Abstract
Background
Sedation is common in critical care. It is unknown if sedative self-administration is a safe or acceptable intervention for mechanically ventilated (MV) patients.
Objectives
To evaluate whether patient self-administered sedation with dexmedetomidine (Precedex®) (PST-DEX) is safe and acceptable for self-management of anxiety during ventilatory support.
Methods
Randomized pilot trial in three ICUs. Intubated patients were randomly assigned to PST-DEX (n=17) or usual care (n=20). PST-DEX was administered via standard PCA pumps with a basal infusion (0.1–0.7 mcg/kg/hour) titrated by the number of patient-triggered doses (0.25 mcg/kg/dose). PST-DEX safety goals were heart rate > 40 bpm, systolic BP > 80 mm Hg, diastolic BP > 50 mm Hg. Acceptability was determined by patients’ self-reported satisfaction and ability to administer DEX. A 100-mm visual analog scale assessed patients’ anxiety daily (VAS-A).
Results
Patients (N = 37) were 60% male, 89% Caucasian. Mean age was 50.6 ±15 years; APACHE III 60.1 ± 32.6 and protocol duration 3.4 ± 1.6 days [median = 4]. Five PST-DEX patients had BP and/or HR readings below safety parameters necessitating short-term treatment. Nursing adherence to safety parameter reporting was 100%; adherence to PST-DEX titration algorithm was 73%. Overall baseline VAS-A was 38.4 ± 28.0 and did not change significantly over time [βday = 2.1((SE=2.5), p = 0.4)]. Most PST-DEX patients (92%) were satisfied/very satisfied with their ability to self-administer medication.
Conclusions
For select patients, PST-DEX is a safe and acceptable alternative to clinician-administered sedation. Additional research to determine the efficacy of PST DEX is warranted.
Keywords: critical illness, respiration, artificial, dexmedetomidine
Administration of sedative medications to critically ill patients receiving mechanical ventilatory support is a common intensive care unit (ICU) practice. These medications are administered for numerous reasons, including reducing anxiety as well as promoting patient comfort with mechanical ventilator breaths. Recent practice guidelines suggest that administration of these medications be targeted to achieve a “lightly sedated, interactive patient” when medically feasible.1 Scales to guide sedation levels rely on clinicians to administer medications based on subjective assessment and observation of a patient’s arousal and motor activity. While clinicians desire that patients remain comfortable and in synchrony with ventilator breaths, the ideal means to achieve this goal is not consistent across providers.
Patient-controlled analgesia (PCA) has been used for many years to promote effective patient self-management of pain; PCA is superior to clinician-administered analgesic therapy with high patient satisfaction.2 It is not known if a similar parallel between PCA and sedation self-administration by mechanically ventilated (MV) patients exists. Findings from our previous proof-of-principle study documents that mechanically ventilated patients are willing, able and satisfied with their ability to self-administer their own sedative medication to manage anxiety.3 However, this study recruited a highly selective group of patients who all self-administered their own sedative therapy limited to 24 hours. The next logical step was to build on these promising findings by examining whether self-management of sedative therapy in a larger group of patients for greater than 24 hours is safe. Thus, the aims of this study were to determine whether patient self-administered sedative therapy with dexmedetomidine (PST-DEX) is a safe and acceptable sedation option for MV patients.
METHODS
Study Aims
The primary goal was to establish safety and acceptability of PST-DEX in a small randomized pilot trial for up to five days versus standard, nurse-administered sedative practice. Safety was determined by the occurrence of study-defined adverse events, adverse hemodynamic effects or self-extubations. Protocol deviations related to the study drug, research protocol or infusion pump were also collated. Acceptability of PST-DEX was defined as the patient’s appraisal of their ability to self-administer dexmedetomidine for relaxation, including level of relaxation, anxiety. Secondary aims were to determine adherence to the PST-DEX safety alert notification parameters and patient-care registered nurses’ (RNs) adherence to the infusion titration algorithm. Approval for the use of human subjects in research was obtained from the University of [removed for peer review] Institutional Review Board, which included limitations on proxy consent.
Patients and Setting
Adult intubated patients expected to require mechanical ventilation for at least an additional 48 hours were screened at three ICUs in the [removed for peer review] area. The ICUs consisted of a medical ICU (14 beds) and a surgical ICU (21 beds) at an academic medical center, and a 24-bed community medical-surgical ICU. The ICUs used the same electronic medical record (EMR), ventilator management, weaning, and sedation medication order sets. Nursing care was provided by RNs typically in a 1 nurse to 2 patient ratio. Medical care was provided by faculty intensivists across units. Standard practices on the ICU consisted of daily respiratory therapist assessment of patients’ for weaning readiness and attainment of criteria for a spontaneous breathing trial.
