Abstract
The epidemic emergence of Zika virus (ZIKV) in 2015-2016 has been associated with congenital malformations and neurological sequela. Current efforts to develop a ZIKV vaccine build on technologies that successfully reduced infection or disease burden against closely related flaviviruses or other RNA viruses. Subunit-based (DNA plasmid and modified mRNA), viral vectored (adeno- and measles viruses) and inactivated viral vaccines are already advancing to clinical trials in humans after successful mouse and non-human primate studies. Among the greatest challenges for the rapid implementation of immunogenic and protective ZIKV vaccines will be addressing the potential for exacerbating Dengue virus infection or causing Guillain-Barré syndrome through production of cross-reactive immunity targeting related viral or host proteins. Here, we review vaccine strategies under development for ZIKV and the issues surrounding their usage.
Introduction
Historically, Zika virus (ZIKV) infection caused a mild, self-limiting febrile illness that was associated with conjunctivitis, rash, headache, myalgia, and arthralgia [1]. However, during the recent epidemics in Asia and the Americas, more severe and unusual clinical consequences have been observed. Infection of fetuses during pregnancy, particularly during the first trimester, has been associated with placental insufficiency and congenital malformations including cerebral calcifications, microcephaly, and miscarriage [2–6]. In adults, ZIKV infection is linked to an increased incidence of Guillain-Barré syndrome (GBS), an autoimmune disease characterized by ascending paralysis and polyneuropathy [7] that occurs during the acute phase of ZIKV infection or shortly afterward [8–10].
ZIKV was identified in 1947 from a sentinel Rhesus monkey in the Zika Forest of Uganda [11,12]. Prior to 2007, seroprevalence studies in Asia and Africa suggested ZIKV infections occurred periodically without evidence of severe disease [1,13]. Contemporary outbreaks of ZIKV arose in 2007 on Yap Island in the Federated States of Micronesia followed by an epidemic in French Polynesia in 2013 [14]; these events were associated with a high prevalence of infection, with greater than 11% of people on the islands presenting with ZIKV-associated symptoms [7,14]. A study in French Polynesia of patients diagnosed with GBS during the outbreak found that all had neutralizing antibodies against ZIKV compared to 56% of patients presenting to hospitals with non-febrile illnesses [7]. The next ZIKV outbreak began in late 2014 in northeastern Brazil, which was followed by a rapid spread to many other countries in the Americas in 2015 and 2016, including locally-transmitted infections in Florida and Texas in the United States [15–17]. Associated with this ZIKV epidemic were cases of GBS and congenital defects that correlated temporally with the growing number of infections [9]. Aedes aegypti and Aedes albopictus mosquitoes have tested positive for ZIKV and are believed to be primary agents of transmission [18,19]. In addition to mosquito vectors, sexual transmission of ZIKV was established from male-to-female [20,21] and subsequently from male-to-male and female-to-male [22,23]. Diagnostic studies have confirmed viral RNA in semen, sperm, and vaginal secretions of symptomatic patients up to 6 months following the onset of symptoms [24–26].
ZIKV belongs to the Flavivirus genus of the Flaviviridae family of positive-stranded, enveloped RNA viruses. ZIKV has an ∼11 kb RNA genome and one open reading frame. Translation of infectious viral RNA in the cytoplasm generates a polyprotein that is cleaved into three structural proteins (capsid (C), pre-membrane/membrane (prM/M), and envelope (E)) and seven non-structural proteins (NS1, NS2A, NS2B, NS3, NS4A, NS4B, and NS5). ZIKV strains are classified into two lineages, African and Asian/American. As the African lineage shows greater divergence [27], some studies have divided them into two African subtypes [28]. The existence of multiple lineages, however, does not impact antibody neutralization significantly and thus, ZIKV has been classified as a single serotype [29]. ZIKV is related genetically to several pathogens that cause disease globally including Dengue (DENV), yellow fever (YFV), West Nile (WNV), Japanese encephalitis (JEV), and tick-borne encephalitis (TBEV) viruses. Of these viruses, ZIKV is most closely related to the four serotypes of DENV and shares 54 to 59% amino acid identity across the viral E protein [30]. The sequence similarity between ZIKV and DENV poses unique issues for diagnosis and vaccination, and has implications for disease pathogenesis due to antibody cross-reactivity [30–33].
Studies on related flaviviruses have shown that antibody responses against the viral E protein can serve as correlates of protection in animals and humans [34–38]. The historical efficacy of the YFV, TBEV, and JEV vaccines in preventing infection and epidemics suggests that an effective vaccine targeting all strains of ZIKV should be feasible, especially given the limited (∼3 to 5%) amino acid variability between E proteins of the two lineages [27]. In terms of prioritization, pre-pubescent children and men and women of child-bearing age living within or traveling to endemic areas might be priority recipients in a ZIKV vaccination campaign (Figure 1) [39].
Figure 1. ZIKV vaccine candidates, targets, and challenges.
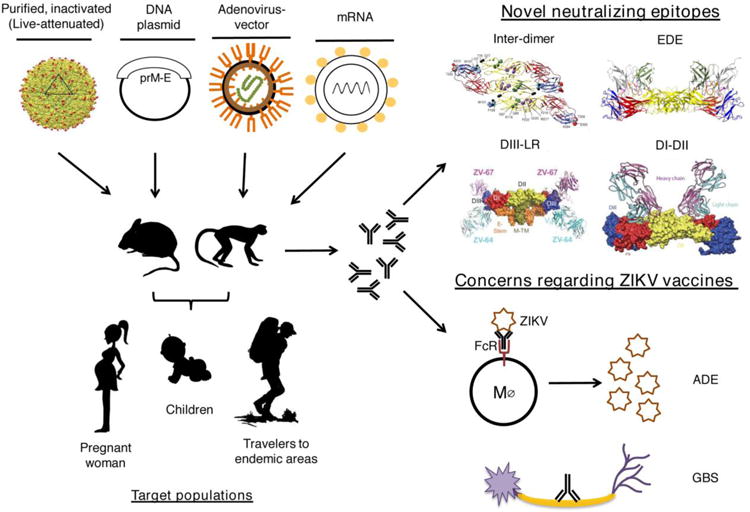
(Left) Current platforms entering Phase 1 clinical trials in humans include purified, inactivated virus (adapted from [97]), DNA plasmid, adenovirus-vectored, and modified mRNA vaccines, all of which have demonstrated pre-clinical efficacy in mice and non-human primates. The primary target populations are indicated. (Right, top) Structural analysis of monoclonal antibodies derived from infected mice and human subjects identified protective epitopes for vaccine targeting: Inter-dimer (adapted from [32]), Intra-dimer (EDE) (adapted from [51]), DIII-LR (adapted from [47]), and DI-DII (adapted from [33]). (Right, bottom) Concerns for ZIKV vaccine development and deployment include immune-mediated enhancement (ADE) of DENV infection and Guillain-Barré syndrome (GBS) due to the possible induction of autoreactive antibodies and/or T cells (latter not shown).
