Abstract
We present the case of a 29-year-old man with mutation-positive Duchenne muscular dystrophy and mutation-positive hypertrophic cardiomyopathy. His cardiac phenotype has characteristics of both disorders; he manifests sub-epicardial left ventricular free wall late gadolinium enhancement that is consistent with Duchenne cardiomyopathy, as well as asymmetric ventricular septal hypertrophy, hyperdynamic left ventricular systolic function, and septal mid-myocardial late gadolinium enhancement, which are characteristic of hypertrophic cardiomyopathy.
Keywords: Duchenne muscular dystrophy, hypertrophic cardiomyopathy, cardiac magnetic resonance imaging
Duchenne muscular dystrophy is a genetic disorder resulting from mutations in the dystrophin gene and occurs in ~1:4000 males. Clinical manifestations of Duchenne muscular dystrophy include progressive skeletal muscle disease and the development of cardiomyopathy. Left ventricular systolic dysfunction typically presents in the second decade of life and in the era of improved pulmonary care is a major source of morbidity and mortality. Pathologically, the cardiomyopathy is characterised by sub-epicardial fibrosis of the left ventricular free wall1 that manifests as late gadolinium enhancement on post-contrast cardiac magnetic resonance imaging.2
Hypertrophic cardiomyopathy is a genetic, primary myocardial disorder for which over 1400 mutations in more than 20 genes associated with the sarcomere, cytoskeleton, Z-disc, and calcium handling have been described. Hypertrophic cardiomyopathy occurs in ~1:500 people and its clinical manifestations include left ventricular hypertrophy without an identifiable haemodynamic cause. Pathologically, hypertrophic cardiomyopathy is characterised by myocyte hypertrophy and disarray, and diffuse interstitial myocardial fibrosis that is often concentrated in the hypertrophied ventricular septum. This fibrosis is also evident on post-contrast cardiac magnetic resonance as late gadolinium enhancement.3 We present the case of a 29-year-old man with dystrophin mutation-positive Duchenne muscular dystrophy who has asymmetric septal hypertrophy and preserved, hyperdynamic left ventricular systolic function, who was found to have a co-occurring sarcomeric gene mutation known to cause hypertrophic cardiomyopathy.
Case report
The patient presented with gross motor delays and calf pseudohypertrophy and was diagnosed with Duchenne muscular dystrophy at age 7. Genetic testing demonstrated a duplication of dystrophin exons 3 and 4, a frequently reported mutation resulting in alteration of the reading frame. He began prednisone at age 13, which was discontinued at age 15; he was restarted on deflazacort at age 23. His initial echocardiogram at age 9 – before steroid therapy – demonstrated marked asymmetric septal hypertrophy with hyperdynamic left ventricular systolic function. Over time, he developed progressive left ventricular and septal hypertrophy and he was started on propranolol for left ventricular outflow tract obstruction at age 19.
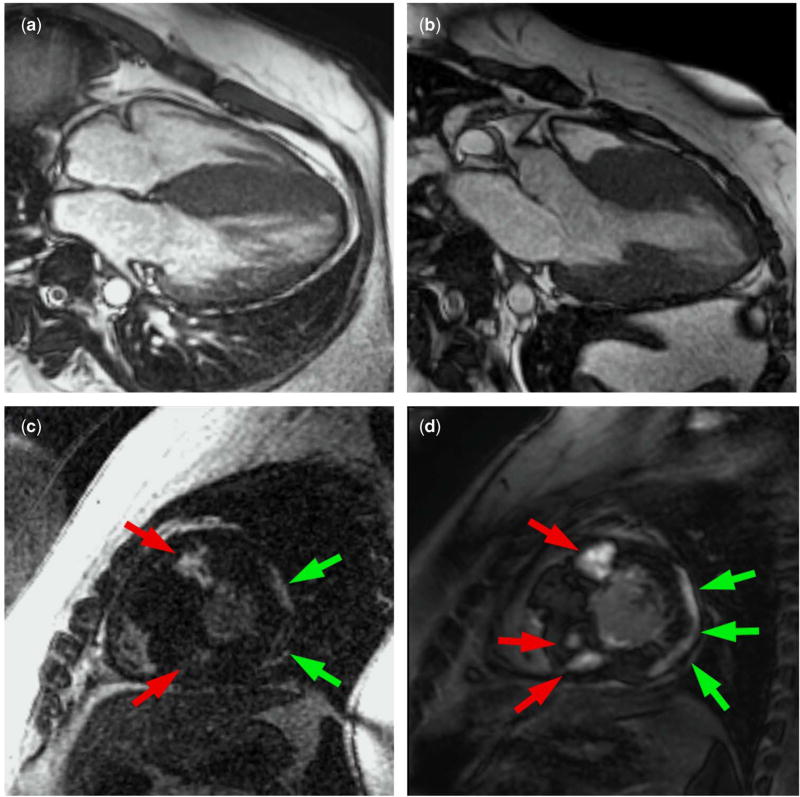
His initial cardiac magnetic resonance at age 22 demonstrated severe asymmetric septal hypertrophy (Fig 1a and b), near-obliteration of the mid-left ventricle in systole, and systolic anterior motion of the mitral valve. There was hyperdynamic global left ventricular systolic function, in agreement with previous echocardiograms. On post-contrast imaging (Fig 1c), diffuse sub-epicardial late gadolinium enhancement in the left ventricular free wall (green arrows) and multiple focal septal areas of mid-myocardial late gadolinium enhancement (red arrows) were seen. Subsequent cardiac magnetic resonance studies have demonstrated progressive late gadolinium enhancement. His latest cardiac magnetic resonance study at age 27 (Fig 1d) demonstrates sub-epicardial partial thickness late gadolinium enhancement in large areas of the anterior, lateral, and inferior left ventricular free wall segments (green arrows) and mid-myocardial late gadolinium enhancement in the anterior and inferior ventricular septum starting at the level of the papillary muscles and extending to the apex (red arrows). The patient has continued to exhibit preserved, hyperdynamic left ventricular systolic function (Supplemental Movie 1). The asymmetric septal hypertrophy prompted genetic testing for mutations known to cause hypertrophic cardiomyopathy. Results showed a heterozygous p.Glu542Gln mutation in myosin binding protein C (cardiac) and a heterozygous p.Glu195Lys mutation in troponin T type 2 (cardiac), both of which have been reported as disease-causing in individuals with autosomal-dominant hypertrophic cardiomyopathy. No other family members have completed genetic testing for hypertrophic cardiomyopathy, but his parents have normal echocardiograms.
