Abstract
Preventive chemotherapy (PC), the large-scale distribution of anthelminthic drugs to population groups at risk, is the core intervention recommended by the WHO for reducing morbidity and transmission of the four main helminth infections, namely lymphatic filariasis, onchocerciasis, schistosomiasis and soil-transmitted helminthiasis. The strategy is widely implemented worldwide but its general theoretical foundations have not been described so far in a comprehensive and cohesive manner. Starting from the information available on the biological and epidemiological characteristics of helminth infections, as well as from the experience generated by disease control and elimination interventions across the world, we extrapolate the fundamentals and synthesise the principles that regulate PC and justify its implementation as a sound and essential public health intervention. The outline of the theoretical aspects of PC contributes to a thorough understanding of the different facets of this strategy and helps comprehend opportunities and limits of control and elimination interventions directed against helminth infections.
Keywords: Preventive chemotherapy, Disease control, Helminth infection
1. Introduction
The focus of the public health strategy against helminth infections has shifted over the years from measures targeting extrahuman stages of the life cycle of the worms, such as vector control or environmental sanitation, to measures targeting the human host, and specifically treatment with chemotherapeutics at regular intervals. In 2006, the existing disease-specific recommendations developed by the WHO and its partners on large-scale treatment of individuals were aggregated and combined into a single strategy, denominated preventive chemotherapy (PC).1 PC, which does not represent a new concept but rather a novel integrated approach to a well established series of interventions, is nowadays implemented on a worldwide scale and is gradually becoming a ‘routine’ intervention in a number of countries. In 2008, over 680 million individuals living in endemic areas received anthelminthic drugs against at least one of the four target infections.2 The aim of this paper was to examine and illustrate the multiple facets of PC in a comprehensive manner.
2. Definition and characteristics of preventive chemotherapy
PC is ‘the use of anthelminthic drugs, either alone or in combination, as a public health tool against helminth infections’1 and is the key public health strategy recommended by the WHO to reduce morbidity and transmission of lymphatic filariasis (LF), onchocerciasis, schistosomiasis and soil-transmitted helminthiasis (STH).1 Three key characteristics define PC as a public health intervention:
-
1
population-based diagnosis;
-
2
population-based treatment; and
-
3
implementation at regular intervals.
2.1. Population-based diagnosis
Population-based diagnosis consists of assessing the significance of a helminth infection in a population through surveys applied to a sample of its individuals. Appropriate diagnostic tests, or standard questionnaires screening for pathognomonic symptoms or signs or for behaviours associated with risk of infection, can be used alternatively. Population-based diagnosis can also be carried out retrospectively by analysing existing epidemiological data. Based on its results, an appropriate intervention is selected. Population-based diagnosis distinguishes PC from the clinical approach in which diagnosis is performed at the individual level prior to treatment.
2.2. Population-based treatment
In PC, administration of anthelminthic drugs is not the outcome of a personalised, case management treatment approach performed by specialised personnel on individuals reporting to health facilities. It rather entails active finding of population groups at risk, large-scale delivery of single-administration medicines by non-medical personnel (teachers, volunteers or community drug distributors) and the use of non-medical settings as advanced outposts and entry points to target population groups.
2.3. Implementation at regular intervals
PC is implemented at regular intervals of time; the appropriate interval of re-treatment (i.e. the period between two consecutive PC interventions) is based on the epidemiological characteristics of the disease as measured by the population-based diagnosis; the intervention is repeated without the need for further diagnostic interventions,1 although implementation of a monitoring system is recommended.
3. Criteria of eligibility for preventive chemotherapy
The following four characteristics strongly suggest considering a helminth infection eligible for PC; they are linked to biological features of the target diseases as well as to operational aspects of the interventions directed against them: 3,4
late or unclear onset of the clinical symptomatology;
slow increase in the likelihood of an infected host to develop morbidity and to transmit infection;
high efficacy, safety and easiness of treatment procedures; and
low cost of the PC intervention (diagnostics, drugs, operational costs).
3.1. Late or unclear onset of the clinical symptomatology
Individuals suffering from infections characterised by mild and/or non-specific symptoms in their initial stages are frequently unaware of their condition and do not seek treatment5. The consequence is that the associated morbidity can progress without impediment into advanced stages that may no longer be reversible by treatment. An intervention actively distributing drugs, such as PC, is therefore necessary to address the problem.