Screening and enrollment by trained research personnel followed a rigorous, three-step procedure to ensure only those MV patients willing and able to self-manage sedative therapy were appropriately offered study participation.
Step #1: Pre-screening
Research personnel initially screened via the EMR to determine the presence of exclusion criteria: 1) aggressive ventilatory support (e.g., PEEP > 15 cm of water, prone positioning, high-frequency oscillator ventilation); 2) condition potentially worsened by dexmedetomidine, e.g., systolic [BP] < 85 mmHg, second- or third-degree heart block or bradycardia (HR) < 50 beats/min; 3) condition preventing use of the push-button device (e.g., paralysis); 4) positive pregnancy test; 5) acute hepatitis or liver failure; 6) general anesthesia within the prior 24 hours; 7) acute stroke or uncontrolled seizures; 8) acute myocardial infarction; 9) receipt of medications known to interact with dexmedetomidine (e.g., isoniazid, clonidine, fluoxetine, hydrocodone); and 10) severe cognition or communication problems (e.g., coma, deafness without signing literacy, physician-documented dementia).
Step #2: Patient Screening
Eligible patients were next assessed for ability to communicate, follow commands, and depress the push-button medication infusion device. The Confusion Assessment Method for the ICU (CAM-ICU) was used to determine the presence of delirium. The CAM-ICU is a widely used, valid, and reliable assessment tool for the presence (CAM-ICU positive) or absence of delirium (CAM-ICU negative).4–8 Inter-rater reliability has been reported to range from k = 0.92–0.995; sensitivities and specificities were high and showed no significant differences between subgroups.4,6 Patients were required to be CAM-ICU negative for enrollment.
Step #3: Informed Consent Procedures
If step #2 was passed and the attending physician approved enrollment, the study was explained in greater detail, and the patient was offered the opportunity to enroll. Patients were consented directly when possible. The content of the consent form was read verbatim to the patient by research personnel. A list of yes-no questions concerning the consent process was used to ensure understanding. If a patient correctly answered the consent questions and agreed to participate, he/she then signed the consent form. In specific cases where a patient was unable to self-consent, a proxy consent procedure with the legally authorized representative (LAR) was implemented. Obtaining proxy consent occurred when patients were too fatigued or weak to participate in a lengthy consent process, had decreased ability to maintain concentration, or were more sedated for a short duration for a bedside procedure such as bronchoscopy. If proxy consent was necessary, the LAR provided written consent with patient assent.
Data Collection Procedures
Demographic and descriptive data were recorded upon enrollment and included age, gender, race, ethnicity, weight, medical diagnoses, comorbidities, indication for ventilatory support, all medications, and ventilator settings. Acute Physiology and Chronic Health Evaluation (APACHE) III scores were calculated based on EMR data during the first 24 hours of ICU admission.
Daily measures on protocol for all patients
Research personnel assessed for delirium daily using the CAM-ICU and positive findings were reported to the primary care team. Anxiety, defined as a state marked by apprehension, agitation, arousal, increased motor activity, and fearful withdrawal,9,10 was assessed daily with a 100-mm vertical visual analog scale (VAS-A). All patients responded to the question, “How anxious are you feeling today?” by marking their current level of anxiety from 0/not anxious at all, to 100/most anxious ever. Scores were derived by the distance in millimeters from the bottom anchor to the mark placed by the patient.11–14 The vertical presentation of the VAS-A is more sensitive and easier for patients to use, particularly for those with a narrowed visual field or when under stress.15–17 The VAS-A is an accurate and sensitive measure of anxiety state, is a reliable measure of anxiety in MV patients12 and can easily be completed by MV patients.18
Length of mechanical ventilatory support and ICU stay
Length of MV support was defined as the time (in days) from intubation to clinician-ordered extubation, withdrawal of ventilatory support, or death. Unplanned self-extubations and re-intubations were recorded. Length-of-ICU stay was defined as the time (in days) from ICU admission to ICU discharge or death.
Study Treatments
To prevent any unconscious selection bias by preferentially recruiting only those patients who were thought to be ideal for PST-DEX, patients were randomly allocated via consecutive opaque envelopes to either the experimental PST-DEX protocol or usual ICU care. Given the necessity to first establish safety of PST-DEX, clinicians were not blinded to the experimental treatment. Likewise, it was important for the medical safety officers to know which medications the patient was receiving to address any acute changes in condition. Further, medical staff not affiliated with the study team were concerned that blinding was possibly unsafe given the limited knowledge surrounding PST-DEX. Patients remained in the study for up to 5 days, or until they withdrew, were extubated, transferred from the ICU, or died.
