Abstract
Purpose
Acrylamide was classified as ‘probably carcinogenic’ to humans in 1994 by the International Agency for Research on Cancer. In 2002, public health concern increased when acrylamide was identified in starchy, plant-based foods, processed at high temperatures. The purpose of this study was to identify which food groups and lifestyle variables were determinants of hemoglobin adduct concentrations of acrylamide (HbAA) and glycidamide (HbGA) in 801 non-smoking postmenopausal women from 8 countries in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort.
Methods
Biomarkers of internal exposure were measured in red blood cells (collected at baseline) by HPLC/MS/MS (high-performance liquid chromatography/tandem mass spectrometry). In this cross-sectional analysis four dependent variables were evaluated: HbAA, HbGA, sum of total adducts (HbAA+HbGA), and their ratio (HbGA/HbAA). Simple and multiple regression analyses were used to identify determinants of the four outcome variables. All dependent variables (except HbGA/HbAA), and all independent variables were log-transformed (log2) to improve normality. Median (25th-75thpercentile) HbAA and HbGA adducts levels were 41.3 (32.8-53.1) pmol/g Hb and 34.2 (25.4-46.9) pmol/g Hb, respectively.
Results
The main food group determinants of HbAA, HbGA, HbAA+HbGA were biscuits, crackers, and dry cakes. Alcohol intake and body mass index were identified as the principal determinants of HbGA/HbAA. The total percent variation in HbAA, HbGA, HbAA+HbGA, and HbGA/HbAA explained in this study was 30%, 26%, 29%, and 13%, respectively.
Conclusions
Dietary and lifestyle factors explain a moderate proportion of acrylamide adduct variation in non-smoking postmenopausal women from the EPIC cohort.
Keywords: acrylamide, glycidamide, hemoglobin adducts, biomarkers, diet, nutrition
Introduction
Acrylamide was identified in food in 2002, and is mainly formed through the Maillard reaction whereby a carbonyl compound (a reducing sugar, such as glucose or fructose) reacts with the amino group of asparagine processed at high temperatures (>120 ºC; i.e. frying, baking, or roasting) [1, 2]. Nevertheless, acrylamide has also been found in foods cooked at temperatures lower than 100ºC (e.g. prune juice) [3]. Thus, levels of acrylamide in foods depend on factors such as temperature and length of cooking time, water content, and the amount of both reducing sugars and asparagine levels present in foods [4]. Freisling et al. assessed the principal food group determinants of acrylamide intake based on a 24-h dietary recall (24hDR) in 13,486 men and 23,508 women from the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort, and identified bread, crisp bread, rusks, coffee, and potatoes [5].
Once acrylamide is consumed, it is absorbed in the gastrointestinal tract and, via the circulation, is distributed to peripheral tissues [6, 7]. In the body, acrylamide is metabolized to glycidamide mainly by the cyp2e1 enzyme complex, and is conjugated with reduced glutathione for elimination. Acrylamide is neurotoxic in animals and in humans, but only glycidamide is considered to have mutagenic and genotoxic properties [1, 8]. As a consequence of animal and in vitro studies, the International Agency for Research on Cancer (IARC) classified acrylamide as ‘probably carcinogenic’ to humans [9].
Acrylamide and glycidamide can bind to N-terminal valine of hemoglobin (Hb) in red blood cells, and form adducts, both of which are considered valid biomarkers that reflect human internal exposure within the last 120 days (the average lifespan of erythrocytes) [1, 10]. Tobacco smoking is an important source of acrylamide exposure, and smokers have been observed to have mean Hb adducts levels three to four times higher than non-smokers [11–13].
Two previous EPIC studies evaluated biomarkers of acrylamide measured as acrylamide and glycidamide Hb-adducts (HbAA and HbGA, respectively). The first study, published by Vesper et al., aimed to determine acrylamide exposure variability (both at the individual- and group/country-level) in 240 men and 270 women, and at the same time, determine which non-dietary factors could play a role in this variability [14]. The second study, published by Ferrari et al., was conducted with the intention to compare HbAA and HbGA levels with total estimated dietary acrylamide intakes assessed using dietary questionnaires (DQs), and a 24hDR in 240 men and 270 women to estimate the validity of the EPIC dietary acrylamide assessment [15]. The main objective of the present study, which differed from the two former EPIC studies, was to identify which food groups and lifestyle factors (assessed through country-specific DQs and lifestyle questionnaires) were determinants of HbAA and HbGA concentrations in a subgroup of non-smoking postmenopausal women from the EPIC cohort. The relation between intakes of several food items using DQs, and HbAA and HbGA adducts levels has been previously evaluated in three different studies [16–18].
Materials and Methods
Study population and data collection
The EPIC study comprises 23 research centers in 10 European countries, and was designed to evaluate the relation between nutrition and lifestyle factors and the incidence of cancer and other chronic diseases. The present study includes 8 of the 10 participating EPIC countries: France, Germany, Spain, the United Kingdom (UK), Greece, Italy, The Netherlands, and Sweden (Umeå). Norway and Denmark did not participate in this analysis (EPIC-Denmark published their results separately as the Diet, Cancer, and Health cohort) [17].
The methodology of the EPIC study has been previously described [19]; all local ethics committees, and/or the IARC ethical review boards approved the study. Briefly, recruitment started between 1992-1998, and all EPIC participants provided information on habitual diet through country-specific validated DQs, referring to the year before recruitment. Information on tobacco smoking, physical activity, and education were assessed using country-specific questionnaires. Anthropometric measures (height, weight, and waist or hip circumference) were obtained at baseline by trained personnel; however, participants from France and Oxford (UK) cohorts self-reported their height and weight. Umeå (Sweden), did not collect information on waist or hip circumference, and only 29% participants from France had information on these anthropometric measures.
Blood samples (serum, plasma, white blood cells, and erythrocytes) were collected at recruitment for 385,747 of the over 500,000 EPIC participants, and were stored in liquid nitrogen (-196ºC) at the central biological bank located at IARC; blood samples from Umeå were kept in freezers (-80ºC) at local repositories [19]. The present study population comprises control women from two published nested case-control studies of acrylamide hemoglobin adducts levels and ovarian and endometrial cancers risk in EPIC [20, 21]. The selection of cases and controls for these two nested-case control studies followed the protocol that has been previously described by Cust et al. and Peeters et al. [22, 23]. Briefly, two controls (free of cancer, with the exception of non-melanoma skin cancer) were randomly selected using an incidence density sampling protocol, for each case subject (ovarian or endometrial cancer case) at the time of diagnosis. Matching criteria for both cases and controls included study center, menopausal status (premenopausal, postmenopausal, ‘undefined’), age at recruitment, (±6 months), time of day of blood draw (±1 hour), fasting status (<3, 3-6, >6 hours). For the present study, only women who were postmenopausal at blood draw, and non-smokers at recruitment were selected, since it has been postulated that acrylamide may disrupt hormonal balance, and it is known that smoking contributes to Hb-adducts levels. Postmenopausal status refers to women who reported having had the last menstrual period more than 1 year before recruitment, or when they were more than 55 years old [23]. The category of non-smoking women includes those women who reported being never smokers or having quit smoking 5 or more years before recruitment.