3.2. Slow increase in the likelihood of an infected host to develop morbidity and to transmit infection
Such slow evolution of the disease entails that no immediate medical action is required and that the PC’s spaced treatment intervals are sufficient to reduce the likelihood of developing morbidity and transmitting the infection.
3.3. High efficacy, safety and easiness of treatment procedures
The efficacy of the drugs used allows for spaced interventions. The other characteristics are necessary to ensure that population-based treatment in non-medical settings—and without direct medical supervision—is implemented with high compliance rates and without unnecessary risks.
3.4. Low cost of the preventive chemotherapy intervention (diagnostics, drugs, operational costs)
The overall low cost of the resources employed ensures the feasibility of PC on a large scale and in resource-poor settings, and therefore its viability as a public health measure. The cost effectiveness of PC should ideally be higher than that of individual case management.
4. Modalities of implementation of preventive chemotherapy
There are three modalities by which PC interventions can be implemented. The relevant terminology was originally developed for STH but is now applied to all PC-eligible helminth infections:1,6,7
mass drug administration (MDA): when the entire population of an area (e.g. state, region, province, district, subdistrict, village) is administered anthelminthic drugs at regular intervals, irrespective of the individual infection status;
targeted chemotherapy: when specific risk groups in the population, defined by age, sex or other social characteristics such as the profession (e.g. school-age children, fishermen), are administered anthelminthic drugs at regular intervals, irrespective of the individual infection status; and
selective chemotherapy: when, as a result of a regular screening exercise in a population group living in an endemic area, all individuals found (or suspect) to be infected are administered anthelminthic drugs.
Selective chemotherapy is the intervention that most resembles clinical practice, as it involves individual-level diagnosis; however, the large-scale, population-based application of individual screening followed by treatment of positive cases at regular intervals fulfils the characteristics mentioned in the definition of PC.
5. Criteria for selection of the most appropriate modality of implementation
Selection of the most appropriate PC intervention against an eligible helminth infection depends on a number of factors that are linked to the epidemiological characteristics of that infection:
demographic characteristics of infection and disease;
proportion of infected individuals in the target area (prevalence of infection); and
chances of achieving elimination of transmission.
5.1. Demographic characteristics of infection and disease
If a given infection (as a consequence of more frequent exposure to infective stages of the parasite) or its associated morbidity (as a consequence of higher vulnerability), is uniformly widespread throughout a population, MDA or selective chemotherapy can both be considered appropriate choices; if on the contrary its burden is strongly associated with a given demographic group (defined by age, gender or other characteristics such as type of work), then the PC intervention can be focused on that high-risk group (targeted chemotherapy).
5.2. Proportion of infected individuals in the target area (prevalence of infection)
More comprehensive modalities of implementation, such as MDA, are appropriate for settings in which the prevalence of infection is high; more conservative ones, such as selective chemotherapy, are rather indicated as the prevalence of infection diminishes.
5.3. Chances of achieving elimination of transmission
The more a transmission cycle is fragile or an infection’s transmission rates are low, i.e. the more the infection has a low efficiency of transmission (see below), the higher are the chances of eliminating (i.e. interrupting) transmission through PC alone. In this case, ensuring treatment of all infected individuals with the aim of neutralising all possible sources of transmission is the key objective to be achieved, and MDA naturally appears as the most appropriate choice.