Experimental PST-DEX Protocol
We selected dexmedetomidine (Precedex®, Hospira, Inc. Lake Forest, IL, USA) because of its pharmacokinetic profile including light sedative properties whereby patients can easily be awakened, and its successful use in our preliminary proof-of-principle study.3 Dexmedetomidine is Food and Drug Administration (FDA)-approved for continuous infusion for up to 24 hours, has a rapid distribution half-life of 6 minutes, a terminal elimination half-life of 2 hours and linear kinetics in dosages of 0.2 to 0.7 mcg/kg/hour, the maximum FDA-approved dose.19 This study operated under FDA IND #111693 [removed for peer review]. Study medication preparation and distribution were the responsibility of the [removed for peer review] Investigational Drug Services (IDS) pharmacy.
PST-DEX Administration Protocol
We used the LifeCare™ PCA Infusion System (#20709-04, Hospira, Inc. Lake Forest, IL, USA) to administer the PST-DEX medication in the PCA + continuous infusion mode. The IDS pharmacy prepared bar-coded syringes for this infusion device. We utilized the same PCS dosing algorithm as in our preliminary study: a loading dose (0.5 mcg/kg) followed by a continuous basal infusion of 0.2 mcg/kg/hour, up to a maximum infusion of 0.7 mcg/kg/hour.3 Patients were allowed three self-boluses of dexmedetomidine per hour (0.25 mcg/kg) with a 20-minute lock-out.3 PST-DEX patients were instructed to depress the push-button device when feeling anxious or if they desired medication for relaxation.
Patient-care RNs increased or decreased the basal infusion rate based on the number of bolus attempts from the patient in the prior 2 hours. Details are published elsewhere.3 Because the study aims were to evaluate the safety and acceptability of PST-DEX, these patients did not experience daily sedative reduction trials. If a patient remained on ventilatory support after 5 days, the PST-DEX protocol was discontinued and the sedative regimen reverted to medication(s) ordered by the primary care team.
PST-DEX Safety Monitoring
An extensive safety monitoring plan was required for this pilot trial. Research personnel abstracted every 4-hour HR and BP recordings from the medical record. Research personnel or patient-care RNs reported study-defined adverse events (adverse hemodynamic effects [systolic BP < 80 or > 180 mmHg, diastolic BP < 50 or > 100 mmHg; HR < 40 or > 120 beats/min]), persistent inability to understand rationale for triggering the pushbutton device, or marked worsening of respiratory status requiring aggressive ventilatory support. Any safety issues, change in patient’s medical status, or adverse events were first reported to the attending physician, and then to a study Medical Safety Officer (MSO); both were available at all times by pager and participated in decisions to immediately modify or suspend the protocol. The study physician [removed for peer review] made the final decision on restarting the protocol or withdrawing a patient from the study.
Protocol deviations
Research personnel reviewed the EMR daily for protocol deviations related to the study drug, infusion pump, or any cause for PST-DEX patients.
Protocol adherence
A daily checklist was used to monitor patient-care RNs’ abilities to adhere to the infusion algorithm across all shifts.
Acceptability of PST-DEX
Patients randomized to PST-DEX were queried at protocol completion regarding their ability to self-administer DEX for relaxation, ability to control anxiety, and level of relaxation experienced using an investigator-created 5-choice Likert-scale questionnaire.
Usual Care
Patients randomized to usual care (UC) continued on their current sedative regimen with doses and frequencies of medications ordered by the primary care team and administered per standard practice by the RNs. UC sedative therapy administration practices consisted of physician orders with parameters to titrate continuous infusions up or down based on a prescribed target Minnesota Sedation Assessment Tool (MSAT)20 or Motor Activity Assessment Scale (MAAS)21. A majority of patients also had as needed (prn) orders for sedative and/or opioid bolus doses. Titration of continuous infusions of sedatives and/or prn bolus doses were administered at the nurses’ discretion based on physician-ordered parameters. If feasible and appropriate, UC patients had reduction or interruption in the continuous sedative infusion to increase wakefulness and re-evaluate sedative requirements.
In addition, patients in both groups were evaluated each morning by a respiratory therapist for readiness for a spontaneous breathing trial (SBT). No data were gathered on differences between groups on daily screening and completion of sedative medication reductions or SBTs.
Statistical Analysis
Descriptive statistics and graphing were used to report summary statistics and illustrated the distribution of the interval measures. Comparisons between patient demographic and clinical characteristics were accomplished using t-tests for normally distributed interval data or Mann-Whitney U tests for skewed distributions. Categorical data were compared using Chi-square Tests of Association. Mixed models were fit to detect any change in anxiety levels over time by group. Analysis was performed using SPSS v19 and SAS v9.3. Results were considered significant at p < .05.