Thus, a total of 802 non-smoking postmenopausal control women (416 and 386 controls from the ovarian and the endometrial nested case-control studies, respectively) were available for the present study. One participant was excluded from analyses because she did not have information on HbGA adduct level, leaving a total of 801 observations
Assessment of dietary acrylamide intake
The methodology followed to create the EPIC acrylamide database has been previously described [15, 24]. In brief, the EPIC acrylamide database was assembled using information on average acrylamide levels in foods from an EU monitoring database (maintained by the European Community Institute for Reference Materials and Measurements; IRMM), and the frequency of consumption of these foods using country-specific DQs and the EPIC-Soft food classification.
Measurement of acrylamide and glycidamide hemoglobin adducts
The methodology to measure HbAA and HbGA in EPIC has been previously described [14, 15]. Briefly, 300 μL of hemolysed erythrocytes were used to measure HbAA and HbGA, and were analyzed by HPLC/tandem mass spectrometry (HPLC/MS/MS) as has been published elsewhere [12, 25]. All blood samples were measured and analyzed in a randomized and blinded manner. Two independent adduct measures per sample were performed. Hemoglobin adduct concentrations were reported as the average of these two measurements relative to the amount of hemoglobin. The detection limits for this method were 3 and 4 pmol/g Hb for HbAA and HbGA, respectively. Additionally, 42 (5%) blood samples from the same participants were sent in duplicate to evaluate the reproducibility of the hemoglobin adducts measurements.
Statistical methods
All continuous variables included in the analysis were assessed for normality using the Kolmogorov-Smirnov test, and were log-transformed (log2) in order to reduce skewness. To account for zeroes in dietary and lifestyle variables, a log2(x + 0.1) transformation was applied. Regarding adduct values, four outcomes were evaluated: log-transformed HbAA adducts (log2HbAA), log2HbGA, sum of total adducts [log2(HbAA + HbGA)], and HbGA/HbAA ratio. Simple and multiple linear regression analyses were used to assess the associations between each of the four outcome variables and food consumption and lifestyle data.
The following dietary variables and food groups were evaluated to build the HbAA, HbGA, and HbAA+HbGA final models: total energy, total carbohydrates, total fat, total fiber, total proteins, starch, potatoes, ‘vegetables’, ‘fruits, nuts and seeds’, ‘cereal and cereal products’, ‘meat and meat products’[26], ‘cakes and biscuits’, ‘flour, flakes, starches, and semolina’, ‘pasta, rice, and other grains’, ‘bread, crisp bread, and rusks’, ‘breakfast cereals’, ‘salty biscuits, aperitif biscuits, and crackers’, ‘dry cakes and biscuits’, ‘bread’, ‘pastries’, ‘olives’, ‘deep frying fats’, ‘chocolate, candy, paste, confetti’, ‘snacks’, ‘bread, and pizza dough’, ‘olive oil’, ‘coffee’, ‘decaffeinated coffee’, and ‘tea’. Then, a correlation matrix was performed to identify interdependency between dietary variables. Variables that were not correlated (r <0.6), that were matching factors for both nested case-control studies (country was used instead of center due to the number of observations), and ‘type of control’ (endometrial, ovarian control) were selected for building the final models. Lifestyle variables such as alcohol intake (g/day) and body mass index (kg/m2; BMI) were also investigated as they may affect the activity of Cyp2e1 [5, 27].
Stepwise selection was used to build models for HbAA, HbGA, and HbAA+HbGA adduct outcome variables. Matching factor and ‘type of control’ variables were forced to be included in the stepwise selection, and covariates were included in the model if they met the 0.10 significance level. Stepwise selection was also performed with all food items energy-adjusted using the residual method [28], but according to the Akaike Information Criterion (AIC) [29], these models were not optimal compared to those without energy-adjusted food items.
Lifestyle variables such as physical activity using the Cambridge index [30], education level (none, primary, technical/professional, secondary, and higher education), history of diabetes (yes, no, unknown), ever use of oral contraceptives (OCs), and ever use of hormone replacement therapy (HRT) were also evaluated; however, were not included in HbAA, HbGA, and HbAA+HbGA final models because they did not have an effect on β-estimates.
The HbGA/HbAA ratio model only included lifestyle variables described above (BMI, physical activity, education level, history of diabetes, OCs use, HRT use, and alcohol intake), since the ratio of HbGA/HbAA may reflect the metabolism of acrylamide to glycidamide.
Intraclass correlation coefficients (ICC) were estimated to evaluate the reproducibility of acrylamide measurements using 42 duplicate blood samples from the same participants [31]. Analyses stratified by alcohol intake (never drinkers, drinkers), by BMI (<25, 25-30, ≥30 kg/m2), and by European region (Northern countries: the UK, The Netherlands, Germany, Sweden; Southern countries: France, Italy, Spain, Greece) were also performed. A Wilcoxon signed-rank test was used to assess differences in Hb-adducts levels by alcohol intake, BMI, and second-hand smoke exposure (SHS). Variables for SHS were not evaluated in final models due to the large number of missing values (>50%). R square (R2) values were used to describe the percent variation in Hb-adduct levels explained by the independent variables. Partial R2 values for each of the selected variables in the models were estimated using Type II sums of squares. Pearson’s correlation coefficients for continuous variables were also estimated. To test whether the slope of a regression line differed significantly from zero, Student’s t statistics and the corresponding P-values were used. All statistical tests were evaluated at α-level 0.05.
All analyses were performed using SAS v. 9.1 (Cary, North Carolina, USA). Graphics were created using R v. 3.1.
Results
Correlation Matrix
Total carbohydrates was correlated with total fiber, total proteins, starch, and ‘cereal and cereal products’. Total fat was correlated with total proteins. Starch was correlated with ‘cereal and cereal products’. Thus, total carbohydrates, total fat, and starch were excluded from the analyses because these were larger groups of foods.
Dietary acrylamide intake and baseline characteristics
The median (25th-75th percentile) acrylamide intake at baseline based on DQ information was 20.3 (13.5-29.9) μg/day. The median (25th-75th percentile) estimated dietary acrylamide in relation to body weight was 0.3 (0.2-0.5) μg/kg-body weight/day. The highest median intakes were found in the UK, the Netherlands, and Germany, whereas Italy had the lowest median intake (Table1).