Figure 1.
(a, b): Four-chamber (a) and three-chamber (b) bright blood cardiac magnetic resonance imaging at age 22 showing asymmetric septal hypertrophy. (c): Short-axis post-contrast imaging at age 22 showing diffuse sub-epicardial left ventricular free wall late gadolinium enhancement (green arrows) and multiple focal septal areas of mid-myocardial late gadolinium enhancement (red arrows). (d): Short-axis post-contrast imaging at age 27 showing progression of sub-epicardial left ventricular free wall enhancement (green arrows) and progression in terms of both number and thickness of focal areas of mid-myocardial septal enhancement (red arrows).
Discussion
This case illustrates genetically proven co-occurrence of two disorders, Duchenne muscular dystrophy and hypertrophic cardiomyopathy. Our patient exhibits the typical skeletal muscle phenotype of Duchenne muscular dystrophy, but the cardiac phenotype is atypical. The asymmetric ventricular septal hypertrophy, hyperdynamic left ventricular systolic function, and septal mid-myocardial late gadolinium enhancement are characteristic of hypertrophic cardiomyopathy, 3 whereas the sub-epicardial left ventricular free wall late gadolinium enhancement is consistent with the Duchenne muscular dystrophy cardiac phenotype.2 We postulate that the cytoskeletal pathways mediated by dystrophin and the sarcomeric pathways mediated by myosin binding protein C (cardiac) and troponin T type 2 (cardiac) are both deranged, resulting in a cardiac phenotype with characteristics of both Duchenne muscular dystrophy and hypertrophic cardiomyopathy. However, the downstream interaction of these two pathways has abrogated the typical Duchenne cardiomyopathy expected in patients his age, exemplified by the lack of significant left ventricular systolic dysfunction and the presence of myocardial hypertrophy. Although there has been a report of cardiac hypertrophy in a patient with Becker muscular dystrophy,4 to our knowledge this is the first report of a patient with genetically proven co-occurring Duchenne muscular dystrophy and hypertrophic cardiomyopathy.
Supplementary Material
Acknowledgments
The authors are grateful to Larry W. Markham, MD, Kan N. Hor, MD, John L. Jefferies, MD, and Stephanie M. Ware, MD, PhD for their insights and clinical management of the patient.
Footnotes
Conflicts of Interest
None.
Consent
Informed consent was obtained from the patient for publication of this case report and any accompanying images.
To view supplementary material for this article, please visit http://dx.doi.org/10.1017/S1047951114000067
References
- 1.Frankel KA, Rosser RJ. The pathology of the heart in progressive muscular dystrophy: epimyocardial fibrosis. Hum Pathol. 1976;7:375–386. doi: 10.1016/s0046-8177(76)80053-6. [DOI] [PubMed] [Google Scholar]
- 2.Puchalski MD, Williams RV, Askovich B, et al. Late gadolinium enhancement: precursor to cardiomyopathy in Duchenne muscular dystrophy? Int J Cardiovasc Imaging. 2009;25:57–63. doi: 10.1007/s10554-008-9352-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rubinshtein R, Glockner JF, Ommen SR, et al. Characteristics and clinical significance of late gadolinium enhancement by contrast-enhanced magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Circ Heart Fail. 2010;3:51–58. doi: 10.1161/CIRCHEARTFAILURE.109.854026. [DOI] [PubMed] [Google Scholar]
- 4.Park OY, Ahn Y, Park WS, et al. Rapid progression from hypertrophic cardiomyopathy to heart failure in a patient with Becker’s muscular dystrophy. Eur J Heart Fail. 2005;7:684–688. doi: 10.1016/j.ejheart.2004.07.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.