RESULTS
Demographic and Clinical Characteristics of Enrolled Patients
Of the 37 enrolled patients, 60% were male, with race 89% White, 3% Asian, 5% Black, and 3% Native Hawaiian/Pacific Islander. Mean age was 50.6 (± 15) years. Mean illness severity (APACHE III) was 60.1 (± 32.6) (Table 1). A majority of patients had a respiratory-related ICU admission diagnosis with a number of co-morbidities (Table 2).
Table 1.
Demographic and Clinical Characteristics of Patients by Group (N=37)
| PST-DEX (n=17) |
Usual Care (n=20) |
p-value | |
|---|---|---|---|
| Age, mean (SD), y | 53.4 (15.3) | 48.3 (14.9) | .31 |
| Gender: male, No. (%) | 9 (52.9) | 13 (65.0) | .46 |
| APACHE III score, mean (SD) | 65.6 (32.0) | 55.2 (33.3) | .34 |
| Total days in ICU, mean (range), d | 16.5 (3–40) | 15.5 (3–45) | .16 |
| Total mechanical ventilator days, mean (range), d |
3.9 (1–12) | 6.7 (1–24) | .08 |
| Length of ICU stay prior to study enrollment, mean (range), d |
8.1 (1–36) | 9.9 (0–32) | .77 |
| Length of ventilator support prior to study enrollment, mean (range), d |
3.9 (0–20) | 7.8 (0–25) | .18 |
| Length of time enrolled on protocol, mean (range), d |
3.1 (1–5) | 3.6 (1–5) | .99 |
Table 2.
Primary Admission Diagnosis, Co-morbidities and Indication for Ventilatory Support (N=37)
| PST-DEX (n=17) |
Usual Care (n=20) |
|
|---|---|---|
| Primary ICU admission, No. (%) | ||
| CABG | 0(0) | 1(5) |
| Hypotension | 1(6) | 0(0) |
| ARDS | 1(6) | 1(5) |
| COPD | 3(18) | 0(0) |
| Pneumonia | 4(24) | 3(15) |
| Pulmonary Fibrosis | 1(6) | 2(10) |
| Shortness of Breath | 6(6) | 6(30) |
| Respiratory Failure | 2(12) | 4(20) |
| Cancer | 2(12) | 1(5) |
| Sepsis | 2(12) | 1(5) |
| Abdominal Pain | 0(0) | 2(10) |
| Gastrointestinal Bleed | 0(0) | 2(10) |
| Pancreatitis | 1(6) | 0(0) |
| Acute Renal Failure | 2(12) | 0(0) |
| Surgery | 2(12) | 3(15) |
| Co-Morbidities, No. (%) | ||
| Cardiovascular | 7(41) | 11(55) |
| Respiratory | 10(59) | 11(55) |
| Neurologic | 5(29) | 4(20) |
| Renal | 3(18) | 3(15) |
| Gastrointestinal | 5(29) | 7(35) |
| Metabolic/Endocrine | 9(53) | 7(35) |
| Malignancy | 3(18) | 5(20) |
| Infection | 4(24) | 0(0) |
| Hematologic | 3(18) | 3(15) |
| Musculoskeletal | 3(18) | 5(20) |
| Transplant | 5(29) | 1(5) |
| Obesity | 2(12) | 2(10) |
| Indication for Mechanical Ventilation, No. (%) | ||
| Airway Protection | 0(0) | 2(10) |
| ARDS | 2(12) | 1(5) |
| COPD | 1(6) | 0(0) |
| Hypoxia | 2(12) | 2(10) |
| Pneumonia | 3(18) | 4(20) |
| Respiratory Arrest | 1(6) | 1(5) |
| Shortness of Breath | 8(47) | 10(50) |
| Respiratory Failure | 9(53) | 8(40) |
| Tachypnea | 1(6) | 1(5) |
ARDS, adult respiratory distress syndrome; CABG, coronary artery bypass graft; COPD, chronic obstructive pulmonary disease