ZIKV vaccine epitope targets of humoral immunity
The ZIKV E protein is composed of three ectodomains (DI, DII, and DIII), which are displayed on the surface of the virion and contribute to entry into susceptible cells. A large proportion of anti-ZIKV antibodies generated during human infection target the fusion loop present in DII (DII-FL), which is highly conserved across flaviviruses. Animal studies have shown some protective activity of DII-FL antibodies in the context of flavivirus infection even though they generally have poor neutralizing capacity in vitro [40,41]. Most DII-FL antibodies are not ideal from a protection perspective because their epitope is partially inaccessible on the mature virion [42] and they require Fc-dependent effector functions for in vivo activity, the latter of which also is responsible for antibody dependent enhancement (ADE) of infection (see below) [43]. DIII adopts an immunoglobulin-like fold and is believed to participate in viral attachment and entry to host cells, which could influence cellular tropism and host range [44–46]. The lateral ridge epitope within DIII (DIII-LR) is recognized by type-specific, strongly neutralizing anti-ZIKV antibodies [32,47] (e.g., ZV-67) that likely block infection by preventing E protein rearrangements required for fusion [48,49]. Additionally, several classes of conformational anti-ZIKV antibodies that potently neutralize infection and recognize quaternary epitopes formed by adjacent E proteins have been described. E-dimer-dependent (EDE) antibodies (e.g., C10) bind to conserved sites along the E dimer interface to cross-link the E protein in a prefusion state. Specifically, EDE antibodies bind to DII-FL and additional sites of DII (b strand and ij loop) of one E subunit along with residues in DI and DIII of the opposite E subunit of the dimer [50,51]. Although originally identified in the context of a humoral response to DENV [50], cross-reactive EDE antibodies neutralize ZIKV infection in cell culture and protect against lethal infection in mice [30,51–53]. Another conformational epitope is recognized by neutralizing antibodies (e.g., ZIKV-117) that bind across two adjacent ZIKV E protein dimers in DII. These inter-dimer binding antibodies can prevent fetal infection and disease in pregnancy models of ZIKV in mice [32]. A group of protective human antibodies with distinct binding activity was described recently [33]; Z3L1 and Z23 preferentially recognize ZIKV-specific epitopes in DI and DIII, respectively, whereas Z20 binds to an epitope in DII across the E dimer interface but in a distinct pattern from EDE antibodies [33]. Collectively, these studies define a suite of protective antibodies that bind distinct epitopes and suggest that vaccines capable of targeting accessible epitopes on the soluble E protein or conformational epitopes on the virion should elicit polyclonal antibody responses with broad protective activity against most, if not all, ZIKV strains.
ZIKV vaccine approaches
Many approaches have been used for developing flavivirus vaccines against YFV, DENV, JEV, WNV, and TBEV including subunit-based (protein or DNA plasmid), chemically inactivated, and live-attenuated vaccines. Moreover, novel lipid-encapsulated modified mRNA vaccines [54,55] and viral vectored vaccines [56] have recently been adapted for ZIKV. Remarkably, in less than one year, several of these vaccines have progressed beyond pre-clinical studies in animals and are advancing into phase 1 human trials (Table 1). Additional platforms (e.g., live-attenuated vaccines) are in pre-clinical testing and expected to enter human trials in 2017 [57].
Table 1.
ZIKV vaccine candidates entering humans in 2016-2017.
| Vaccine Name | Technology | Target | Strain | Sponsor | Clinical Trial Identifier | Phase | Citation |
|---|---|---|---|---|---|---|---|
| VRC-ZKADNA0 85-00-VP | DNA vaccine | prM-E | H/PF/2013 | NIH Vaccine Research Center | NCT02840487 | 1 | [56,58] |
| GLS-5700 | DNA vaccine | prM-E | ZIKV consensus | GeneOne Life Science |
NCT02887482 NCT02809443 |
1 | [59] |
| VRC-ZKADNA0 90-00-VP | DNA vaccine | prM-E | H/PF/2013 | NIH Vaccine Research Center | NCT02996461 | 1 | |
| ZPIV | Inactivated virus vaccine | Whole virion | PRVABC59 | WRAIR/NIAID |
NCT02937233 NCT02952833 NCT02963909 NCT03008122 |
1 | [56,58,60] |
| MV- ZIKA | Viral vector (measles) | E | Themis Bioscience | NCT02996890 | 1 | ||
| mRNA-1325 | mRNA vaccine | prM-E | Micronesia 2007 | Moderna Therapeutics | NCT03014089 | 1/2 | [54] |
DNA and adenovirus-vectored vaccines
Leading candidates for ZIKV immunization include DNA plasmid-based and adenovirus-vectored vaccines incorporating the prM and E genes to produce a secreted E protein or subviral particle that elicits neutralizing antibody response. DNA plasmid-based vaccines have utility due to their ease of production, relative stability, and low reactogenicity [61]. Additionally, they lack risk of reversion, as can be observed with some live-attenuated virus vaccines. One limitation of DNA plasmid vaccines is that they must be introduced into cells (e.g., by electroporation) for optimal protein production [62]. Their low reactogenicity, however, makes this vaccine class a candidate for use in pregnant women [61,63]. Adenovirus-vectored vaccines share ease of production and stability with DNA plasmid vaccines; additionally, they have broad cellular tropism and can be manufactured to high titer, which allows for optimal delivery and immunogenicity. Limitations for adenovirus vaccines include their ability to induce toxic inflammatory responses at high doses, the potential for pre-existing immunity to naturally occurring human adenoviruses that results in accelerated clearance and dampened immunogenicity, and a size limit on the gene inserted [64]. Reactogenicity has been circumvented by deletion of genes required for replication, which also allows for larger inserts [64]. Identification of monkey adenoviruses as vaccine vectors can bypass pre-existing immunity to human adenoviruses [65].
Full-length prM-E (amino acids 93-794) DNA vaccines from a French Polynesian ZIKV strain (H/PF/2013) in a cytomegalovirus promoter-driven plasmid vector were constructed with mutations in the signal sequence or the E protein stem and transmembrane regions to improve expression [58]. Immunization of six rhesus macaques using a prime and boost scheme induced humoral immunity and protected against viremia independent of the challenge dose of a heterologous ZIKV strain (PRVABC59) when administered eight weeks after the boost [66]. Analysis of the pre-and post-challenge serum of immunized animals demonstrated an inverse correlation between neutralizing antibody titer and viremia [66].
Engineering of the M-E genes (amino acids 216-794) of a Brazilian ZIKV isolate (BeH815744) into a mammalian expression plasmid yielded high levels of humoral and cellular immunity in BALB/c mice when assessed at three weeks following a single immunization [58]. Upon challenge of BALB/c mice with homologous or heterologous strains of ZIKV four weeks following immunization, the M-E plasmid vaccine abrogated ZIKV viremia. Antibody-mediated responses were sufficient to confer protection, as CD4+ or CD8+ T cell depletion did not impact vaccine efficacy and passive transfer of vaccine-derived antibody to naïve mice protected against challenge [58]. Intramuscular immunization of four rhesus macaques with this M-E plasmid induced protective humoral and cellular immune responses against a homologous strain of ZIKV, but only after boosting [56].