6. Morbidity in helminth infections
The presence of a helminth within a human body (infection) is not necessarily associated with morbidity (disease).8,9 Morbidity results from the sum of the damage produced by each single worm infecting an individual. When the number of worms is small, the damage they cause is counterbalanced by the human host, and morbidity does not develop until a certain threshold of intensity of infection is reached, above which no compensation is possible: conventionally, this is defined as the threshold of high intensity. When such a threshold is exceeded, the burden of morbidity is positively correlated with the number of worms: if such a number just exceeds the threshold, the damage produced by the worms will be limited, but if their number is significant, as in the case of continuing re-infection episodes, morbidity will be substantial. The natural history of morbidity in helminth infections is such that an infection initially produces pathological changes associated with acute inflammation; however, if the cause of inflammation (i.e. the presence of the worm) persists (as in the case of lack of treatment), such inflammation will progress into chronic stages and will be associated with a number of negative outcomes, most notably the development of fibrosis. Anthelminthic treatment, and consequently PC, acts by killing the worms harboured in the human body: as such, it will arrest the inflammation process sustained by the presence of the worms in the human tissues. If inflammation is still in its acute stage at the time of treatment, the associated morbidity will be largely reversible; however, if inflammation has already progressed into a chronic stage, the existing morbidity will gradually be less and less reversible, up to a stage when tissue destruction, cellular necrosis and diffuse fibrosis determine sequelae that are irreversible and permanent. These sequelae are frequently macroscopic and detectable by clinical examination or imaging techniques such as ultrasound; they can be responsible for disability and disfigurement that are likely to have a significant impact on the quality of life of affected individuals.10 Severe sequelae are frequent in patients who have been repeatedly exposed to re-infection episodes and have therefore progressively increased the number of worms within their body and maintained it above the threshold of high intensity for long periods of time. As a result of such continuing re-infection episodes, these individuals are constantly and concomitantly affected by different time stages of inflammation and morbidity that will inexorably lead to the development of sequelae. Paradoxically, however, sequelae can be detected in individuals who no longer harbour worms and are therefore no longer infected. This happens when the last exposure to the infectious agent has occurred several years before, with the consequence that infecting worms have all died at time of detection.
7. Transmission in helminth infections
A given worm population circulating in a geographical area tends to reach an equilibrium such that the number of worms generated in that area is equivalent to the number of worms that die.6 The number of circulating worms at equilibrium is dependent on a series of epidemiological factors that can be grouped into two main categories:
biological factors that are the product of the specific characteristics of each infection and that determine the overall basic reproductive rate (R0) of that worm population;6,9 and
environmental factors that can modulate the biological factors.
Both biological and environmental factors can be combined so as to determine the efficiency of transmission (EoT) of an infection. EoT is a dimensionless parameter somewhat comparable with the force of infection (λ): whilst the latter quantifies transmissibility from a human point of view (i.e. the risk of acquiring a given infection), the former does so from a disease point of view, thus expressing the capability of a given infection to perpetuate itself in a given environment. Whilst biological factors are intrinsic to each type of infection and are therefore constant, environmental factors are susceptible to variation and can determine a change of EoT (ΔEoT). Pharmacological pressure exerted by chemotherapeutic agents can be considered one of the environmental factors capable of affecting the EoT of a helminth infection. Any change in EoT is associated with an increase or decrease in the number of worms circulating in that area. For diseases in which humans host the parasite, this higher or lower number of circulating worms can be measured in terms of prevalence or mean intensity of infection in the human host population living in that area. In summary, EoT of a given infection in a given area at a given point in time corresponds to a given number of worms circulating in that area, which can be quantified as a level of prevalence or mean intensity of infection in the human population living in that area. The latter indicators can thus be considered as proxy measures of EoT.
8. Outcomes of preventive chemotherapy
PC prevents progression of the disease to its more advanced stages; in addition, although taking anthelminthic drugs does not confer immunity against infection and does not directly protect the recipients from acquiring new infections, it reduces the chances that such individuals are a source of infection for the community in which they live. PC is therefore both a secondary and a primary prevention measure, whose two respective immediate outcomes are:
reduction of morbidity among treated individuals; and
reduction of transmission in the target area.
Two additional, possible longer-term outcomes, respectively related and consequential to the former two, are then:
-
3.
elimination of morbidity in the target area; and
-
4.
elimination of transmission in the target area.
Both the additional outcomes are used variously (and somewhat inconsistently among different diseases) as criteria for the achievement of ‘elimination as a public health problem’, a term of frequent usage in public health11.