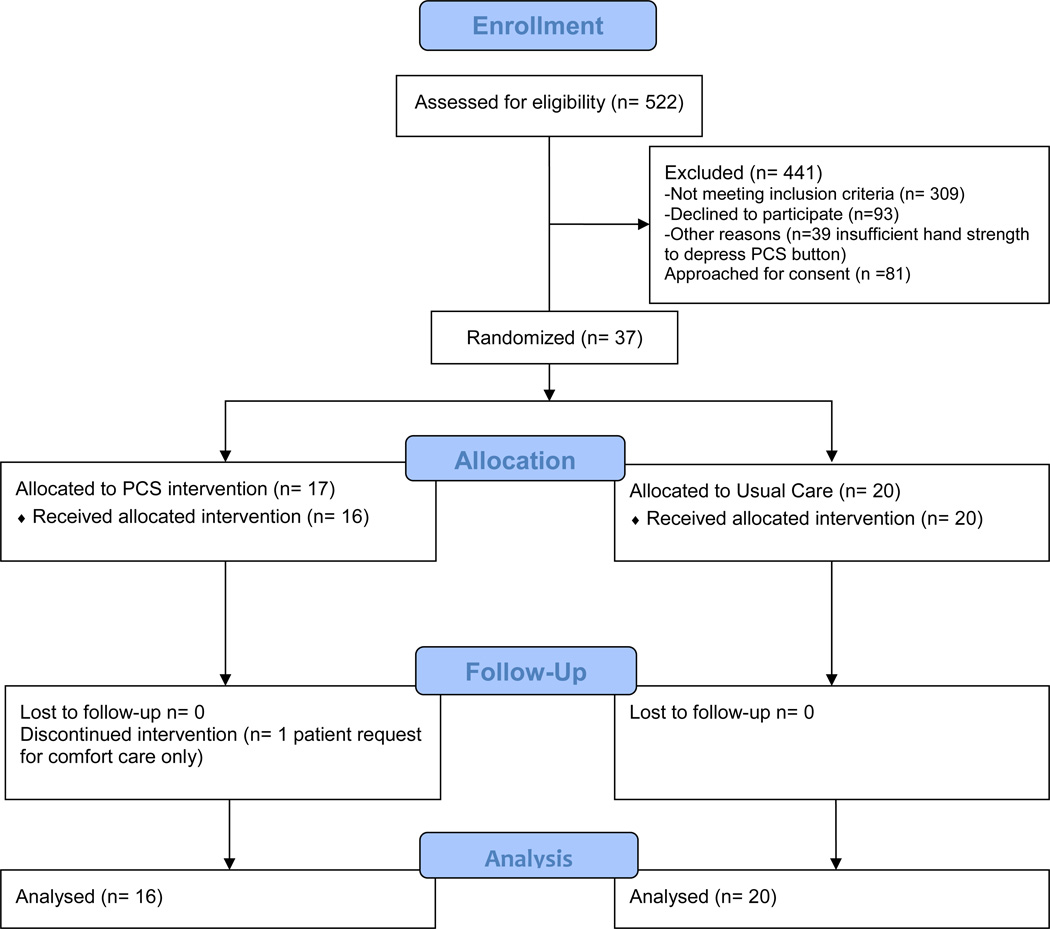
Five hundred twenty-two patients were eligible after the first chart review screen. 81 remained eligible after secondary screening; 37 participants were enrolled (46% consent rate) with 2 consented by proxy (5%) (Figure 1). The main exclusions were aggressive ventilatory support, vasopressors, chemical paralysis, stroke, myocardial infarction, or delirium. Over half (56%) of those patients passing the pre-screen were assessed as CAM-ICU positive on the secondary screen. Further, patients frequently lacked adequate hand strength to depress the push-button device. Patients approached for consent who declined to participate indicated they were too tired, not interested, or felt like they had too much going on.
Figure 1.
CONSORT Flow Diagram
Seventeen (17) patients were assigned to the experimental PST-DEX condition (46%), 20 to UC (54%) resulting in an unbalanced randomization. There were no statistically significant differences between the two groups on baseline variables (Table 1).
Patients were enrolled for an average of 3.4 (±1.6) days, median 4; range 1–5. Patients randomized to PST-DEX were on protocol for a mean 3.1 (±1.5) days (median = 2.0), UC patients 3.6 (±1.7) days (median = 4.0). Three UC patients were extubated while enrolled on protocol. A total of 7 patients received a tracheostomy while enrolled; 4 UC, 3 PST-DEX. There were no patient deaths while enrolled on protocol. Zero patients randomized to PST-DEX were assessed as CAM-ICU positive while on protocol; four patients randomized to UC were assessed CAM-ICU positive (delirium present), p= .058.
Daily Anxiety Ratings
Overall mean anxiety ratings at study entry were 38.4 ± 28. Mixed models analyses were fit to determine any changes in anxiety scores over the 5-day protocol. There was no significant change in VAS-A ratings over the 5-day study period [βday = 2.1((SE=2.5), p = .4)].
Supplemental Medications
Over the 5-day study period, 59% of PST-DEX patients received an average of 3.75 (SD 7.2) supplemental medication doses (mode = 0, median = 1). Medications included midazolam, fentanyl and hydromorphone bolus doses.
Safety
Study Defined Hemodynamic Effects or Adverse Events
Five PST-DEX patients (29%) experienced study-defined hemodynamic alterations. In all cases, the hypotension and/or bradycardia resolved after temporarily decreasing or suspending the infusion and/or administering fluids (Table 3). No PST-DEX patient was removed from the study due to safety concerns. One UC patient self-extubated and required re-intubation. No PST-DEX patient self-extubated while on protocol.