Full-length prM-E (amino acids 93-794) from a ZIKV consensus sequence was incorporated into a eukaryotic plasmid (pVax1) with the addition of an IgE leader sequence to improve expression [67]. Serial immunization of wild-type and immunodeficient mice induced humoral and cellular immunity that protected against challenge with a virulent American strain of ZIKV (ZIKV-PR209). Notably, vaccination also reduced disease severity in immunodeficient mice. Primary immunization of five rhesus macaques promoted a humoral response that was enhanced upon boosting [59].
A rhesus adenovirus serotype 52 (RhAd52) vaccine encoding the M-E genes from ZIKV BeH815744 induced broadly neutralizing humoral and cellular immunity after a single dose in four rhesus macaques [56]. The M-E sequence also was codon optimized and inserted into a replication-defective adenovirus [68]. A single immunization of female C57BL/6 mice with RhAd52-M-E induced ZIKV-specific neutralizing IgG that was augmented upon boosting. Immunized female mice were mated with naïve sires, and neonatal mice were challenged with a heterologous ZIKV strain at day 7 after birth and followed for 21 days [68]. Maternally transmitted vaccine immunity protected suckling mice against ZIKV-induced weight loss and lethality.
Modified mRNA vaccines
Although lipid encapsulated modified mRNA vaccines have been developed in the oncology field [69], more recently they have been adapted for viral vaccines, with two now described for ZIKV [54,55]. Many mRNA vaccines are non-amplifying and all platforms lack the capacity to integrate into the genome [69]. Modified mRNA vaccines contain a 5′ type I cap, a poly(A) tail, and untranslated regions that optimize translation efficiency and intracellular stability as well as nucleoside modifications (e.g., introduction of pseudouridine bases) to minimize the indiscriminate activation of innate immunity.
A lipid encapsulated mRNA vaccine encoding full-length prM-E of an Asian (Micronesia 2007) strain of ZIKV induced robust neutralizing antibody responses in mice against ZIKV [54]. Challenge studies with a heterologous African ZIKV strain (Dakar 41519) in immunodeficient (AG129) or immunocompetent (C57BL/6 and BALB/c) mice showed protection against weight loss and lethality when a prime and boost regimen was administered intramuscularly, and this effect was durable even 18-weeks after initial vaccination. A modified prM-E mRNA vaccine encoding mutations destroying the conserved fusion-loop epitope in domain II of the E protein protected against ZIKV and diminished production of antibodies enhancing DENV infection in cells or mice [54].
A single intradermal dose of nucleoside-modified lipid encapsulated mRNA vaccine encoding prM-E of ZIKV H/PF/2013 (French Polynesia) induced a strong antibody response in C57BL/6 and BALB/c mice that persisted for 12- and 20-weeks, respectively [55]. Challenge with a heterologous Asian-American ZIKV (PRVABC59, Puerto Rico) at 2- and 20-weeks post vaccination yielded no detectable viremia. Furthermore, a single intradermal dose inoculation of rhesus macaques also induced ZIKV strain (PRVABC59) at 5-weeks following mRNA vaccination were protected from developing viremia compared to placebo-immunized animals [55].
Inactivated virus vaccines
Purified, inactivated whole virus vaccines have been developed to circumvent issues associated with live-attenuated vaccines. This approach eliminates the possibility of viral replication yet retains, to varying degrees, the antigenicity of the structural proteins. Inactivated viral vaccines are considered desirable, especially for populations that are relatively immunocompromised (newborns, elderly, acquired or genetic immune deficiencies, or pregnant women) where live-attenuated virus vaccines may be contraindicated [70,71]. Inactivated whole virus vaccines have been used successfully for several flaviviruses including YFV, JEV, TBEV, and WNV (the latter for veterinary use only) [72].
An inactivated ZIKV vaccine (ZPIV) was developed based on a previous vaccine targeting JEV [73]. A Puerto Rican strain (PRVABC59) of ZIKV was cultured to high-titer in Vero cells, purified, and inactivated with formalin treatment [58]. A single immunization of BALB/c mice with alum-adjuvant ZPIV yielded ZIKV specific IgG titers (∼1/100) that correlated with protection against challenge with a heterologous strain of ZIKV [58]. ZPIV testing in rhesus macaques also induced neutralizing antibodies and cellular immunity after two doses [56,60]. Subsequent challenge of nonhuman primates with homologous or heterologous strains of ZIKV resulted in complete protection against plasma viremia, or viral RNA in urine, cerebrospinal fluid, colorectal, and cervicovaginal secretions [56].
Live-attenuated vaccines
Arguably, the most successful flavivirus vaccine is YF-17D, a live-attenuated virus that was generated in the 1930's after 176 serial passages of the parent YFV Asibi strain in mouse and chicken tissues [74,75]. A single YF-17D dose induces high levels of neutralizing antibodies in most individuals and confers protection in 95% of recipients, which can last up to 40 years [74]. A chimeric vaccine against JEV was developed by substituting the prM-E genes of JEV into the backbone of the YF-17D capsid and non-structural protein genes. Immunization of subjects in endemic regions with ChimerVax JE™ resulted in responses that neutralized JEV strains of multiple genotypes [76,77] and is available in Australia, Malaysia, Philippines, Thailand, and Myanmar [78]. This chimeric vaccine platform also was adapted for DENV. Different industry groups have refined tetravalent formulations incorporating either chimeric DENV-YFV virus strains (approved as Dengvaxia®) or DENV-DENV chimera (phase 3 trials of TAK-003) to achieve an attenuated strains for vaccination [79,80]. Although a multi-dose regimen of Dengvaxia® protected flavivirus-immune individuals from subsequent symptomatic DENV infection, it had less efficacy for naïve subjects [81,82].
Live-attenuated vaccines are a favored immunization strategy against flaviviruses because of their ability to induce durable and effective adaptive immunity at relatively low production cost [83]. However, they generally are avoided in immunocompromised populations (including pregnant women) due to possible reversion and pathogenicity.
For YF-17D, there have been rare cases of vaccine associated neurotropic and viscerotropic disease following immunization, especially in the elderly [70,74]. Several groups have stated an intention of developing live-attenuated ZIKV vaccines although to date, no data showing immunogenicity or protection has yet been published [57]. Even if such vaccines were highly immunogenic, questions remain as to their relative safety in some of the target populations.