8.1. Reduction of morbidity
Administration of anthelminthic drugs leads to a reduction in the number of worms (adults and/or larvae) harboured by the human host;12–17 because of the fact that such a number is positively correlated with morbidity, the outcome produced by PC in a treated individual is a reduction of existing reversible morbidity.1,4,5 If the number of worms infecting an individual is decreased below the threshold of high intensity, reversible morbidity will be fully reverted and progression of such morbidity into more advanced stages will be fully prevented. If such a status is continuously maintained over time, the individual will be ‘protected’ from morbidity or, in other words, ‘control of morbidity’ will be achieved in such an individual5,11. Administration of anthelminthic drugs at regular intervals has in fact the specific purpose of keeping the intensity of infection below such a threshold despite re-infection episodes. To control morbidity, there is therefore no need to kill all the worms harboured by the target individual, but just to keep their number below the threshold over time. This is achieved by ensuring that the interval of re-treatment is shorter than the time necessary for a treated individual to reach again, through re-infection episodes, the threshold: intervals of re-treatment between 6 months and 12 months are the most widely used compromise between logistic feasibility and the chances of producing an impact, although shorter or longer intervals are recommended in specific cases.1,5,18,19
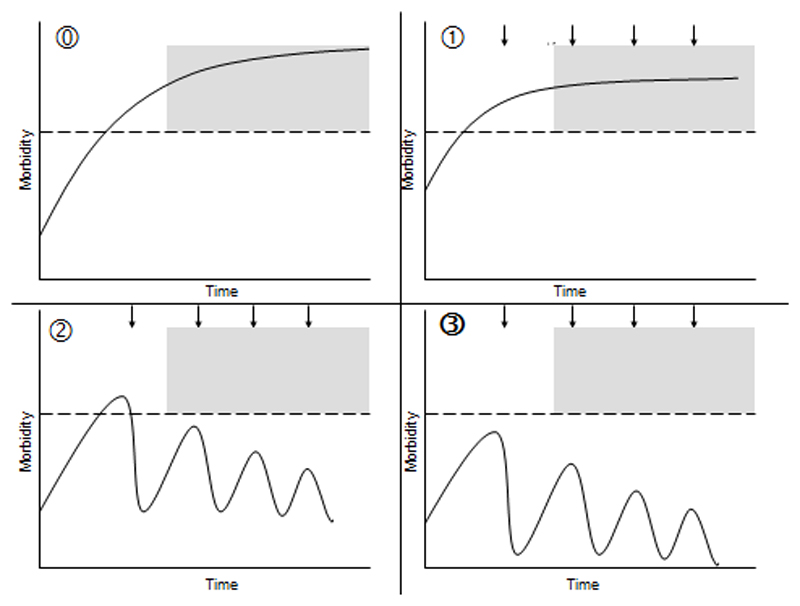
A graphical representation of the evolution of morbidity associated with helminth infections in the absence of PC interventions is provided in Figure 1A, whilst Figure 1B–D shows the three possible scenarios of the impact produced by PC on morbidity, according to an infected individual’s intensity of infection and duration of exposure to the worms.4,5,20,21
Figure 1.
Graphical representation of the evolution of morbidity associated with helminth infections (continuous line), at individual level, in the absence of preventive chemotherapy (PC) interventions (A) and of the three possible scenarios showing the impact of PC on morbidity (B–D). In each of the four figure parts, the grey area represents irreversible morbidity, the dotted line represents the threshold of morbidity (threshold of high intensity) and the arrows represent the treatment rounds. (A) PC is not implemented: acute inflammation progresses into chronic stages associated with irreversible sequelae; morbidity is not controlled. (B) PC targets a chronically infected patient who has already developed irreversible sequelae. In this case, no reversion of such chronic morbidity can be achieved. However, as in endemic areas this individual is likely to concomitantly suffer from different time stages of morbidity, treatment will revert the early-stage morbidity consequent to more recent re-infection episodes. (C) PC targets an individual who has developed reversible morbidity but not yet its irreversible stages. In this case, all the existing morbidity will be reverted and its burden reduced. (D) PC targets an individual who has not yet developed any morbidity and is implemented throughout the period of exposure to the infectious agents, with an interval of re-treatment that is shorter than the period of time needed for re-infection episodes to increase the number of worms above the morbidity threshold. In this case, PC will fully prevent morbidity and the infected subject will never develop any of the pathological consequences of high-intensity infections.