Table 1. Estimated dietary acrylamide intake and hemoglobin acrylamide and glycidamide adducts levels by EPIC country in a subgroup of non-smoking postmenopausal women.
| Acrylamide intake | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| EPIC country | Total observations | Endometrial controls | Ovarian controls | Acrylamide intake (μg/day) | Acrylamidea intake (μg/day) | μg/ kg-body weight/day | HbAA pmol/g of Hb | HbGA pmol/g of Hb | HbAA+HbGA pmol/g of Hb | Ratio of HbGA/HbAA |
| n (%) | n (%) | n (%) | Median (QR) | Median (QR) | Median (QR) | Median (QR) | Median (QR) | Median (QR) | Median (QR) | |
| France | 65 (8) | 35 (9) | 30 (7) | 20.0 (14.5- 25.6) | 17.6 (13.5- 21.6) | 0.3 (0.2- 0.4) | 38.5 (34.2- 47.3) | 29.4 (24.6- 36.8) | 66.4 (60.1- 82.9) | 0.7 (0.7- 0.8) |
| Italy | 126 (16) | 74 (19) | 52 (12) | 8.6 (5.5- 11.5) | 7.8 (4.1- 10.7) | 0.1 (0.1- 0.2) | 35.8 (26.7- 44.9) | 28.9 (21.9- 38.2) | 64.7 (49.8- 87.7) | 0.8 (0.7- 0.9) |
| Spain | 127 (16) | 72 (19) | 55 (13) | 16.6 (9.8- 25.9) | 19.0 (13.0- 25.4) | 0.2 (0.1- 0.4) | 42.4 (33.8- 53.8) | 39.8 (28.6- 48.6) | 82.4 (62.9- 101.5) | 0.9 (0.8- 1.0) |
| United Kingdom | 153 (19) | 59 (15) | 94 (23) | 31.4 (23.8- 40.4) | 30.5 (24.2- 39.5) | 0.5 (0.3- 0.6) | 55.6 (44.4- 69.0) | 47.1 (35.2- 61.3) | 102.9 (83.1- 133.4) | 0.8 (0.7- 1.0) |
| The Netherlands | 116 (14) | 38 (10) | 78 (19) | 28.8 (19.4- 40.0) | 30.0 (22.1- 40.4) | 0.4 (0.3- 0.6) | 45.3 (37.2- 54.4) | 38.6 (29.5- 51.7) | 80.9 (68.0- 105.2) | 0.9 (0.7- 1.0) |
| Greece | 59 (7) | 16 (4) | 43 (10) | 17.7 (14.4- 21.9) | 19.1 (17.5- 22.8) | 0.2 (0.2- 0.3) | 26.4 (21.1- 35.3) | 22.0 (16.5- 31.8) | 50.4 (40.1- 67.1) | 0.9 (0.7- 1.0) |
| Germany | 102 (13) | 56 (15) | 46 (11) | 22.8 (18.5- 29.5) | 24.9 (19.9- 29.2) | 0.3 (0.2- 0.5) | 36.2 (29.7- 43.8) | 29.3 (23.1- 38.5) | 67.2 (53.0- 78.5) | 0.8 (0.7- 0.9) |
| Sweden | 53 (7) | 34 (9) | 19 (5) | 18.3 (14.8- 21.0) | 22.6 (20.5- 25.0) | 0.3 (0.2- 0.3) | 38.5 (34.9- 46.9) | 34.6 (25.9- 42.2) | 72.8 (62.9- 87.8) | 0.9 (0.8- 1.0) |
| Total | 801 | 384 | 417 | 20.3 (13.5-29.9) | 21.9 (14.6-29.2) | 0.3 (0.2-0.5) | 41.3 (32.8-53.1) | 34.2 (25.4-46.9) | 75.5 (58.5-100.0) | 0.8 (0.7-1.0) |
EPIC, European Prospective Investigation into Cancer and Nutrition; QR, quartile range, HbAA, hemoglobin adducts of acrylamide; HbGA, hemoglobin adducts of glycidamide; Hb, hemoglobin
Energy-adjusted using the residual method
The median (25th-75th percentile) age in the present subcohort of women was 59 (54-63) years. Means and standard deviations, as well as medians and 25th-75th percentiles are presented for all dietary and lifestyle variables that were used in all analyses (Supplemental Table1).
Hemoglobin adducts of acrylamide and glycidamide
The ICC for the present study based on 42 duplicates was 0.96 for HbAA and 0.95 for HbGA.
The median (25th-75th percentile) HbAA and HbGA adducts level were 41.3 (32.8-53.1) and 34.2 (25.4-46.9) pmol/g of Hb, respectively (Table1). The highest median HbAA adduct level was observed in the UK, The Netherlands, and Spain, while Greece had the lowest HbAA levels, followed by Italy. Regarding HbGA adduct levels, the highest medians were found in the UK, Spain, and The Netherlands, and the lowest median was also found in Greece and Italy (Table1). The geometric means for HbAA and HbGA adducts in the total dataset were 5.3 and 5.1 pmol/g Hb respectively (data not shown). Values for HbAA+HbGA, and HbGA/HbAA by EPIC country are also presented in Table 1.
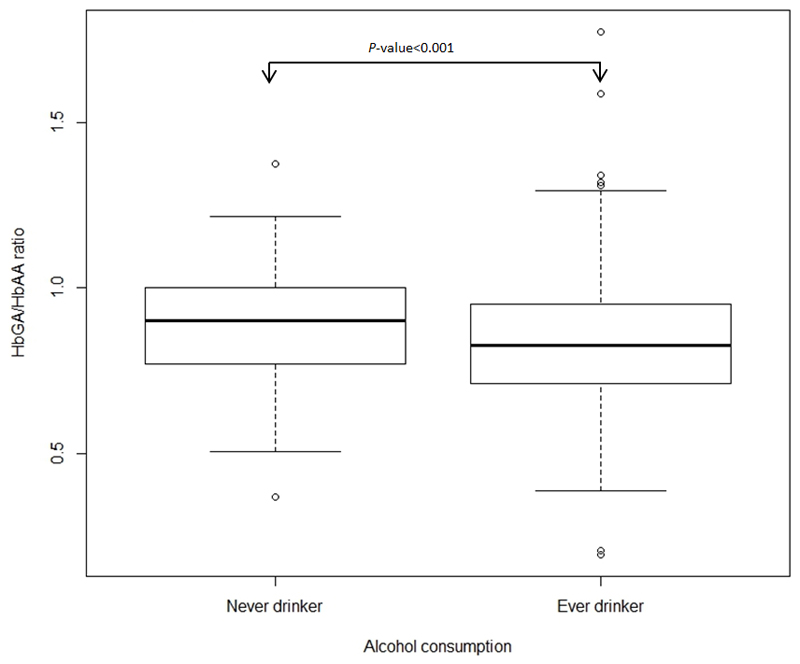
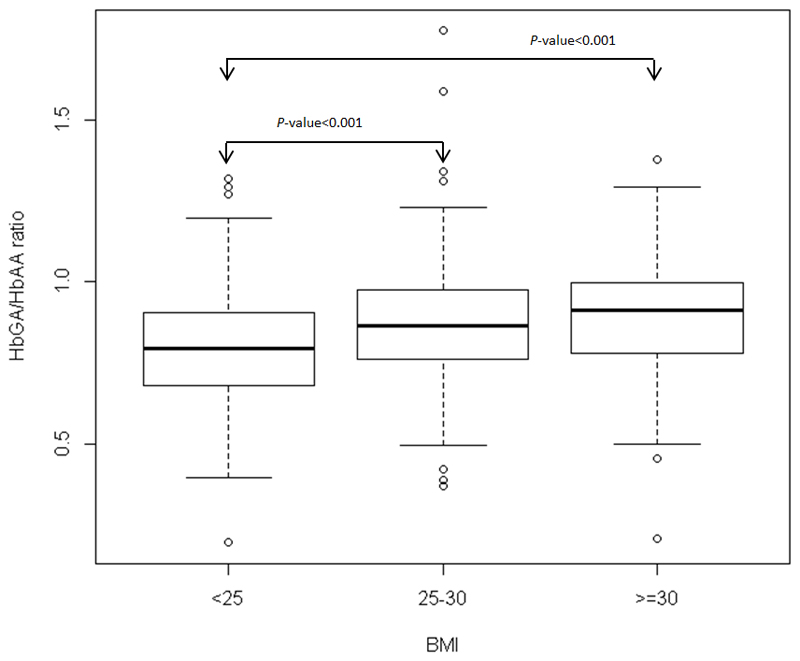
Regarding differences in Hb-adducts levels by alcohol intake and by BMI, only the ratio HbGA/HbAA differed (never vs ever drinkers, P-value <0.0001; <25, 25 to <30, ≥30 kg/m2, P-value <0.0001) (Figure 1, and Figure 2 respectively). No statistically significant differences in Hb-adduct levels were observed between women who reported being exposed to SHS (n=95) and who were not exposed (n=149) (data not shown).