ZIKV vaccine challenges
Beyond the generation of an immunogenic vaccine that elicits protective humoral and cell-mediated immunity, there are unique challenges to developing a ZIKV vaccine:
(a) Immune enhancement of heterologous DENV infection
The DENV complex is comprised of four genetically related serotypes. Whereas primary infection with DENV generates a protective antibody response that protects durably against the homologous serotype, secondary infection with a heterologous DENV serotype can result in a severe capillary permeability shock syndrome. This disease is attributed in part to ADE, whereby cross-reactive antibodies from the first DENV infection bind but fail to neutralize the second DENV serotype, and instead augment infection in myeloid cells expressing Fc-gamma receptors [43]. This phenomenon could be relevant to ZIKV vaccination because DENV and ZIKV are related closely to one another, the two viruses co-circulate, and their infections produce cross-reactive antibodies targeting the highly conserved DII-FL epitope of the E protein. Indeed, studies in cell culture have confirmed that ADE can occur reciprocally, with DENV and ZIKV antibodies augmenting infection of ZIKV and DENV, respectively [30,84–86]. Moreover, anti-ZIKV human monoclonal antibodies can enhance DENV infection and disease in mice [87]. If ZIKV antibody responses are shown to augment DENV infection and disease in humans, vaccine strategies that minimize the generation of cross-reactive antibodies may be required to avoid sensitizing ZIKV vaccine recipients to severe DENV infections. In this case, soluble E protein or virus-like particle (prM-E) antigens that abrogate the DII-FL epitope but retain other protective epitopes may be useful [44,54,88,89].
(b) Guillain-Barré syndrome
Currently, there is an epidemiological association between ZIKV infection and GBS, although a causal link has not yet been established. The pathogenesis of GBS might be due to direct ZIKV infection of neurons and glial cells in the spinal cord or to autoimmune-mediated targeting, possibly due to antibodies or T cells that cross-react between viral and host antigens [10]. Prior to deployment of a ZIKV vaccine, it will be important to confirm that the elicited humoral or cellular anti-ZIKV responses in humans does not promote the development of GBS.
(c) Pregnancy
Many vaccines are avoided during pregnancy due to the possible risks of infection or inflammation to the developing fetus. Indeed, vaccination prior to pregnancy remains the desired approach. Notwithstanding this, retrospective analysis of administered live-attenuated or inactivated vaccines have failed to establish conclusively adverse outcomes in fetuses of vaccinated mothers [90–92]. The current recommendation is to administer vaccines if the disease risk outweighs the potential of vaccine related effects [93]. Several recent studies suggest a relatively high frequency (85-100%) of adverse neurodevelopmental effects of fetuses of symptomatic and asymptomatic pregnant women following ZIKV infection [6,94–96]. With current information, it remains difficult to determine whether the risk of exposure to ZIKV in utero surpasses that associated with immunization with certain classes of vaccines.
Conclusions
The consequences of the ZIKV epidemic highlight the need for rapid development and introduction of a vaccine. Decades of work on related flaviviruses have provided mature vaccine technologies and platforms, many of which can be adapted for use in immunocompromised and susceptible populations including children and pregnant women. Currently, DNA plasmid and purified, inactivated vaccines have demonstrated immunogenicity and protection in mice and nonhuman primates and now are entering Phase 1 clinical testing in humans. While optimism remains high for generating protective vaccines against ZIKV across multiple platforms, questions remain about their safety because of the unique clinical manifestations of ZIKV and its genetic and serological relatedness to DENV. Parallel discovery and epidemiological efforts are needed to address these issues prior to widespread implementation of a ZIKV vaccine.
Highlights.
- Subunit and inactivated vaccines against ZIKV have entered phase 1 trials in humans
- Multiple epitopes recognized by neutralizing mAbs against ZIKV have been identified
- Animal models are now developed to establish vaccine correlates of protection
- Guillain-Barre syndrome and antibody enhancement may delay vaccine implementation
Acknowledgments
NIH grants (R01 AI073755 and R01 AI104972) to M.S.D supported this work. E.F. was supported by an NIH Pre-doctoral training grant award (T32 AI007163).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could a ect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weaver SC, Costa F, Garcia-Blanco MA, Ko AI, Ribeiro GS, Saade G, Shi P, Vasilakis N. Zika virus: History, emergence, biology, and prospects for control. Antiviral Res. 2016;130:69–80. doi: 10.1016/j.antiviral.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brasil P, Pereira Junior JP, Raja Gabaglia C, Damasceno L, Wakimoto M, Ribeiro Nogueira RM, Carvalho de Sequeira P, Machado Siqueira A, Abreu de Carvalho LM, Cotrim da Cunha D, et al. Zika Virus Infection in Pregnant Women in Rio de Janeiro — Preliminary Report. N Engl J Med. 2016 doi: 10.1056/NEJMoa1602412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika Virus and Birth Defects — Reviewing the Evidence for Causality. N Engl J Med. 2016;374:1981–1987. doi: 10.1056/NEJMsr1604338. (*) The authors analyzed available epidemiological data to establish that fetal anomalies are caused by ZIKV infection during the first trimester. [DOI] [PubMed] [Google Scholar]
- 4.van der Eijk AA, van Genderen PJ, Verdijk RM, Reusken CB, Mögling R, van Kampen JJA, Widagdo W, Aron GI, GeurtsvanKessel CH, Pas SD, et al. Miscarriage Associated with Zika Virus Infection. N Engl J Med. 2016;375:1002–1004. doi: 10.1056/NEJMc1605898. [DOI] [PubMed] [Google Scholar]
- 5.Schaub B, Vouga M, Najioullah F, Gueneret M, Monthieux A, Harte C, Muller F, Jolivet E. Analysis of blood from Zika virus-infected fetuses : a prospective case series. 2017;3099:26–28. doi: 10.1016/S1473-3099(17)30102-0. [DOI] [PubMed] [Google Scholar]
- 6.Honein MA, Dawson AL, Petersen EE, Jones AM, Lee EH, Yazdy MM, Ahmad N, Macdonald J, Evert N, Bingham A, et al. Birth Defects Among Fetuses and Infants of US Women With Evidence of Possible Zika Virus Infection During Pregnancy. Jama. 2016;30333:59–68. doi: 10.1001/jama.2016.19006. [DOI] [PubMed] [Google Scholar]