The direct beneficial effects of PC on morbidity are limited to the recipients of the drugs; from a public health perspective, the overall impact of a PC intervention, in terms of burden of morbidity prevented or reduced, will be a function of the proportion of infected individuals who have received treatment (P) and will increase proportionately to the number of infected human hosts who are treated.
8.2. Elimination of morbidity
When reversible morbidity associated with an infection is controlled in each of the individuals living in the target area, ‘elimination of morbidity’ (also referred to as ‘elimination of disease’) is achieved in that area, as the risk of developing morbidity by its inhabitants is equal to zero (reduction to zero of the incidence of disease)22. Irreversible morbidity will still be present in chronic patients who were not administered treatment on time, but will gradually disappear as such individuals die out. Continued interventions are required to maintain the status of elimination of morbidity until transmission in the target area is interrupted.11,22
8.3. Reduction of transmission
The decrease in prevalence and mean intensity of infection observed after a PC intervention is the outcome of two mechanisms: the first is the drug-induced worm clearing that occurs in treated individuals; and the second is the reduced number of re-infection episodes consequent to the reduced contamination of the environment by worm eggs or larvae discharged by such individuals.5,12,13,17 Both mechanisms sustain a reduction in the number of worms circulating in the target area and therefore a decrease in EoT, which will benefit any individuals living in that area, irrespective of whether they have been treated or not. As a result, for any linear increase in P achieved by the PC intervention, there will be a greater than linear increase in the overall impact on transmission (ΔEoT) produced. Such a concept is somewhat comparable with that of ‘herd immunity’ in vaccination,23 which implies that when a large proportion of a population is immunised, protection is also conferred to unvaccinated individuals. Because of this ‘amplification’ mechanism, the ΔEoT produced by a number of repeated rounds of PC can be so significant that ‘elimination of transmission’ (i.e. the reduction to zero of the incidence of an infection in a geographical area, also referred to as ‘elimination of infection’)11,22 can be envisaged.
8.4. Elimination of transmission
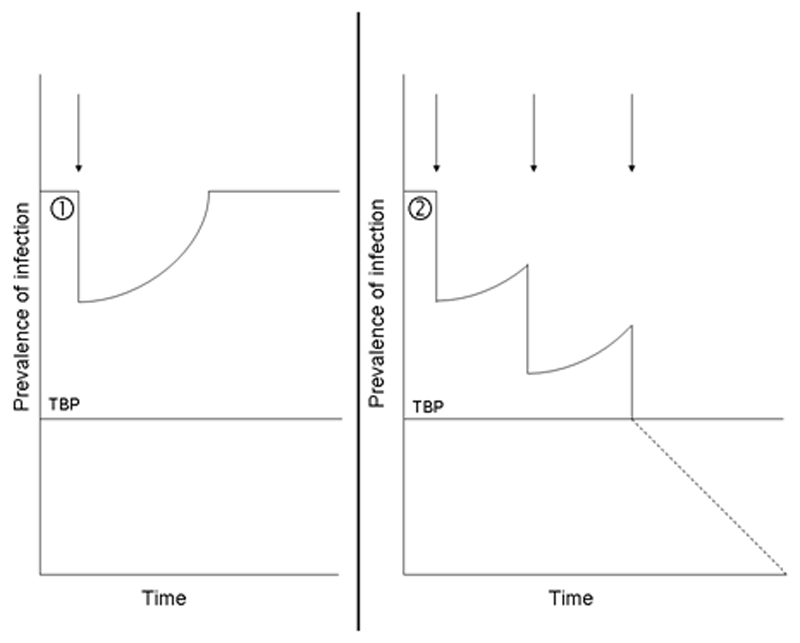
The concept of elimination is based on the assumption that transmission of a helminth infection can be interrupted by reducing the number of worms circulating in the target area below a certain threshold or transmission breakpoint (TBP).24 This is an adaptation to helminth infections of the concept developed by Ross in his writings about the ‘malaria model’.24,25 For diseases in which humans host the parasite, this threshold has most frequently been expressed as a threshold of prevalence of infection measured in the human population living in an area.9 Figure 2 graphically exemplifies this concept. When a PC intervention is implemented, the existing equilibrium between the number of worms that are generated and those that die in the target area is broken; the number of worms circulating in the area is consequently decreased and the measured prevalence of infection is reduced. When the PC intervention is discontinued, the worm killing induced by the chemotherapeutic agents ceases, with the consequence that the system tends to regain its former equilibrium: the pre-intervention EoT, number of circulating worms and corresponding level of prevalence are therefore re-established (Figure 2A). However, if one or repeated PC interventions succeed in decreasing the prevalence of infection below the level associated with the TBP, transmission will eventually be interrupted (Figure 2B). This happens because below the TBP, even in absence of any PC intervention, the worm population is so small that the number of new worms generated by successful completion of the life cycle is lower than the number of worms that die; such a population is therefore not able to perpetuate itself and will eventually disappear, thus leading to interruption of transmission.26 The level of prevalence associated with the TBP is typically very low9 and is influenced both by biological and environmental factors. In fact, it is inversely related to the infection’s EoT prior to the PC intervention: the