Figure 1.
Box-and-whisker plot of HbGA/HbAA ratio versus alcohol consumption (never drinkers, ever drinkers). Arrow marks significant differences between groups. P-value based on a Wilcoxon rank sum test.
Figure 2.
Box-and-whisker plot of HbGA/HbAA ratio versus body mass index (BMI; <25, 25-30, ≥30 kg/m2).Arrow marks significant differences between groups. P-values based on a Wilcoxon rank sum test.
Simple linear regression analyses
The crude Pearson’s correlation coefficient between log2-acrylamide intake and log2HbAA was 0.36, between log2-acrylamide intake and log2HbGA was 0.35, between log2-acrylamide intake and log2(HbAA+HbGA) was 0.37, and between log2HbAA and log2HbGA was 0.86 (all P-values <0.0001). Log2HbAA was inversely associated with BMI (P-value <0.0001) and positively associated with alcohol intake (P-value= 0.04). A statistically significant inverse association was found between HbGA/HbAA ratio and alcohol intake (P-value <0.0001), whereas a positive association was observed between HbGA/HbAA ratio and BMI (P-value <0.0001) (Table 2).
Table 2. Simple linear regression with β-estimates for the association between dietary and lifestyle variables and log-transformed (log2) HbAA, HbGA, HbAA+HbGA, and HbGA/HbAA.
| Variables | Log2HbAA | Log2HbGA | HbAA+HbGA | HbGA/HbAA | ||||
|---|---|---|---|---|---|---|---|---|
| β | P-value | β | P-value | β | P-value | β | P-value | |
| Acrylamide intake (µg/day) | 0.20 | <.0001 | 0.23 | <.0001 | 0.21 | <.0001 | 0.02 | 0.02 |
| Age at recruitment (y) | -0.003 | 0.26 | 0.001 | 0.79 | -0.002 | 0.60 | 0.002 | 0.03 |
| Body mass index (kg/m2) | -0.32 | <.0001 | -0.01 | 0.91 | -0.18 | 0.02 | 0.17 | <.0001 |
| Alcohol intake (g/day) | 0.01 | 0.04 | -0.01 | 0.09 | 0.002 | 0.79 | -0.01 | <.0001 |
| Total dietary fiber (g/day) | 0.17 | <.0001 | 0.15 | 0.002 | 0.16 | 0.0001 | -0.01 | 0.53 |
| Total proteins (g/day) | 0.15 | 0.0003 | 0.18 | 0.0003 | 0.17 | 0.0002 | 0.01 | 0.30 |
| Meat and meat products (g/day) | -0.02 | 0.20 | 0.00 | 0.78 | -0.01 | 0.58 | 0.01 | 0.01 |
| Potatoes and other tubers (g/day) | 0.04 | <.0001 | 0.05 | <.0001 | 0.05 | <.0001 | 0.01 | 0.16 |
| Vegetables (g/day) | 0.05 | 0.01 | 0.03 | 0.17 | 0.04 | 0.03 | -0.01 | 0.10 |
| Fruits, nuts, and seeds (g/day) | -0.02 | 0.21 | -0.02 | 0.32 | -0.02 | 0.25 | 0.002 | 0.78 |
| Olives (g/day) | -0.05 | <.0001 | -0.05 | <.0001 | -0.05 | <.0001 | 0.001 | 0.79 |
| Cereal and cereal products (g/day) | -0.02 | 0.51 | -0.02 | 0.47 | -0.02 | 0.51 | 0.00003 | 1.00 |
| Flour, flakes, starches, semolina (g/day) | -0.07 | <.0001 | -0.06 | <.0001 | -0.06 | <.0001 | 0.004 | 0.25 |
| Pasta, rice, other grains (g/day) | -0.02 | 0.02 | -0.03 | 0.01 | -0.02 | 0.01 | -0.002 | 0.48 |
| Bread, crisp bread and rusks (g/day) | -0.04 | 0.01 | -0.03 | 0.10 | -0.04 | 0.03 | 0.007 | 0.19 |
| Breakfast cereals (g/day) | 0.04 | <.0001 | 0.03 | <.0001 | 0.04 | <.0001 | -0.002 | 0.34 |
| Salty biscuits, aperitif biscuits, crackers (g/day) | 0.04 | <.0001 | 0.04 | <.0001 | 0.04 | <.0001 | -0.003 | 0.21 |
| Dry cakes, biscuits (g/day) | 0.02 | 0.0003 | 0.03 | <.0001 | 0.03 | <.0001 | 0.005 | 0.02 |
| Pastries (g/day) | -0.02 | 0.06 | -0.04 | 0.0001 | -0.03 | 0.004 | -0.01 | <.0001 |
| Cakes, biscuits (g/day) | 0.03 | 0.0003 | 0.04 | <.0001 | 0.03 | <.0001 | 0.004 | 0.14 |
| Chocolate, candy, paste, confetti (g/day) | 0.03 | <.0001 | 0.03 | <.0001 | 0.03 | <.0001 | 0.001 | 0.51 |
| Confectionery non-chocolate, candied fruits (g/day) | 0.02 | 0.004 | 0.03 | 0.004 | 0.02 | 0.003 | 0.002 | 0.41 |
| Snacks (g/day) | 0.05 | <.0001 | 0.06 | <.0001 | 0.05 | <.0001 | 0.002 | 0.35 |
| Olive oil (g/day) | -0.04 | <.0001 | -0.04 | <.0001 | -0.04 | <.0001 | 0.002 | 0.33 |
| Deep frying fats (g/day) | 0.03 | 0.04 | 0.03 | 0.03 | 0.03 | 0.03 | 0.004 | 0.36 |
| Tea (ml/day) | 0.02 | <.0001 | 0.02 | 0.0001 | 0.02 | <.0001 | -0.002 | 0.06 |
| Coffee (ml/day) | 0.02 | 0.001 | 0.02 | 0.01 | 0.02 | 0.002 | -0.0001 | 0.98 |
| Decaffeinated coffee (ml/day) | 0.02 | <.0001 | 0.03 | <.0001 | 0.03 | <.0001 | 0.002 | 0.24 |
| Energy intake (kcal/day) | 0.13 | 0.01 | 0.13 | 0.02 | 0.13 | 0.01 | -0.0001 | 0.99 |
HbAA, hemoglobin adducts of acrylamide, HbGA, hemoglobin adducts of glycidamide
All independent variables were log-transformed (log2) to improve normality.