Table 3.
PST-DEX Protocol Hemodynamic Alternations: Interventions and Outcomes
| Patient | Hypotension | Bradycardia | Intervention | Outcome |
|---|---|---|---|---|
| 1 | During night shift BP decreased triggered alert parameter notification |
Dexmedetomidine infusion suspended overnight for approximately 8 hours; restarted in morning |
No further hemodynamic alterations; continued on protocol |
|
| 2 | During night shift BP decrease triggered alert parameter notification |
During night shift HR decrease triggered alert parameter notification |
Patient remained stable and did not require any additional intervention other than continued observation |
No further hemodynamic alterations; continued on protocol |
| 3 | During night shift BP decrease triggered alert parameter notification |
Dexmedetomidine infusion suspended for approximately 1.5 hours; restarted without incident |
No further hemodynamic alterations; continued on protocol |
|
| 4 | HR decreased more than 30% triggered alert parameter notification |
Patient remained stable and did not require any additional intervention other than continued observation |
No further hemodynamic alterations; continued on protocol |
|
| 5 | Diastolic BP consistently below 50 overnight triggered alert parameter notification |
500mL saline bolus plus Dexmedetomidine infusion suspended for approximately 2 hours; restarted without incident |
No further hemodynamic alterations; continued on protocol |
Safety Alert Notification
Patient-care RNs appropriately made calls to the Medical Safety Officer for patient needs 100% of the time and made recommended changes in the drug infusion rate or care interventions 100% of the time.
Protocol Deviations
Two protocol deviations occurred related to the infusion pump. In the first case, the patient-care RN documented that the infusion pump ceased infusing for an unknown period of time; there were no adverse effects to the patient. In the second case, the infusion pump drug library did not ‘recognize’ the medication syringe barcode, preventing initiation of the protocol. This patient was removed from the study with the primary care team reinstituting the previous sedative therapy without incident.
Patient Acceptability of PST-DEX
Acceptability was evaluated by three investigator-created questions (Table 4). 76% (13/17) of PST-DEX patients responded; there were 4 non-responses due to extubation and transfer from the ICU, change in medical condition, or patient decision to withdraw ventilatory support. A majority of PST-DEX patients were satisfied/very satisfied with their ability to self-administer medication (92%) and control anxiety (62%).
Table 4.
Acceptability of Patient-Managed Sedative Therapy with Dexmedetomidine (n = 13)+
| Question | Very satisfied N (%) |
Satisfied N (%) |
Neutral N (%) |
Unsatisfied N (%) |
Very Unsatisfied N (%) |
|---|---|---|---|---|---|
|
Ability to self- administer medication |
6 (46) | 6 (46) | 1 (8) | 0 | 0 |
|
Ability to control anxiety |
6 (46) | 3 (23) | 3 (23) | 1 (8) | 0 |
|
Ability to achieve relaxation |
5 (39) | 3 (23) | 2 (15) | 2(15) | 1 (8) |
Note:
3 patients did not complete the satisfaction survey at the conclusion of the protocol; 1 patient did not receive the intervention due to infusion pump issues
Adherence to Medication Algorithm Protocol
Patient-care RNs, not research nurses, adhered to the previously published titration algorithm3 78% of the time on days, 75% on evenings, and 65% on night shifts. Adherence to the dexmedetomidine basal infusion titration algorithm across all shifts was 73%.
DISCUSSION
The aims of this randomized clinical pilot study were to determine safety and acceptability of PST-DEX in MV patients. Findings document that PST-DEX is safe as defined by a priori criteria for a select sample of patients during the later, more stable portion of ventilator treatment. We observed changes in HR and mild hypotension during PST-DEX comparable to its use during clinician-administered sedation.22–24 These hemodynamic alterations resolved with minor clinical interventions; no PST-DEX patients were removed from the study for safety reasons. Busy patient-care RNs were able to adhere to the protocol’s safety alert parameters and correctly adhered to the PST-DEX titration algorithm a majority of the time. There were no self-extubations by patients randomized to PST-DEX.
Likewise, a majority of patients were satisfied with their ability to self-administer dexmedetomidine to control anxiety and achieve relaxation. Patients were able to use PST-DEX based on their individual needs under the conditions and limited duration of this trial. While there was no significant change in anxiety over time for either group, these findings suggest that patients randomized to PST-DEX were able to manage anxiety with self-administration of sedative therapy comparable to patients who received clinician-administered sedative therapy. This is congruent with the majority of PST-DEX patients who were satisfied with their ability to control anxiety. These pilot data can be used to adequately power future clinical trials testing PST-DEX to determine if sedative self-administration is efficacious for symptom management in MV patients.