- 7.Cao-Lormeau VM, Blake A, Mons S, Lastère S, Roche C, Vanhomwegen J, Dub T, Baudouin L, Teissier A, Larre P, et al. Guillain-Barré Syndrome outbreak associated with Zika virus infection in French Polynesia: a case-control study. Lancet. 2016;387:1531–1539. doi: 10.1016/S0140-6736(16)00562-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.dos Santos T, Rodriguez A, Almiron M, Sanhueza A, Ramon P, de Oliveira WK, Coelho GE, Badaró R, Cortez J, Ospina M, et al. Zika Virus and the Guillain– Barré Syndrome — Case Series from Seven Countries. N Engl J Med. 2016;375:1598–1601. doi: 10.1056/NEJMc1609015. [DOI] [PubMed] [Google Scholar]
- 9.Santana do Rosario M, Antonio Pereira de Jesus P, Vasilakis N, Farias DS, Antônio Caires Novaes M, Rodrigues SG, Martins LC, Fernando da Costa Vasconcelos P, Ko AI, Carlos Junior Alcantara L, et al. Case Report : Guillain – Barré Syndrome after Zika Virus Infection in Brazil. Am J Trop Med Hyg. 2016;95:1157–1160. doi: 10.4269/ajtmh.16-0306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parra B, Lizarazo J, Jimenez- Arango JA, Zea- Vera AF, Manrique GG, Vargas J, Angarita JA, Zuñiga G, Gonzalez RL, Beltran CL, et al. Guillain- Barre Syndrome Associated with Zika Virus Infection in Colombia. N Engl J Med. 2016;373:1513–1523. doi: 10.1056/NEJMoa1605564. [DOI] [PubMed] [Google Scholar]
- 11.Dick GW, Kitchen S, Haddow A. Zika Virus (I). Isolations and serological specificity. Trans R Soc Trop Med Hyg. 1952;46:509–520. doi: 10.1016/0035-9203(52)90042-4. [DOI] [PubMed] [Google Scholar]
- 12.Dick GWA. Zika Virus (II). Pathogenicity and Physical Properties. Trans R Soc Trop Med Hyg. 1952;46:521–534. doi: 10.1016/0035-9203(52)90043-6. [DOI] [PubMed] [Google Scholar]
- 13.Zanluca C, dos Santos CND. Zika virus – an overview. Microbes Infect. 2016;18:295–301. doi: 10.1016/j.micinf.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Musso D, Nilles EJ, Cao-Lormeau VM. Rapid spread of emerging Zika virus in the Pacific area. Clin Microbiol Infect. 2014;20:O595–O596. doi: 10.1111/1469-0691.12707. [DOI] [PubMed] [Google Scholar]
- 15.Campos G, Bandeira A, Sardi S. Zika Virus Outbreak, Bahia Brazil. Emerg Infect Dis. 2015;21:1885–6. doi: 10.3201/eid2110.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frieden TR. Zika Virus 6 Months Later. 2017;30333:2016–2017. doi: 10.1001/jama.2016.11941. [DOI] [PubMed] [Google Scholar]
- 17.Castro LA, Fox SJ, Chen X, Liu K, Bellan SE, Dimitrov NB, Galvani AP, Meyers LA. Real-time Zika risk assessment in the United States. bioRxiv. 2016 doi: 10.1101/056648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grard G, Caron M, Mombo IM, Nkoghe D, Mboui Ondo S, Jiolle D, Fontenille D, Paupy C, Leroy EM. Zika Virus in Gabon (Central Africa) - 2007: A New Threat from Aedes albopictus? PLoS Negl Trop Dis. 2014;8:1–6. doi: 10.1371/journal.pntd.0002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marchette NJ, Garcia R, Rudnick A. Isolation of Zika virus from Aedes aegypti: mosquitoes in Malaysia. Am J Trop Med Hyg. 1969;18:411–415. doi: 10.4269/ajtmh.1969.18.411. [DOI] [PubMed] [Google Scholar]
- 20.Foy BD, Kobylinski KC, Foy JLC, Blitvich BJ, da Rosa AT, Haddow AD, Lanciotti RS, Tesh RB. Probable Non-Vector-borne Transmission of Zika Virus, Colorado, USA. Emerg Infect Dis. 2011;17:880–882. doi: 10.3201/eid1705.101939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Russell K, Hills SL, Oster AM, Porse CC, Danyluk G, Cone M, Brooks R, Scotland S, Schiffman E, Fredette C, et al. Male-to-Female Sexual Transmission of Zika Virus — United States, January–April 2016. Clin Infect Dis. 2016;64:ciw692. doi: 10.1093/cid/ciw692. [DOI] [PubMed] [Google Scholar]
- 22.Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected Female-to-Male Sexual Transmission of Zika Virus — New York City, 2016. MMWR Morb Mortal Wkly Rep. 2016;65:716–717. doi: 10.15585/mmwr.mm6528e2. [DOI] [PubMed] [Google Scholar]
- 23.Deckard DT, Chung WM, Brooks JT, Smith JC, Woldai S, Hennessey M, Kwit N, Mead P. Male-to-Male Sexual Transmission of Zika Virus — Texas, January 2016. MMWR Morb Mortal Wkly Rep. 2016;65:372–374. doi: 10.15585/mmwr.mm6514a3. [DOI] [PubMed] [Google Scholar]
- 24.Gornet M, Bracero N, Segars J. Zika Virus in Semen: What We Know and What We Need to Know. Semin Reprod Med. 2016;34:285–292. doi: 10.1055/s-0036-1592312. [DOI] [PubMed] [Google Scholar]
- 25.Mansuy JM, Dutertre M, Mengelle C, Fourcade C, Marchou B, Delobel P, Izopet J, Martin-Blondel G. Zika virus: High infectious viral load in semen, a new sexually transmitted pathogen? Lancet Infect Dis. 2016;16:405. doi: 10.1016/S1473-3099(16)00138-9. [DOI] [PubMed] [Google Scholar]
- 26.Murray KO, Gorchakov R, Carlson AR, Berry R, Lai L, Natrajan M, Garcia MN, Correa A, Patel SM, Aagaard K, et al. Prolonged Detection of Zika Virus in Vaginal Secretions and Whole Blood. Emerg Infect Dis. 2017;23:99–101. doi: 10.3201/eid2301.161394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haddow AD, Schuh AJ, Yasuda CY, Kasper MR, Heang V, Huy R, Guzman H, Tesh RB, Weaver SC. Genetic Characterization of Zika Virus Strains: Geographic Expansion of the Asian Lineage. PLoS Negl Trop Dis. 2012;6:e1477. doi: 10.1371/journal.pntd.0001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shen S, Shi J, Wang J, Tang S, Wang H, Hu Z, Deng F. Phylogenetic analysis revealed the central roles of two African countries in the evolution and worldwide spread of Zika virus. Virol Sin. 2016;31:118–130. doi: 10.1007/s12250-016-3774-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dowd KA, DeMaso CR, Pelc RS, Speer SD, Smith ARY, Goo L, Platt DJ, Mascola JR, Graham BS, Mulligan MJ, et al. Broadly Neutralizing Activity of Zika Virus-Immune Sera Identifies a Single Viral Serotype. Nature. 2016;11:1485–91. doi: 10.1016/j.celrep.2016.