Drosophila melanogaster using a large transposable element. EMBO J. 1982;1(1):93–98. doi: 10.1002/j.1460-2075.1982.tb01130.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafen E., Levine M., Garber R. L., Gehring W. J. An improved in situ hybridization method for the detection of cellular RNAs in Drosophila tissue sections and its application for localizing transcripts of the homeotic Antennapedia gene complex. EMBO J. 1983;2(4):617–623. doi: 10.1002/j.1460-2075.1983.tb01472.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelrigg T., Levis R., Rubin G. M. Transformation of white locus DNA in drosophila: dosage compensation, zeste interaction, and position effects. Cell. 1984 Feb;36(2):469–481. doi: 10.1016/0092-8674(84)90240-x. [DOI] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judd B H. Direct Proof of a Variegated-Type Position Effect at the White Locus in Drosophila Melanogaster. Genetics. 1955 Sep;40(5):739–744. doi: 10.1093/genetics/40.5.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer-Safer P. R., Levine M., Ward D. C. Immunological method for mapping genes on Drosophila polytene chromosomes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4381–4385. doi: 10.1073/pnas.79.14.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepesant J. A., Kejzlarova-Lepesant J., Garen A. Ecdysone-inducible functions of larval fat bodies in Drosophila. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5570–5574. doi: 10.1073/pnas.75.11.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M., Hafen E., Garber R. L., Gehring W. J. Spatial distribution of Antennapedia transcripts during Drosophila development. EMBO J. 1983;2(11):2037–2046. doi: 10.1002/j.1460-2075.1983.tb01697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis R., Bingham P. M., Rubin G. M. Physical map of the white locus of Drosophila melanogaster. Proc Natl Acad Sci U S A. 1982 Jan;79(2):564–568. doi: 10.1073/pnas.79.2.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechler B., Rabbitts T. H. Membrane-bound ribosomes of myeloma cells. IV. mRNA complexity of free and membrane-bound polysomes. J Cell Biol. 1981 Jan;88(1):29–36. doi: 10.1083/jcb.88.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan T. H. SEX LIMITED INHERITANCE IN DROSOPHILA. Science. 1910 Jul 22;32(812):120–122. doi: 10.1126/science.32.812.120. [DOI] [PubMed] [Google Scholar]
- O'hare K., Levis R., Rubin G. M. Transcription of the white locus in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6917–6921. doi: 10.1073/pnas.80.22.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrotta V., Bröckl C. Transcription of the Drosophila white locus and some of its mutants. EMBO J. 1984 Mar;3(3):563–568. doi: 10.1002/j.1460-2075.1984.tb01847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirrotta V., Hadfield C., Pretorius G. H. Microdissection and cloning of the white locus and the 3B1-3C2 region of the Drosophila X chromosome. EMBO J. 1983;2(6):927–934. doi: 10.1002/j.1460-2075.1983.tb01523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RIZKI M. T. Intracellular localization of kynurenine in the fatbody of Drosophila. J Biophys Biochem Cytol. 1961 Mar;9:567–572. doi: 10.1083/jcb.9.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ready D. F., Hanson T. E., Benzer S. Development of the Drosophila retina, a neurocrystalline lattice. Dev Biol. 1976 Oct 15;53(2):217–240. doi: 10.1016/0012-1606(76)90225-6. [DOI] [PubMed] [Google Scholar]
- Rozek C. E., Davidson N. Drosophila has one myosin heavy-chain gene with three developmentally regulated transcripts. Cell. 1983 Jan;32(1):23–34. doi: 10.1016/0092-8674(83)90493-2. [DOI] [PubMed] [Google Scholar]
- Smith G. E., Summers M. D. The bidirectional transfer of DNA and RNA to nitrocellulose or diazobenzyloxymethyl-paper. Anal Biochem. 1980 Nov 15;109(1):123–129. doi: 10.1016/0003-2697(80)90019-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sullivan D. T., Bell L. A., Paton D. R., Sullivan M. C. Purine transport by malpighian tubules of pteridine-deficient eye color mutants of Drosophila melanogaster. Biochem Genet. 1979 Jun;17(5-6):565–573. doi: 10.1007/BF00498891. [DOI] [PubMed] [Google Scholar]
- Sullivan D. T., Kitos R. J., Sullivan M. C. Developmental and genetic studies on kynurenine hydroxylase from Drosophila melanogaster. Genetics. 1973 Dec;75(4):651–661. doi: 10.1093/genetics/75.4.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan D. T., Sullivan M. C. Transport defects as the physiological basis for eye color mutants of Drosophila melanogaster. Biochem Genet. 1975 Oct;13(9-10):603–613. doi: 10.1007/BF00484918. [DOI] [PubMed] [Google Scholar]
- Zachar Z., Bingham P. M. Regulation of white locus expression: the structure of mutant alleles at the white locus of Drosophila melanogaster. Cell. 1982 Sep;30(2):529–541. doi: 10.1016/0092-8674(82)90250-1. [DOI] [PubMed] [Google Scholar]
- Zipursky S. L., Venkatesh T. R., Teplow D. B., Benzer S. Neuronal development in the Drosophila retina: monoclonal antibodies as molecular probes. Cell. 1984 Jan;36(1):15–26. doi: 10.1016/0092-8674(84)90069-2. [DOI] [PubMed] [Google Scholar]