Interestingly, no patients randomized to PST-DEX developed delirium after enrollment whereas four patients randomized to UC developed delirium while on protocol. This post-hoc finding requires confirmation in larger studies. As PST-DEX involves medication, it is not a “drug-free” intervention. However, dexmedetomidine has been shown to accelerate the resolution of delirium22 and allow patients to be more interactive with caregivers.23 PST-DEX is consistent with the goals of the 2013 clinical practice guidelines (ICU-PAD) for interactive, more alert mechanically ventilated patients when compared to other commonly used ICU sedatives such as midazolam or propofol.23
Limitations
Due to the requirement that patients be awake enough to understand the sedation self-management concept, PST-DEX was not appropriate for many MV patients, especially in the first few days of respiratory failure. Thus, the generalizability of the study findings is limited to those mechanically ventilated patients with clinical characteristics similar to our participants. On the other hand, as ICUs increasingly adopt a “lightly sedated strategy,” more patients would be eligible for a sedation self-management protocol similar to this trial. A MV patient on an early mobility protocol would likely be appropriate for a sedation self-management protocol.
This trial compared usual care to a combined experimental arm of dexmedetomidine delivered by continuous infusion plus patient self-initiated boluses. A study design of PST-DEX versus nurse-directed sedation using dexmedetomidine was considered. However, such a trial would have limited generalizability because dexmedetomidine is not a first-line sedative in usual clinical practice.25,26 Because of resource limitations, we did not assess patients for physical or mental limitations after they left the ICU. Lastly, we did not evaluate RN satisfaction with PST-DEX in this study given our previous work has documented overall nursing staff satisfaction with patient self-administration of sedative therapy.3
CONCLUSIONS
MV patient self-administration of sedative therapy is safe. Patients are satisfied and able to self-administer PST-DEX to manage anxiety and achieve relaxation. The application of patient self-administration of sedative therapy logically fits in the contemporary practice of sedation management which aims to have patients more alert and participating in their care. Only a larger, adequately powered study can determine whether PCS can achieve clinically relevant outcomes such as shorter ventilator duration, decreases in patient symptoms such as anxiety, prevention of delirium and improved recovery after critical illness.
Acknowledgments
Financial Support: Supported in part by the National Institute of Nursing Research, National Institutes of Health grant number 1R21 NR012795, and Hospira, Inc. The content is solely the responsibility of the authors and does not represent the official views of the National Institute of Nursing Research, the National Institutes of Health or Hospira, Inc.
This study operated under FDA IND number 111693 (C. Weinert). Clinical Trials registration number: NCT#01606852
We are grateful to the patients and the staff on the Medical ICU and Surgical ICU at the University of Minnesota Medical Center, and the ICU at Fairview-Southdale Hospital for their cooperation with this study.
Footnotes
Institution in which the work was performed: University of Minnesota Medical Center, Minneapolis, MN and Fairview-Southdale Hospital, Edina MN
REFERENCES
- 1.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. doi: 10.1097/CCM.0b013e3182783b72. [DOI] [PubMed] [Google Scholar]
- 2.Momeni M, Crucitti M, De Kock M. Patient-controlled analgesia in the management of postoperative pain. Drugs. 2006;66(18):2321–2337. doi: 10.2165/00003495-200666180-00005. [DOI] [PubMed] [Google Scholar]
- 3.Chlan L, Weinert C, Skaar D, et al. Patient-controlled sedation: A novel approach to sedation management for mechanically ventilated patients. CHEST. 2010;138(5):1045–1053. doi: 10.1378/chest.09-2615. [DOI] [PubMed] [Google Scholar]
- 4.Ely EW, Margolin R, Francis J. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the intensive care unit (CAM-ICU) Crit Care Med. 2001;29(7):1370–1379. doi: 10.1097/00003246-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 5.Truman B, Ely EW. Monitoring delirium in critically ill patients using the Confusion Assessment Method for the intensive care unit. Critical Care Nurse. 2003;23(2):25–36. [PubMed] [Google Scholar]