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dejnirattisai W, Supasa P, Wongwiwat W, Rouvinski A, Barba-spaeth G, Duangchinda T, Sakuntabhai A, Malasit P, Rey FA, Mongkolsapaya J, et al. Dengue virus sero-cross-reactivity drives antibody- dependent enhancement of infection with zika virus. Nat Publ Gr. 2016;17:1102–1108. doi: 10.1038/ni.3515. (**) The authors showed that plasma from DENV-infected individuals could cross-react with and augment cellular infection of ZIKV at low concentrations. They determined this was due to antibody-dependent enhancement of infection. This study highlights the potential for ZIKV disease enhancement by cross-reactive anti-DENV antibodies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Priyamvada L, Quicke KM, Hudson WH, Onlamoon N, Sewatanon J, Edupuganti S, Pattanapanyasat K, Chokephaibulkit K, Mulligan MJ, Wilson PC, et al. Human antibody responses after dengue virus infection are highly cross-reactive to Zika virus. Proc Natl Acad Sci U S A. 2016;113:7852–7. doi: 10.1073/pnas.1607931113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sapparapu G, Fernandez E, Kose N, Bin Cao, Fox JM, Bombardi RG, Zhao H, Nelson CA, Bryan AL, Barnes T, et al. Neutralizing human antibodies prevent Zika virus replication and fetal disease in mice. Nature. 2016;540:443–447. doi: 10.1038/nature20564. (**) The authors isolated human monoclonal antibodies agains ZIKV, defined a new protective epitope at the inter-dimer interface, and showed that passive prophylaxis or therapy can protect the fetus against infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Q, Yang H, Liu X, Dai L, Ma T, Qi J, Wong G, Peng R, Liu S, Li J, et al. Molecular determinants of human neutralizing antibodies isolated from a patient infected with Zika virus. Sci Transl Med. 2016;8:1–11. doi: 10.1126/scitranslmed.aai8336. (*) The authors identified a panel of ZIKV-specific human antibodies, some of which can protect against ZIKV infection in mice. X-ray crystal structures of the antibodies with ZIKV E protein identfied three epitopes that were distinct from those reported previously for other flavivirues. [DOI] [PubMed] [Google Scholar]
- 34.Vogt MR, Moesker B, Goudsmit J, Jongeneelen M, Austin SK, Oliphant T, Nelson S, Pierson TC, Wilschut J, Throsby M, et al. Human monoclonal antibodies against West Nile virus induced by natural infection neutralize at a postattachment step. J Virol. 2009;83:6494–507. doi: 10.1128/JVI.00286-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Throsby M, De Kruif J. Isolation and characterization of human monoclonal antibodies from individuals infected with West Nile Virus. J Virol. 2006;80:6982–6992. doi: 10.1128/JVI.00551-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kimura-Kuroda J, Yasui K. Protection of mice against Japanese encephalitis virus by passive administration with monoclonal antibodies. J Immunol. 1988;141:3606–3610. [PubMed] [Google Scholar]
- 37.Mason PW, Dalrymple JM, Gentry MK, McCown JM, Hoke CH, Burke DS, Fournier MJ, Mason TL. Molecular characterization of a neutralizing domain of the Japanese encephalitis virus structural glycoprotein. J Gen Virol. 1989;70:2037–2049. doi: 10.1099/0022-1317-70-8-2037. [DOI] [PubMed] [Google Scholar]
- 38.Gotuzzo E, Yactayo S, Córdova E. Review article: Efficacy and duration of immunity after yellow fever vaccination: Systematic review on the need for a booster every 10 years. Am J Trop Med Hyg. 2013;89:434–444. doi: 10.4269/ajtmh.13-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marston HD, Lurie N, Borio LL, Fauci AS. Considerations for Developing a Zika Virus Vaccine. N Engl J Med. 2016;375:1209–1212. doi: 10.1056/NEJMp1607762. [DOI] [PubMed] [Google Scholar]
- 40.Vogt MR, Dowd KA, Engle M, Tesh RB, Johnson S, Pierson TC, Diamond MS. Poorly Neutralizing Cross-Reactive Antibodies against the Fusion Loop of West Nile Virus Envelope Protein Protect In Vivo via Fc Receptor and Complement-Dependent Effector Mechanisms. J Virol. 2011;85:11567–11580. doi: 10.1128/JVI.05859-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dai L, Song J, Lu X, Deng YQ, Musyoki AM, Cheng H, Zhang Y, Yuan Y, Song H, Haywood J, et al. Structures of the Zika Virus Envelope Protein and Its Complex with a Flavivirus Broadly Protective Antibody. Cell Host Microbe. 2016;19:696–704. doi: 10.1016/j.chom.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 42.Nelson S, Jost CA, Xu Q, Ess J, Martin JE, Oliphant T, Whitehead SS, Durbin AP, Graham BS, Diamond MS, et al. Maturation of West Nile Virus Modulates Sensitivity to Antibody-Mediated Neutralization. PLoS Pathog. 2008;4:e1000060. doi: 10.1371/journal.ppat.1000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Halstead SB, Mahalingam S, Marovich MA, Ubol S, Mosser DM. Intrinsic antibody-dependent enhancement of microbial infection in macrophages: disease regulation by immune complexes. Lancet Infect Dis. 2010;10:712–722. doi: 10.1016/S1473-3099(10)70166-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crill WD, Roehrig JT. Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J Virol. 2001;75:7769–73. doi: 10.1128/JVI.75.16.7769-7773.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee E, Lobigs M. Substitutions at the putative receptor-binding site of an encephalitic flavivirus alter virulence and host cell tropism and reveal a role for glycosaminoglycans in entry. J Virol. 2000;74:8867–8875. doi: 10.1128/jvi.74.19.8867-8875.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mandl CW, Allison SL, Holzmann H, Meixner T, Heinz FX. Attenuation of tick-borne encephalitis virus by structure-based site-specific mutagenesis of a putative flavivirus receptor binding site. J Virol. 2000;74:9601–9. doi: 10.1128/jvi.74.20.9601-9609.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhao H, Fernandez E, Dowd KA, Speer SD, Platt DJ, Gorman MJ, Govero J, Nelson CA, Pierson TC, Diamond MS, et al. Structural Basis of Zika Virus-Specific Antibody Protection. Cell. 2016;166:1016–1027. doi: 10.1016/j.cell.2016.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nybakken GE, Oliphant T, Johnson S, Burke S, Diamond MS, Fremont DH. Structural basis of West Nile virus neutralization by a therapeutic antibody. Nature. 2005;437:764–769. doi: 10.1038/nature03956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dai L, Wang Q, Qi J, Shi Y, Yan J, Gao GF. Molecular basis of antibody-mediated neutralization and protection against flavivirus. IUBMB Life. 2016;68:783–791. doi: 10.1002/iub.1556. [DOI] [PubMed] [Google Scholar]
- 50.Rouvinski A, Guardado-Calvo P, Barba-Spaeth G, Duquerroy S, Vaney MC, Kikuti CM, Navarro Sanchez ME, Dejnirattisai W, Wongwiwat W, Haouz A, et al. Recognition determinants of broadly neutralizing human antibodies against dengue viruses. Nature. 2015;520:109–113. doi: 10.1038/nature14130. [DOI] [PubMed] [Google Scholar]