Multiple linear regression analyses
Four multivariable linear regression analyses (all models included country, age at recruitment, date of blood donation, fasting status, and type of control) were performed for each of the four outcome variables with the aim to identify independent determinants of log2HbAA, log2HbGA, log2(HbAA+HbGA), and HbGA/HbAA (Table 3). Analyses stratified by alcohol intake and by BMI were performed, but no major differences were observed, thus, only overall results are presented.
Table 3. Multiple linear stepwise regression with β-estimates and standard errors for the association between dietary and lifestyle variables and log-transformed (log2) HbAA, HbGA, HbAA+HbGA, and HbGA/HbAA.
| Hemoglobin | Dietary and/or life style variables | β (SE) | P-value | Partial R2 | Model R2 |
|---|---|---|---|---|---|
| adducts | |||||
| Log2HbAA | Salty biscuits, aperitif biscuits, crackers | 0.03 (0.01) | 0.0001 | 0.01 | 0.3 |
| Dry cakes, biscuits | 0.02 (0.01) | 0.003 | 0.01 | ||
| Tea | -0.01 (0.005) | 0.02 | 0.01 | ||
| Vegetables | 0.05 (0.02) | 0.02 | 0.005 | ||
| BMI | -0.15 (0.07) | 0.05 | 0.004 | ||
| Deep frying fats | 0.04 (0.02) | 0.08 | 0.003 | ||
| Log2HbGA | Dry cakes, biscuits | 0.03 (0.01) | 0.0001 | 0.02 | 0.26 |
| Salty biscuits, aperitif biscuits, crackers | 0.03 (0.01) | 0.0002 | 0.01 | ||
| Alcohol at recruitment | -0.02 (0.01) | 0.01 | 0.01 | ||
| Deep frying fats | 0.07 (0.03) | 0.02 | 0.01 | ||
| Coffee | 0.01 (0.01) | 0.06 | 0.003 | ||
| Tea | -0.01 (0.01) | 0.07 | 0.003 | ||
| Log2 (HbAA+HbGA) | Salty biscuits, aperitif biscuits, crackers | 0.03 (0.01) | 0.0001 | 0.01 | 0.29 |
| Dry cakes, biscuits | 0.02 (0.01) | 0.0005 | 0.01 | ||
| Tea | -0.01 (0.005) | 0.02 | 0.005 | ||
| Deep frying fats | 0.05 (0.02) | 0.03 | 0.004 | ||
| Fiber | 0.07 (0.04) | 0.05 | 0.003 | ||
| Coffee | 0.01 (0.01) | 0.08 | 0.003 | ||
| HbGA/HbAA | BMI | 0.14 (0.03) | <0.0001 | 0.03 | 0.13 |
| Alcohol at recruitment | -0.01 (0.002) | 0.00002 | 0.02 | ||
| Education level | |||||
| None | reference | - | 0.006 | ||
| Primary school completed | -0.01 (0.02) | 0.73 | |||
| Technical/professional school | -0.02 (0.03) | 0.51 | |||
| Secondary school | -0.02 (0.03) | 0.61 | |||
| Higher education | -0.06 (0.03) | 0.05 | |||
| Not specified | 0.02 (0.05) | 0.70 | |||
| Missing | -0.04 (0.07) | 0.53 | |||
| Ever use of OCs | |||||
| No | reference | - | |||
| Yes | -0.02 (0.01) | 0.12 | 0.003 | ||
| Missing | 0.04 (0.06) | 0.53 | |||
| Physical activity | |||||
| Inactive | reference | - | 0.003 | ||
| Moderately inactive | 0.02 (0.02) | 0.28 | |||
| Moderately active | -0.02 (0.02) | 0.29 | |||
| Active | 0.01 (0.02) | 0.77 | |||
| Missing | -0.03 (0.09) | 0.74 | |||
| Ever use of HRT | |||||
| No | reference | - | |||
| Yes | 0.004 (0.02) | 0.81 | 0.002 | ||
| Missing | -0.05 (0.04) | 0.22 | |||
| Diabetes | |||||
| No | reference | - | |||
| Yes | -0.02 (0.03) | 0.47 | 0.001 | ||
| Missing | -0.03 (0.07) | 0.64 | |||
SE, standard error; HbAA, hemoglobin adducts of acrylamide; HbGA, hemoglobin adducts of glycidamide; BMI, body mass index (kg/m2); HRT, hormonal replacement therapy; OC, oral contraceptive.
All models are adjusted for country, age at recruitment (y), date of blood donation, fasting status (no, in between, yes), and type of control (endometrial, ovarian control). All independent continuous variables were log-transformed (log2) to improve normality.
The HbAA model explained 30% of the variation in HbAA levels, and the food groups ‘salty biscuits, aperitif biscuits, crackers’, ‘dry cakes, biscuits’, and ‘vegetables’ were statistically significant positively associated with log2HbAA values, whereas ‘tea’ was inversely associated .
The HbGA model explained 26% of the variation of HbGA levels, and the following groups were statistically significantly associated with HbGA-log levels: ‘salty biscuits, aperitif biscuits, crackers’, ‘dry cakes, biscuits’, and ‘deep frying fats’. Alcohol intake was inversely associated with log2HbGA values.
The HbAA+HbGA model explained 29% of the variation of the sum of total adducts levels, and only ‘salty biscuits, aperitif biscuits, crackers’, ‘dry cakes, biscuits’, and ‘deep frying fats’ were significantly associated with the sum of total adducts levels.
The HbGA/HbAA model explained 13% of the variation in HbGA/HbAA ratio, and BMI was positively associated, whereas alcohol consumption at recruitment was inversely associated with the HbGA/HbAA ratio. No other lifestyle variable explained variation in the ratio.
Multiple linear regression analyses were also performed by European region (data not shown). The three different models from the northern countries explained a higher variation in HbAA, HbGA, and HbAA+HbGA levels than the southern countries; however, the food groups identified as dietary determinants of Hb-adducts levels by European region were, in general, similar to those presented in table 3.
Discussion
The present study was carried out with the aim to identify independent determinants of HbAA, HbGA, HbAA+HbGA, and HbGA/HbAA. We investigated these associations in a cross-sectional study of 801 non-smoking postmenopausal women from the EPIC cohort. The most important determinants of HbAA, HbGA, and HbAA+HbGA adduct levels were ‘salty biscuits, aperitif biscuits, and crackers’, and ‘dry cakes and biscuits’; whereas alcohol intake and BMI (inversely and positively associated, respectively) were identified as the two main determinants of HbGA/HbAA ratio.
To our knowledge, there are only three published studies that evaluated the relation between specific food group determinants and Hb-adducts of acrylamide and glycidamide, and all of them obtained dietary information through DQs [16–18]. The first study was based on a Norwegian population, and included 19 men and 31 women (n=50) of which 14% of the subjects were smokers. The second study comprised 296 female, non-smoking, pre- and postmenopausal women from the Nurses’ Health Study II (NHS-II). The Danish study, similar to the present study, was based on postmenopausal women who reported being non-smokers at baseline (n=537).
The current study had the highest estimated intake of acrylamide compared to the Norwegian and the NHS-II studies. The Norwegian study reported a median acrylamide intake of 12.8 µg/day among non-smoking women (in EPIC, 20.3 µg/day), and the NHS-II study reported a mean energy-adjusted intake of 19.3 µg/day (in EPIC, 21.9 µg/day). The Danish study did not report information on overall dietary acrylamide intake.