- 6.Ely EW, Inouye S, Bernard G. Delirium in mechanically ventilated patients: validity and reliability of the Confusion Assessment Method for the intensive care unit (CAM-ICU) JAMA. 2001;286(21):2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 7.Gusmao-Flores D, Figueira Salluh JI, Chalhub RÁ, Quarantini LC. The confusion assessment method for the intensive care unit (CAM-ICU) and intensive care delirium screening checklist (ICDSC) for the diagnosis of delirium: a systematic review and meta-analysis of clinical studies. Crit Care. 2012;16(4):R115. doi: 10.1186/cc11407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomasi CD, Grandi C, Salluh J, et al. Comparison of CAM-ICU and ICDSC for the detection of delirium in critically ill patients focusing on relevant clinical outcomes. J Crit Care. 2012;27(2):212–217. doi: 10.1016/j.jcrc.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 9.McCartney JR, Boland RJ. Anxiety and delirium in the intensive care unit. Crit Care Clin. 1994;10(4):673–680. [PubMed] [Google Scholar]
- 10.Spielberger CD. Corsini Encyclopedia of Psychology. New Jersey: John Wiley & Sons, Inc; 2010. State-Trait anxiety inventory. [Google Scholar]
- 11.Bergbom-Engberg I, Haljamae H. Assessment of patients' experience of discomforts during respirator therapy. Crit Care Med. 1989;17(10):1068–1072. doi: 10.1097/00003246-198910000-00021. [DOI] [PubMed] [Google Scholar]
- 12.Knebel A, Strider VC, Wood C. The art and science of caring for ventilator-assisted patients: learning from our clinical practice. Critical Care Nursing Clinics of North America. 1994;6(4):819–829. [PubMed] [Google Scholar]
- 13.Davey HM, Barratt AL, Butow PN, Deeks JJ. A one-item question with a Likert or Visual Analog Scale adequately measured current anxiety. J Clin Epidemiol. 2007;60(4):356–360. doi: 10.1016/j.jclinepi.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 14.Tsay SL, Wang JC, Lin KC, Chung UL. Effects of acupressure therapy for patients having prolonged mechanical ventilation support. J Adv Nurs. 2005;52:142–150. doi: 10.1111/j.1365-2648.2005.03576.x. [DOI] [PubMed] [Google Scholar]
- 15.Cline ME, Herman J, Shaw ER, et al. Standardization of the visual analogue scale. Nurs Res. 1992;4(6):378–380. [PubMed] [Google Scholar]
- 16.Gift AG. Visual analogue scales: measurement of subjective phenomena. Nurs Res. 1989;38(5):286–288. [PubMed] [Google Scholar]
- 17.Wewers M, Loew N. A critical review of visual analog scales in the measurement of clinical phenomena. Res Nurs Health. 1990;13(4):227–236. doi: 10.1002/nur.4770130405. [DOI] [PubMed] [Google Scholar]
- 18.Chlan L. Relationship between two anxiety instruments in patients receiving mechanical ventilatory support. J Adv Nurs. 2004;48(5):493–499. doi: 10.1111/j.1365-2648.2004.03231.x. [DOI] [PubMed] [Google Scholar]
- 19.Dexmedetomidine Hydrochloride Injection. [Accessed October 7, 2015]; Available from: http://www.drugs.com/pro/dexmedetomidine-hydrochloride-injection.html. [Google Scholar]
- 20.Weinert C, McFarland L. The state of intubated ICU patients: development of a two-dimensional sedation rating scale for critically ill adults. CHEST. 2004;126(6):1883–1890. doi: 10.1378/chest.126.6.1883. [DOI] [PubMed] [Google Scholar]
- 21.Devlin JW, Boleski G, Mlynarek M, et al. Motor Activity Assessment Scale: a valid and reliable sedation scale for use with mechanically ventilated patients in an adult surgical intensive care unit. Crit Care Med. 1999 Jul 27;(7):1271–1275. doi: 10.1097/00003246-199907000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Reade MC, Eastwood GM, Bellomo R, et al. Effect of Dexmedetomidine added to standard care on ventilator-free time in patients with agitated delirium a randomized clinical trial. JAMA. doi: 10.1001/jama.2016.2707. Published online March 15, 2016. [DOI] [PubMed] [Google Scholar]
- 23.Jakob SM, Ruokonen E, Grounds RM, et al. Dexmedetomidine vs midazolam or propofol for sedation during prolonged mechanical ventilation: two randomized controlled trials. JAMA. 2012;307:1151–1160. doi: 10.1001/jama.2012.304. [DOI] [PubMed] [Google Scholar]
- 24.Ahmed S, Murugan R. Dexmedetomidine use in the ICU: are we there yet? Critical Care (London, England) 2013;17(3):320. doi: 10.1186/cc12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Payen JF, Chanques G, Mantz J, et al. Current practices in sedation and analgesia for mechanically ventilated critically ill patients: a prospective multicenter patient-based study. Anesthesiology. 2007;106:687–695. doi: 10.1097/01.anes.0000264747.09017.da. [DOI] [PubMed] [Google Scholar]
- 26.Wunsch H, Kahn J, Kramer A, et al. Use of intravenous infusion sedation among mechanically ventilated patients in the United States. Crit Care Med. 2009;37:3031–3039. doi: 10.1097/CCM.0b013e3181b02eff. [DOI] [PubMed] [Google Scholar]