- 51.Barba-Spaeth G, Dejnirattisai W, Rouvinski A, Vaney MC, Medits I, Sharma A, Simon-Lorière E, Sakuntabhai A, Cao-Lormeau VM, Haouz A, et al. Structural basis of potent Zika–dengue virus antibody cross-neutralization. Nature. 2016;536:48–53. doi: 10.1038/nature18938. [DOI] [PubMed] [Google Scholar]
- 52.Swanstrom JA, Plante JA, Plante KS, Young EF, McGowan E, Gallichotte EN, Widman DG, Heise MT, de Silva AM, Baric RS. Dengue Virus Envelope Dimer Epitope Monoclonal Antibodies Isolated from Dengue Patients Are Protective against Zika Virus. MBio. 2016;7:e01123–16. doi: 10.1128/mBio.01123-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang S, Kostyuchenko VA, Ng TS, Lim XN, Ooi JSG, Lambert S, Tan TY, Widman DG, Shi J, Baric RS, et al. Neutralization mechanism of a highly potent antibody against Zika virus. Nat Commun. 2016;7:13679. doi: 10.1038/ncomms13679. (*)The authors used cryo-electron microscopy to define structurally how EDE antibodies neutralize ZIKV, by preventing fusion of the virus to the host cell. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richner JM, Himansu S, Dowd KA, Butler SL, Salazar V, Fox JM, Julander JG, Tang WW, Shresta S, Pierson TC, et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell. 2017 doi: 10.1016/j.cell.2017.02.017. [DOI] [PubMed] [Google Scholar]
- 55.Pardi N, Hogan MJ, Pelc RS, Muramatsu H, Andersen H, DeMaso CR, Dowd KA, Sutherland LL, Scearce RM, Parks R, et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017 doi: 10.1038/nature21428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abbink P, Larocca RA, De La Barrera RA, Bricault CA, Moseley ET, Boyd M, Kirilova M, Li Z, Nganga D, Nanayakkara O, et al. Protective efficacy of multiple vaccine platforms against Zika virus challenge in rhesus monkeys. Science (80-) 2016;353:1129–1132. doi: 10.1126/science.aah6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Durbin A. Vaccine Development for Zika Virus—Timelines and Strategies. Semin Reprod Med. 2016;34:299–304. doi: 10.1055/s-0036-1592070. [DOI] [PubMed] [Google Scholar]
- 58.Larocca RA, Abbink P, Peron JPS. Vaccine protection against Zika virus from Brazil. Nature. 2016;536:474–478. doi: 10.1038/nature18952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Muthumani K, Griffin BD, Agarwal S, Kudchodkar SB, Reuschel EL, Choi H, Kraynyak KA, Duperret EK, Keaton AA, Chung C, et al. In vivo protection against ZIKV infection and pathogenesis through passive antibody transfer and active immunisation with a prMEnv DNA vaccine. npj Vaccines. 2016;1:16021. doi: 10.1038/npjvaccines.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Routh J. Testing of Investigational Inactivated Zika Vaccine in Human Beings. 2016 no volume. [Google Scholar]
- 61.Beckett CG, Tjaden J, Burgess T, Danko JR, Tamminga C, Simmons M, Wu SJ, Sun P, Kochel T, Raviprakash K, et al. Evaluation of a prototype dengue-1 DNA vaccine in a Phase 1 clinical trial. Vaccine. 2011;29:960–968. doi: 10.1016/j.vaccine.2010.11.050. [DOI] [PubMed] [Google Scholar]
- 62.Ferraro B, Morrow MP, Hutnick NA, Shin TH, Lucke CE, Weiner DB. Clinical applications of DNA vaccines: Current progress. Clin Infect Dis. 2011;53:296–302. doi: 10.1093/cid/cir334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martin JE, Pierson TC, Hubka S, Rucker S, Gordon IJ, Enama ME, Andrews CA, Xu Q, Davis BS, Nason M, et al. A West Nile Virus DNA Vaccine Induces Neutralizing Antibody in Healthy Adults during a Phase 1 Clinical Trial. J Infect Dis. 2007;196:1732–1740. doi: 10.1086/523650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tatsis N, Ertl HC. Adenoviruses as vaccine vectors. Mol Ther. 2004;10:616–629. doi: 10.1016/j.ymthe.2004.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Abbink P, Maxfield LF, Ng'ang'a D, Borducchi EN, Iampietro MJ, Bricault CA, Teigler JE, Blackmore S, Parenteau L, Wagh K, et al. Construction and evaluation of novel rhesus monkey adenovirus vaccine vectors. J Virol. 2015;89:1512–22. doi: 10.1128/JVI.02950-14. (*)The authors reported a novel vaccine platform using rhesus adenoviruses as vectors that allows circumvention of immune targeting by antibodies against naturally occuring human adenoviruses. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dowd KA, Ko SY, Morabito KM, Yang ES, Pelc RS, DeMaso CR, Castilho LR, Abbink P, Boyd M, Nityanandam R, et al. Rapid development of a DNA vaccine for Zika virus. Science (80-) 2016;354:237–240. doi: 10.1126/science.aai9137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Muthumani K, Griffin BD, Agarwal S, Kudchodkar SB, Reuschel EL, Choi H, Kraynyak KA, Duperret EK, Keaton AA, Chung C, et al. In vivo protection against ZIKV infection and pathogenesis through passive antibody transfer and active immunisation with a prMEnv DNA vaccine. npj Vaccines. 2016;1:16021. doi: 10.1038/npjvaccines.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim E, Erdos G, Huang S, Kenniston T, Falo LD, Gambotto A. Preventative Vaccines for Zika Virus Outbreak: Preliminary Evaluation. EBioMedicine. 2016;13:315–320. doi: 10.1016/j.ebiom.2016.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schlake T, Thess A, Fotin-Mleczek M, Kallen KJ. Developing mRNA-vaccine technologies. RNA Biol. 2012;9:1319–30. doi: 10.4161/rna.22269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Monath TP, Lee CK, Julander JG, Brown A, Beasley DW, Watts DM, Hayman E, Guertin P, Makowiecki J, Crowell J, et al. Inactivated yellow fever 17D vaccine: Development and nonclinical safety, immunogenicity and protective activity. Vaccine. 2010;28:3827–3840. doi: 10.1016/j.vaccine.2010.03.023. [DOI] [PubMed] [Google Scholar]
- 71.Monath TP, Fowler E, Johnson CT, Balser J, Morin MJ, Sisti M, Trent DW. An inactivated cell-culture vaccine against yellow fever. N Engl J Med. 2011;364:1326–1333. doi: 10.1056/NEJMoa1009303. [DOI] [PubMed] [Google Scholar]
- 72.Heinz FX, Stiasny K. Flaviviruses and flavivirus vaccines. Vaccine. 2012;30:4301–4306. doi: 10.1016/j.vaccine.2011.09.114. [DOI] [PubMed] [Google Scholar]
- 73.Tauber E, Kollaritsch H, Korinek M, Rendi-Wagner P, Jilma B, Firbas C, Schranz S, Jong E, Klingler A, Dewasthaly S, et al. Safety and immunogenicity of a Vero-cell-derived, inactivated Japanese encephalitis vaccine: a non-inferiority, phase III, randomised controlled trial. Lancet. 2007;370:1847–1853. doi: 10.1016/S0140-6736(07)61780-2. [DOI] [PubMed] [Google Scholar]