Blood samples from both EPIC and NHS-II studies were measured in the same laboratory using the same protocol. Likewise, the Norwegian and the Danish studies shared the same methodology [16, 17]. The median adduct levels for HbAA and HbGA in the present study (41.3 and 34.2 pmol/g of Hb, respectively) were higher than the values reported in the Norwegian (36.8 and 18.2 pmol/g of Hb), and the Danish (35 and 21pmol/g of Hb) studies; however, the NHS-II study reported the highest values of Hb-adducts (43.9 and 49.4 pmol/g of Hb). The NHS-II study also had the highest median values of HbGA/HbAA (1.10), compared to the Norwegian (0.49), and the present (0.8) study.
The correlation between estimated acrylamide intake (based on DQs), and Hb-adducts of acrylamide and glycidamide was low (ranging from 0.08 to 0.43) in most studies, including EPIC [15, 16, 32–34]. The NHS-II study reported moderate correlations between acrylamide intake and HbAA or HbGA (0.19-0.35). These differences could be due to errors in dietary assessment methods, and to incomplete data on acrylamide content in food composition databases. The NHS-II acrylamide database was mainly based on values from the FDA’s Exploratory Analysis of Acrylamide in Foods, but specific foods that were frequently consumed in the study were further analyzed, such as different brands of breakfast cereals, dried food, bread, and potatoes chips among others [18].
The main food group determinants of Hb-adducts (HbAA, HbGA, and HbAA+HbGA) in the present study were ‘salty biscuits, aperitif biscuits, and crackers’, and ‘dry cakes, and biscuits’; whereas Freisling et al. reported that ‘bread, crisp bread, and rusks’, ‘coffee’, and ‘potatoes’, were the main determinants of dietary acrylamide intake (based on a single 24 hDR) in a different subgroup of EPIC men and women [5]. Coffee was selected in the stepwise procedure as one of the food determinants of HbGA, and HbAA+HbGA in the present study, but was not statistically significant. Inverse associations between ‘tea’ and Hb-adduct levels (HbAA, HbGA, and HbAA+HbGA) were observed in the present study. This result may be explained by the possible effect of tea polyphenols, which have been observed to decrease HbAA levels in animals [35]. The variable ‘vegetables’ was selected as a determinant of log2HbAA; however, this result might has been confounded by other acrylamide containing foods, since ‘vegetables’ includes all forms of cooking methods (including frying and baking). The food groups ‘bread, crisp bread, rusks’, and ‘potatoes’ were not selected in any of the models presented in this study; however the possible effect of ‘potatoes’, especially fried potatoes, might have been represented by the variable ‘deep frying fats’. It is worth noting that the present study was evaluating adducts levels, whereas the Friesling et al. study was evaluating acrylamide intake based on 24 hDR. Further, differences in the results of these two EPIC analyses may reflect differences in the subpopulation studied (e.g, age distribution, sex, menopausal status).
The Norwegian study identified as dietary determinants of HbAA levels ‘potatoes’, ‘chips/snacks’, ‘crisp bread’, and ‘jam/preservatives. Furthermore, ‘chips/snacks’, and ‘crisp bread’ were also recognized as determinants of HbAA+HbGA. Similar to EPIC, the NHS-II study also identified dietary determinants of HbAA, HbGA, and HbAA+HbGA; but the food groups were different from the determinants observed in the present study. The Danish study reported ‘coffee’ to be associated with HbAA and HbGA, ‘chips’ to HbAA, and ‘biscuits/crackers’ to HbGA levels. Dietary habits are different between the US and European countries, so direct comparison of food groups as determinants of HbAA levels may not be possible.
The proportion of response variation (R2) explained by food groups and/or lifestyle variables in the present study varied from 13% in the HbGA/HbAA model to 30% for HbAA. The Danish study obtained a response variation of 17% and 12% in the HbAA and HbGA models respectively; however the highest response variations explained were obtained in the Norwegian study (48% in the HbAA, and 37% in the HbAA+HbGA model) [16, 17]. The NHS-II study did not report this information [18].
The main difference between our study and the other three published studies is that prior analyses only included dietary variables that were suspected to be sources of acrylamide intake. The current study included both known sources of dietary acrylamide together with lifestyle variables, such as alcohol intake and BMI, with the intention to better describe the independent determinants of Hb-adducts and their ratio in non-smoking individuals. Diet is complex, and interactions between acrylamide and food ingredients are possible (i.e, acrylamide uptake in humans has been hypothesized to be impaired by a diet rich in proteins) [26]. Likewise, it has been suggested that alcohol intake and BMI may influence the activity of Cyp2e1, the enzyme complex that metabolizes acrylamide to glycidamide. This enzyme is involved in alcohol metabolism mainly when alcohol concentrations in the blood are high. It has been hypothesized that alcohol may compete with acrylamide as a substrate [36]. This could partially explain the results observed in the present study; in which higher alcohol intake was inversely associated with HbGA and HbGA/HbAA, as has been reported in other studies [14, 36]. The mechanism by which BMI may influence acrylamide metabolism is still unclear, but similar to the present study, other studies have observed that BMI was positively associated with the ratio HbGA/HbAA, and negatively associated with HbAA [14, 37, 38]. Recently, statistically significant differences in the ratio of HbGA/HbAA between vegetarians and non-vegetarians have been reported, suggesting that dietary factors may also contribute to acrylamide/glycidamide metabolism [39].
The present study had the largest sample size compared to the previous published papers, and the laboratory methods used to quantify both acrylamide and glycidamide hemoglobin adducts were standardized and followed rigorous quality assurance/quality control [12]. Tobacco is one of the most important sources of acrylamide, and it is well-established that cigarette smoke influences Hb-adducts levels (smokers have mean HbAA levels at least three to four times higher than non-smokers) [11, 14]. Moreover, it has been hypothesized that acrylamide may influence hormonal homeostasis [40–42]. Thus, the present study was designed to reduce confounding by these factors, and only those women who reported being postmenopausal and never smokers were included in the study. Further, limitations derived from using estimates of dietary acrylamide intake, as described above, were avoided in the present analysis of food groups and biomarkers levels.
There are some limitations that should be acknowledged: a) Hb-adducts of acrylamide and glycidamide are valid biomarkers for acrylamide exposure and internal dose; however, these adducts reflect levels of acrylamide and glycidamide during the average lifespan of erythrocytes, which is around 120 days [1, 10], and all dietary and lifestyle variables in EPIC were assessed through DQs that referred to the previous year before recruitment [19]. b) Cooking methodology, which influences acrylamide levels in food, was not recorded in some EPIC centers; however, relevant information on food preparation was available for potatoes (except in Italy), bread, and breaded meats [15, 24]. c) Only one baseline blood sample was collected for each participant. d) Acrylamide content in foods may vary seasonally [43], which was not accounted for in our analyses; nevertheless, all models were adjusted for date of blood extraction to minimize this effect. (e) Ferrari et al. described variations in dietary patterns across EPIC countries [15], and this may have influenced Hb-adducts levels and our prediction models, although all models included country to adjust for country-level effects (i.e. questionnaire design, dietary habits). f) SHS could not be evaluated in our statistical models due to the large number of missing values (>50%); however, in a subset of women from the current study, no statistically significant differences in Hb-adduct levels were observed between women who were exposed to SHS and who were not exposed to SHS.