higher the EoT, the lower the TBP, because if an infection is very efficient even a small number of circulating worms will be sufficient to sustain its transmission. As a general principle, it is easier to interrupt transmission of infections in which both intrinsic biological factors as well as extrinsic environmental factors related to the specific area of intervention are disadvantageous to transmission, thus producing a low EoT. In contrast, infections with a high pre-intervention EoT will have such a low TBP that it is unlikely that transmission can be interrupted with a small number of rounds, or by PC alone at all. In all cases, the likelihood of interrupting transmission can be increased by complementing PC with other interventions that have an impact on the environmental factors contributing to the EoT, such as, for example, health education, vector control, or provision of safe water and sanitation.
Figure 2.
The transmission breakpoint (TBP) of a given infection corresponds to the quantity of worms circulating in the target area below which they are unable to perpetuate themselves, with the consequence that their population will eventually die out. The TBP can be expressed as a prevalence of infection in the human population, whose level depends on the pre-intervention efficiency of transmission (EoT) of the infection. Preventive chemotherapy (PC) interventions (arrows) reduce the number of circulating worms and decrease the target infection’s EoT, thus moving the prevalence of infection to a level that is lower than the pre-intervention level. (A) If the post-intervention number of circulating worms and the corresponding EoT and prevalence of infection are still above the TBP, after discontinuation of such an intervention the pre-intervention levels of all indicators will be re-established as a consequence of continuing re-infection episodes. (B) If the post-intervention number of circulating worms and the corresponding EoT and prevalence of infection reach the level associated with the TBP, the worm population will eventually die out, thus leading to interruption of transmission of the target infection.
As mentioned, the chances that a given intervention has to reach the TBP are proportionate to its capability of reducing the number of worms circulating in the target area. In the context of a PC intervention, this can be pursued by killing the largest possible number of worms harboured by infected individuals, and therefore by administering highly efficacious drugs to as many such individuals as possible (thus maximising P and ΔEoT). Among the modalities of implementation of PC, MDA is the most widely employed in this regard as it intentionally covers the largest number of individuals and is particularly appropriate when it is logistically difficult to reach and identify each infected individual singularly (e.g. because their number is high or because they are geographically dispersed). In contrast, targeted chemotherapy is inappropriate for interrupting transmission, as the likely presence of infected individuals in those population groups that are deliberately not targeted by the intervention will preclude the possibility of achieving a high ΔEoT. When PC is applied as selective chemotherapy, the chances of achieving a high ΔEoT depend on the logistical capacity to reach each infected individual as well as on the sensitivity of the diagnostic test used to screen the population. The appropriateness of such a measure increases as transmission declines and infected cases are progressively concentrated in residual, isolated foci, thus making screening a more feasible and practical option.
In a real intervention scenario in which not all infected individuals are treated, and not all treated individuals are cured, the number of rounds of PC necessary to reach the TBP of a disease with a given pre-intervention EoT, and therefore the time needed to achieve interruption of its transmission, is inversely related to the sum of the ΔEoTs achieved by each round of treatment and directly related to the interval of re-treatment, as a long interval will allow for a rebound in the prevalence of infection as a consequence of the re-infection episodes occurring in the target area. Therefore, the shorter the interval of re-treatment (i.e. the more frequent are the rounds) and the larger the ΔEoT achieved by each round of treatment, the lower will also be the number of rounds—and consequently the amount of time—needed to interrupt transmission.