- 74.Barrett AD, Teuwen DE. Yellow fever vaccine - how does it work and why do rare cases of serious adverse events take place? Curr Opin Immunol. 2009;21:308–313. doi: 10.1016/j.coi.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 75.Beck AS, Barrett ADT. Current status and future prospects of yellow fever vaccines. Expert Rev Vaccines. 2015;14:1479–92. doi: 10.1586/14760584.2015.1083430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bonaparte M, Dweik B, Feroldi E, Meric C, Bouckenooghe A, Hildreth S, Hu B, Yoksan S, Boaz M. Immune response to live-attenuated Japanese encephalitis vaccine (JE-CV) neutralizes Japanese encephalitis virus isolates from south-east Asia and India. BMC Infect Dis. 2014;14:156. doi: 10.1186/1471-2334-14-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Monath TP, McCarthy K, Bedford P, Johnson CT, Nichols R, Yoksan S, Marchesani R, Knauber M, Wells KH, Arroyo J, et al. Clinical proof of principle for ChimeriVax™: Recombinant live, attenuated vaccines against flavivirus infections. Vaccine. 2002;20:1004–1018. doi: 10.1016/s0264-410x(01)00457-1. [DOI] [PubMed] [Google Scholar]
- 78.Barrett AD. Vaccines Available against JE. 2014 [Google Scholar]
- 79.Guy B, Jackson N. Dengue vaccine: hypotheses to understand CYD-TDV-induced protection. Nat Rev Microbiol. 2016;14:45–54. doi: 10.1038/nrmicro.2015.2. [DOI] [PubMed] [Google Scholar]
- 80.Kirkpatrick BD, Whitehead SS, Pierce KK, Tibery CM, Grier PL, Hynes NA, Larsson CJ, Sabundayo BP, Talaat KR, Janiak A, et al. The live attenuated dengue vaccine TV003 elicits complete protection against dengue in a human challenge model Study participants. Sci Transl Med. 2016;8:3–4. doi: 10.1126/scitranslmed.aaf1517. [DOI] [PubMed] [Google Scholar]
- 81.Capeding MR, Tran NH, Hadinegoro SRS, Ismail HIHM, Chotpitayasunondh T, Chua MN, Luong CQ, Rusmil K, Wirawan DN, Nallusamy R, et al. Clinical efficacy and safety of a novel tetravalent dengue vaccine in healthy children in Asia: A phase 3, randomised, observer-masked, placebo-controlled trial. Lancet. 2014;384:1358–1365. doi: 10.1016/S0140-6736(14)61060-6. [DOI] [PubMed] [Google Scholar]
- 82.Villar L, Dayan GH, Arredondo-García JL, Rivera DM, Cunha R, Deseda C, Reynales H, Costa MS, Morales-Ramírez JO, Carrasquilla G, et al. Efficacy of a Tetravalent Dengue Vaccine in Children in Latin America. N Engl J Med. 2015;372:113–123. doi: 10.1056/NEJMoa1411037. [DOI] [PubMed] [Google Scholar]
- 83.Durbin AP, Kirkpatrick BD, Pierce KK, Carmolli MP, Tibery CM, Grier PL, Hynes N, Opert K, Jarvis AP, Sabundayo BP, et al. A 12-Month–Interval Dosing Study in Adults Indicates That a Single Dose of the National Institute of Allergy and Infectious Diseases Tetravalent Dengue Vaccine Induces a Robust Neutralizing Antibody Response. J Infect Dis. 2016;214:832–835. doi: 10.1093/infdis/jiw067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Charles AS, Christofferson RC. Utility of a Dengue-Derived Monoclonal Antibody to Enhance Zika Infection In Vitro. PLoS Curr. 2016 doi: 10.1371/currents.outbreaks.4ab8bc87c945eb41cd8a49e127082620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kawiecki AB, Christofferson RC. Zika Virus–Induced Antibody Response Enhances Dengue Virus Serotype 2 Replication In Vitro. J Infect Dis. 2016;214:1357–1360. doi: 10.1093/infdis/jiw377. [DOI] [PubMed] [Google Scholar]
- 86.Paul LM, Carlin ER, Jenkins MM, Tan AL, Barcellona CM, Nicholson CO, Trautmann L, Michael SF, Isern S. Dengue Virus Antibodies Enhance Zika Virus Infection. bioRxiv. 2016;5:50112. doi: 10.1038/cti.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stettler K, Beltramello M, Espinosa DA, Graham V, Cassotta A, Bianchi S, Vanzetta F, Minola A, Jaconi S, Mele F, et al. Specificity, cross-reactivity, and function of antibodies elicited by Zika virus infection. Science (80-) 2016;353:823–826. doi: 10.1126/science.aaf8505. [DOI] [PubMed] [Google Scholar]
- 88.Chabierski S, Barzon L, Papa A, Niedrig M, Bramson JL, Richner JM, Palù G, Diamond MS, Ulbert S. Distinguishing West Nile virus infection using a recombinant envelope protein with mutations in the conserved fusion-loop. BMC Infect Dis. 2014;14:1–8. doi: 10.1186/1471-2334-14-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hughes HR, Crill WD, Chang GJJ. Manipulation of immunodominant dengue virus E protein epitopes reduces potential antibody-dependent enhancement. Virol J. 2012;9:115. doi: 10.1186/1743-422X-9-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sur DK, Wallis DH, O'Connell TX. Vaccinations in pregnancy. Am Fam Physician. 2003;68:299–304. [PubMed] [Google Scholar]
- 91.Munoz FM. Infant Protection Against Influenza Through Maternal Immunization. JAMA Pediatr. 2016;170:832. doi: 10.1001/jamapediatrics.2016.1322. [DOI] [PubMed] [Google Scholar]
- 92.Bozzo P, Narducci A, Einarson A. Motherisk Update during pregnancy. 2011;57:555–557. [PMC free article] [PubMed] [Google Scholar]
- 93.Kroger AT, Sumaya CV, Pickering LK, Atkinso WL. General Recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices. MMWR. 2016;60:1–60. [Google Scholar]
- 94.Soares de Oliveira-Szejnfeld P, Levine D. Congenital Brain Abnormalities and Zika Virus: What the Radiologist Can Expect to See Prenatally and Postnatally. Radiology. 2016;281:203–218. doi: 10.1148/radiol.2016161584. (**)Radiological findings of pregnant women with confirmed ZIKV infection and their offspring before and after birth showed a range of brain abnormalities. The authors observed ventriculomegaly, cerebral calcifications, cortical migrational defects, and brainstem hypoplasia. [DOI] [PubMed] [Google Scholar]
- 95.Cuevas EL, Tong VT, Rozo N, Valencia D, Pacheco O, Gilboa SM, Mercado M, Renquist CM, González M, Ailes EC, et al. Preliminary Report of Microcephaly Potentially Associated with Zika Virus Infection During Pregnancy — Colombia, January–November 2016. MMWR Morb Mortal Wkly Rep. 2016;65:1409–1413. doi: 10.15585/mmwr.mm6549e1. [DOI] [PubMed] [Google Scholar]
- 96.van der Linden V, Pessoa A, Dobyns W, Barkovich AJ. Description of 13 Infants Born During October 2015–January 2016 With Congenital Zika Virus Infection Without Microcephaly at Birth — Brazil. MMWR Morb Mortal Wkly Rep. 2016;65:1343–1348. doi: 10.15585/mmwr.mm6547e2. [DOI] [PubMed] [Google Scholar]
- 97.Sirohi D, Chen Z, Sun L, Klose T, Pierson TC, Rossmann MG, Kuhn RJ. The 3.8 Å resolution cryo-EM structure of Zika virus. Science. 2016;352:467–70. doi: 10.1126/science.aaf5316. [DOI] [PMC free article] [PubMed] [Google Scholar]