To conclude, dietary food group and lifestyle variables explain a moderate proportion of HbAA and HbGA adduct level variation in 801 postmenopausal non-smoking women in the EPIC cohort. The main food groups determinants of HbAA, HbGA, and HbAA+HbGA were ‘Salty biscuits, aperitif biscuits, and crackers’, and ‘dry cakes, and biscuits’. Alcohol intake and BMI were identified as the principal determinants for the ratio HbGA/HbAA levels. In this regard, future studies assessing associations between acrylamide and disease risk should take into consideration the use of both biomarkers of acrylamide exposure (HbAA and HbGA), in addition to alcohol intake and BMI.
Supplementary Material
Acknowledgments
This work was supported by the Wereld Kanker Onderzoek Fonds (WCRF NL) [grant WCRF 2011/442] and by the Health Research Fund (FIS) of the Spanish Ministry of Health [Exp PI11/01473]. The coordination of EPIC is financially supported by the European Commission (DG-SANCO) and the International Agency for Research on Cancer. The national cohorts are supported by the Health Research Fund (FIS) of the Spanish Ministry of Health, Regional Governments of Andalucía, Asturias, Basque Country, Murcia [no. 6236], Navarra and the Catalan Institute of Oncology, La Caixa [BM 06-130], Red Temática de Investigación Cooperativa en Cáncer [RD12/0036/0018; RD06/0020/0091] (Spain); Danish Cancer Society (Denmark); Ligue contre le Cancer, Institut Gustave Roussy, Mutuelle Générale de l’Education Nationale, Institut National de la Santé et de la Recherche Médicale (INSERM) (France); Deutsche Krebshilfe, Deutsches Krebsforschungszentrum (DKFZ) and Federal Ministry of Education and Research (Germany); the Hellenic Health Foundation (Greece); Associazione Italiana per la Ricerca sul Cancro (AIRC) and National Research Council (Italy); Dutch Ministry of Public Health, Welfare and Sports (VWS), Netherlands Cancer Registry (NKR), LK Research Funds, Dutch Prevention Funds, Dutch ZON (Zorg Onderzoek Nederland), World Cancer Research Fund (WCRF) and Statistics Netherlands (The Netherlands); Nordic Center of Excellence in Food, Nutrition and Health -Helga (Norway); Swedish Cancer Society, Swedish Scientific Council and Regional Government of Skåne and Västerbotten (Sweden); Cancer Research UK, Medical Research Council (United Kingdom). MO-S is affiliated with the University of Barcelona.
Footnotes
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Friedman M. Chemistry, biochemistry, and safety of acrylamide. A review J AgricFood Chem. 2003;51:4504–4526. doi: 10.1021/jf030204+. [DOI] [PubMed] [Google Scholar]
- 2.Tareke E, Rydberg P, Karlsson P, et al. Analysis of acrylamide, a carcinogen formed in heated foodstuffs. J AgricFood Chem. 2002;50:4998–5006. doi: 10.1021/jf020302f. [DOI] [PubMed] [Google Scholar]
- 3.Becalski A, Brady B, Feng S, et al. Formation of acrylamide at temperatures lower than 100°C: the case of prunes and a model study. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2011;28:726–730. doi: 10.1080/19440049.2010.535217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu Y, Cui B, Ran R, et al. Risk assessment, formation, and mitigation of dietary acrylamide: Current status and future prospects. Food Chem Toxicol. 2014;69C:1–12. doi: 10.1016/j.fct.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 5.Freisling H, Moskal A, Ferrari P, et al. Dietary acrylamide intake of adults in the European Prospective Investigation into Cancer and Nutrition differs greatly according to geographical region. EurJNutr. 2013;52:1369–1380. doi: 10.1007/s00394-012-0446-x. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization. Evaluation of certain food additives and contaminants. World Health Organ Tech Rep Ser. 2013:1–75. back cover. [PubMed] [Google Scholar]
- 7.Zödl B, Schmid D, Wassler G, et al. Intestinal transport and metabolism of acrylamide. Toxicology. 2007;232:99–108. doi: 10.1016/j.tox.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 8.LoPachin RM, Gavin T. Acrylamide-induced nerve terminal damage: relevance to neurotoxic and neurodegenerative mechanisms. JAgricFood Chem. 2008;56:5994–6003. doi: 10.1021/jf703745t. [DOI] [PubMed] [Google Scholar]
- 9.IARC. IARC working group on the evaluation of carcinogenic risks to humans: some industrial chemicals. Lyon, 15-22 February 1994. IARC Monogr EvalCarcinogRisks Hum. 1994;60:1–560. [PMC free article] [PubMed] [Google Scholar]
- 10.Fennell TR, Sumner SC, Walker VE. A model for the formation and removal of hemoglobin adducts. Cancer Epidemiol Biomarkers Prev. 1992;1:213–219. [PubMed] [Google Scholar]
- 11.Bergmark E. Hemoglobin adducts of acrylamide and acrylonitrile in laboratory workers, smokers and nonsmokers. ChemRes Toxicol. 1997;10:78–84. doi: 10.1021/tx960113p. [DOI] [PubMed] [Google Scholar]
- 12.Vesper HW, Bernert JT, Ospina M, et al. Assessment of the relation between biomarkers for smoking and biomarkers for acrylamide exposure in humans. Cancer Epidemiol Biomarkers Prev. 2007;16:2471–2478. doi: 10.1158/1055-9965.EPI-06-1058. [DOI] [PubMed] [Google Scholar]
- 13.EFSA CONTAM Panel (EFSA Panel on Contaminants in the Food Chain) Scientific Opinion on acrylamide in food. EFSA Journal. 2015;13(6):4104. doi: 10.2903/j.efsa.2015.4104. [DOI] [Google Scholar]
- 14.Vesper HW, Slimani N, Hallmans G, et al. Cross-sectional study on acrylamide hemoglobin adducts in subpopulations from the European Prospective Investigation into Cancer and Nutrition (EPIC) Study. JAgricFood Chem. 2008;56:6046–6053. doi: 10.1021/jf703750t. [DOI] [PubMed] [Google Scholar]
- 15.Ferrari P, Freisling H, Duell EJ, et al. Challenges in estimating the validity of dietary acrylamide measurements. Eur J Nutr. 2013;52:1503–1512. doi: 10.1007/s00394-012-0457-7. [DOI] [PubMed] [Google Scholar]
- 16.Bjellaas T, Olesen PT, Frandsen H, et al. Comparison of estimated dietary intake of acrylamide with hemoglobin adducts of acrylamide and glycidamide. ToxicolSci. 2007;98:110–117. doi: 10.1093/toxsci/kfm091. [DOI] [PubMed] [Google Scholar]
- 17.Outzen M, Egeberg R, Dragsted L, et al. Dietary determinants for Hb-acrylamide and Hb-glycidamide adducts in Danish non-smoking women. Br J Nutr. 2011;105:1381–1387. doi: 10.1017/S0007114510005003. [DOI] [PubMed] [Google Scholar]