From an operational perspective, when elimination of transmission is achieved in a limited geographical area (e.g. a country), continued interventions are required to prevent re-introduction of the infection from neighbouring areas and the re-establishment of transmission. But if elimination of transmission is achieved throughout the world (‘eradication’), then intervention measures are no longer needed.11,22
9. Current target infections: reasons for their eligibility for preventive chemotherapy
Helminths responsible for the four diseases currently targeted by PC (LF, onchocerciasis, schistosomiasis and STH) are unable to replicate in humans and require one or more obligate passages outside of them (in an intermediate host, in a vector or in the environment) in order to complete their life cycle.4,5 Direct human-to-human spread is therefore not possible, with the consequence that transmission is a slow process. These facts imply that the increase in the number of worms within an individual is slow as it can only be produced by subsequent re-infection episodes.6 From an individual perspective, both the risk of developing morbidity and the likelihood of transmitting infection are dependent on the number of worms within the human body (intensity of infection). As intensity of infection typically increases slowly, the human host’s risk of developing morbidity also increases slowly: this explains why early-stage manifestations associated with the four target infections are frequently unnoticed, and overt morbidity and symptoms develop only after some time has elapsed.5 The same mechanism explains why the host’s likelihood of transmitting infection also increases slowly. Community diagnostic procedures are available for each of the four diseases.1,27–30 Drug delivery strategies relying on resource persons based in schools or within communities have been developed, and recommended drugs used are low cost or donated.1,12–17,31 All these factors contribute to containing costs and make the PC interventions implemented against the four target diseases amongst the most cost-effective public health measures existing nowadays.32 In addition, all anthelminthic drugs currently used in PC interventions [albendazole (ALB), diethylcarbamazine (DEC), ivermectin (IVM), mebendazole (MBD) and praziquantel (PZQ)] are safe (side effects are rare, mild and transitory) and therefore appropriate for use in interventions targeting infected as well as non-infected individuals.1 Such drugs are also simple to administer, a fact that enables their distribution by non-medical personnel, e.g. school teachers and community drug distributors:1 specifically, they are all available in oral tablet formulations and are given in single administration (i.e. in a single event) and, in the case of MBD and in most uses of ALB, which is always administered at a dose of 400 mg (except in children aged 12–23 months in which the dose is 200 mg), also in single dose (i.e. at a fixed dosage, irrespective of the characteristics of the target individuals). Finally, the mentioned medicines are all characterised by high efficacy. Should decreased drug efficacy arise, new drugs would need to be developed for PC to remain a viable public health intervention.
10. Current target infections: justification for the recommended modality of implementation
10.1. Lymphatic filariasis and onchocerciasis
MDA is the modality of implementation of PC currently recommended by the WHO against both LF and onchocerciasis.1
Demographic characteristics of the infection and disease: all ages are affected by both infections; morbidity accumulates over time through re-infection episodes and is more prominent in adult life.
Proportion of infected individuals in the target area (prevalence of infection): LF is characterised by a low prevalence of infection but a wide geographical distribution, thus usually affecting large numbers of individuals; onchocerciasis can have a high prevalence in areas that are highly suitable for vector populations.