- 18.Wilson KM, Vesper HW, Tocco P, et al. Validation of a food frequency questionnaire measurement of dietary acrylamide intake using hemoglobin adducts of acrylamide and glycidamide. Cancer Causes Control. 2009;20:269–278. doi: 10.1007/s10552-008-9241-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Riboli E, Hunt KJ, Slimani N, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5:1113–1124. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 20.Obón-Santacana M, Freisling H, Peeters PH, et al. Acrylamide and glycidamide hemoglobin adduct levels and endometrial cancer risk: A nested case-control study in nonsmoking postmenopausal women from the EPIC cohort. Int J Cancer. 2015 doi: 10.1002/ijc.29853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Obón-Santacana M, Lujan-Barroso L, Travis RC, et al. Acrylamide and glycidamide hemoglobin adducts and epithelial ovarian cancer: a nested case-control study in non-smoking postmenopausal women from the EPIC cohort. Cancer Epidemiol Biomarkers Prev. 2015 doi: 10.1158/1055-9965.EPI-15-0822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cust AE, Kaaks R, Friedenreich C, et al. Metabolic syndrome, plasma lipid, lipoprotein and glucose levels, and endometrial cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) Endocr Relat Cancer. 2007;14:755–767. doi: 10.1677/ERC-07-0132. [DOI] [PubMed] [Google Scholar]
- 23.Peeters PH, Lukanova A, Allen N, et al. Serum IGF-I, its major binding protein (IGFBP-3) and epithelial ovarian cancer risk: the European Prospective Investigation into Cancer and Nutrition (EPIC) EndocrRelat Cancer. 2007;14:81–90. doi: 10.1677/erc.1.01264. [DOI] [PubMed] [Google Scholar]
- 24.Obon-Santacana M, Slimani N, Lujan-Barroso L, et al. Dietary intake of acrylamide and pancreatic cancer risk in the European Prospective Investigation into Cancer and Nutrition (EPIC) cohort. AnnOncol. 2013;24:2645–2651. doi: 10.1093/annonc/mdt255. [DOI] [PubMed] [Google Scholar]
- 25.Vesper HW, Ospina M, Meyers T, et al. Automated method for measuring globin adducts of acrylamide and glycidamide at optimized Edman reaction conditions. Rapid CommunMass Spectrom. 2006;20:959–964. doi: 10.1002/rcm.2396. [DOI] [PubMed] [Google Scholar]
- 26.Schabacker J, Schwend T, Wink M. Reduction of acrylamide uptake by dietary proteins in a caco-2 gut model. J Agric Food Chem. 2004;52:4021–4025. doi: 10.1021/jf035238w. [DOI] [PubMed] [Google Scholar]
- 27.Wilson KM, Balter K, Adami HO, et al. Acrylamide exposure measured by food frequency questionnaire and hemoglobin adduct levels and prostate cancer risk in the Cancer of the Prostate in Sweden Study. Int J Cancer. 2009;124:2384–2390. doi: 10.1002/ijc.24175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willett W. Nutritional epidemiology. Oxford University Press; 2012. [Google Scholar]
- 29.Akaike H. A new look at the statistical model identification. Automatic Control, IEEE Transactions on. 1974;19:716–723. [Google Scholar]
- 30.Wareham NJ, Jakes RW, Rennie KL, et al. Validity and repeatability of a simple index derived from the short physical activity questionnaire used in the European Prospective Investigation into Cancer and Nutrition (EPIC) study. Public Health Nutr. 2003;6:407–413. doi: 10.1079/PHN2002439. [DOI] [PubMed] [Google Scholar]
- 31.McGraw KO, Wong SP. Forming inferences about some intraclass correlation coefficients. Psychological methods. 1996;1:30. [Google Scholar]
- 32.Kütting B, Uter W, Drexler H. The association between self-reported acrylamide intake and hemoglobin adducts as biomarkers of exposure. Cancer Causes Control. 2008;19:273–281. doi: 10.1007/s10552-007-9090-9. [DOI] [PubMed] [Google Scholar]
- 33.Tran NL, Barraj LM, Murphy MM, Bi X. Dietary acrylamide exposure and hemoglobin adducts--National Health and Nutrition Examination Survey (2003-04) Food Chem Toxicol. 2010;48:3098–3108. doi: 10.1016/j.fct.2010.08.003. [DOI] [PubMed] [Google Scholar]
- 34.Wirfalt E, Paulsson B, Tornqvist M, et al. Associations between estimated acrylamide intakes, and hemoglobin AA adducts in a sample from the Malmo Diet and Cancer cohort. EurJ ClinNutr. 2008;62:314–323. doi: 10.1038/sj.ejcn.1602704. [DOI] [PubMed] [Google Scholar]
- 35.Xie Q, Liu Y, Sun H, et al. Inhibition of acrylamide toxicity in mice by three dietary constituents. J Agric Food Chem. 2008;56:6054–6060. doi: 10.1021/jf0730542. [DOI] [PubMed] [Google Scholar]
- 36.Vikstrom AC, Wilson KM, Paulsson B, et al. Alcohol influence on acrylamide to glycidamide metabolism assessed with hemoglobin-adducts and questionnaire data. Food ChemToxicol. 2010;48:820–824. doi: 10.1016/j.fct.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 37.Huang Y-F, Chen M-L, Liou S-H, et al. Association of CYP2E1, GST and mEH genetic polymorphisms with urinary acrylamide metabolites in workers exposed to acrylamide. Toxicol Lett. 2011;203:118–126. doi: 10.1016/j.toxlet.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 38.Vesper HW, Sternberg MR, Frame T, Pfeiffer CM. Among 10 sociodemographic and lifestyle variables, smoking is strongly associated with biomarkers of acrylamide exposure in a representative sample of the U.S. Population. J Nutr. 2013;143:995S–1000S. doi: 10.3945/jn.112.173013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kotova N, Frostne C, Abramsson-Zetterberg L, et al. Differences in micronucleus frequency and acrylamide adduct levels with hemoglobin between vegetarians and non-vegetarians. Eur J Nutr. 2014 doi: 10.1007/s00394-014-0796-7. [DOI] [PubMed] [Google Scholar]
- 40.Hogervorst JG, Baars BJ, Schouten LJ, et al. The carcinogenicity of dietary acrylamide intake: a comparative discussion of epidemiological and experimental animal research. Crit RevToxicol. 2010;40:485–512. doi: 10.3109/10408440903524254. [DOI] [PubMed] [Google Scholar]
- 41.Hogervorst JG, Fortner RT, Mucci LA, et al. Associations between Dietary Acrylamide Intake and Plasma Sex Hormone Levels. Cancer Epidemiol Biomarkers Prev. 2013;22:2024–2036. doi: 10.1158/1055-9965.EPI-13-0509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nagata C, Konishi K, Tamura T, et al. Associations of acrylamide intake with circulating levels of sex hormones and prolactin in premenopausal Japanese women. Cancer Epidemiol Biomarkers Prev. 2015;24:249–254. doi: 10.1158/1055-9965.EPI-14-0935. [DOI] [PubMed] [Google Scholar]
- 43.Powers SJ, Mottram DS, Curtis A, Halford NG. Acrylamide concentrations in potato crisps in Europe from 2002 to 2011. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2013;30:1493–1500. doi: 10.1080/19440049.2013.805439. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.