Chances of achieving interruption of transmission: in both infections, penetration of the worm’s larvae through the human skin occurs in conjunction with the blood meal taken by the relevant vectors (mosquitoes for LF and blackflies for onchocerciasis); passage of the L3 larvae from the vector to the human body is, however, a difficult and not always successful exercise because of their relatively large size; in addition, replication of the infectious agents within the vector does not occur in either case and consequently their number can only increase following further ingestion of metacercariae.33,34 These considerations, combined with the fact that neither infection has a significant extrahuman reservoir (only LF due to Brugia malayi can be found in animals, but its geographical distribution is limited34), indicate that the EoT of both LF and onchocerciasis is generally low, a fact reflected by the small number of adult worms that are usually found in infected individuals. As a consequence, PC has the potential to affect both morbidity and transmission.16,17
The facts that all ages are concerned by both infections and that the chances of interrupting their transmission through PC are significant represent the key factors leading to the selection of MDA as the most appropriate modality of implementation: all individuals susceptible to contributing to maintaining transmission are therefore targeted for treatment, with the aim of raising P as much as possible. The number of MDA rounds necessary to achieve this goal without any other complementary intervention is currently estimated at 4–6 rounds for LF17 and is under consideration for onchocerciasis,16,35 although 10–13 semiannual rounds have been shown to be successful in interrupting transmission in previously endemic foci in Guatemala36 and annual or biannual rounds for 15–17 years achieved the same goal in Mali and Senegal.37 The different impact on transmission produced by similar PC interventions applied to the same infection in different contexts can be explained by the presence of environmental factors variously modulating the EoT. In the case of onchocerciasis, vector control was extensively used in the past (and is still occasionally implemented in the most affected foci) with the aim of complementing PC in its efforts to reduce the infection’s EoT. As of today, both the Global Programme to Eliminate Lymphatic Filariasis (GPELF) and the Onchocerciasis Elimination Program for the Americas (OEPA) include interruption of transmission among their goals.38–41
10.2. Schistosomiasis and soil-transmitted helminthiasis
Targeted treatment is the main modality of implementation of PC currently recommended by the WHO against both schistosomiasis and STH.1
Demographic characteristics of the infection and disease: peaks in prevalence and intensity of both infections, and consequently reversible morbidity, are usually observed in school-age childhood and, to a lesser degree, in early adult life; women of childbearing age are at higher risk of developing anaemia.
Proportion of infected individuals in the target area (prevalence of infection): prevalence of both infections can vary from low to high, according to the higher or lower suitability of the environmental conditions to sustain transmission.
Chances of achieving interruption of transmission: the transmission cycle of schistosomiasis is very efficient because of a combination of asexual replication in the snail intermediate host and sexual multiplication in the final host.42 The different types of STH also have very efficient transmission cycles: the large number of eggs produced by the parasites,43 the long persistence of the eggs in the soil as well as the absence of a need for vectors or intermediate hosts are all factors that make these infections extremely successful in transmission (approximately one-half of the world’s population is estimated to be infected).44 In most endemic areas, both schistosomiasis and STH are therefore characterised by a high EoT and consequently by a low TBP. This consideration is reflected by the high number of worms typically found in infected individuals and makes PC alone usually effective in controlling morbidity but not sufficient to achieve interruption of transmission, a fact confirmed by the frequent observation that low levels of prevalence are maintained when a constant pharmacological pressure is exerted on the worm population through regular treatment interventions,5,15 but rebounds occur after their discontinuation.45 In the case of schistosomiasis, however, marked and long lasting reductions in the prevalence of infection among a population targeted by PC have been observed, indicating that when defined species (Schistosoma haematobium and S. mansoni) are targeted in defined environmental settings, transmission can in fact be significantly reduced by PC.46–48 This finding has major implications as, according to the general rule explained above, if the number of circulating worms could be pushed below the TBP, then interruption of transmission could be achieved through PC alone. Such a possibility would be more limited in the case of schistosomiasis japonica or mekongi, characterised by an animal reservoir (dogs, pigs, cats, cattle and buffaloes) that can replace humans in the parasite’s transmission cycle,33,34 or in the case of hybridisation occurring between animal and human species of schistosomes (e.g. S. bovis/S. haematobium) as recently described in Senegal.49 In such instances, the ΔEoT would be smaller due to the increased EoT deriving from the presence of the animal reservoir, and from the fact that PC targets only humans and has therefore no impact on the proportion of environmental contamination or on transmission of infection to the vector/intermediate host that is attributable to animals. In all cases, the general rule applies that the impact on transmission can be increased by co-implementation of PC with measures reducing the EoT, such as sanitation and environmental management.
The considerations above are the main reasons justifying the current focus of the WHO-recommended PC strategies against both diseases on morbidity control through targeted chemotherapy.1 In this perspective, the priority treatment target is represented by those population groups at highest risk of infection, such as children, as they will be at highest risk of developing morbidity and also most likely to benefit from treatment due to their expected recent exposure to the infectious agent; in contrast, those who—even if possibly infected and therefore contributing to transmission—are not at risk of developing morbidity (due to less frequent exposure to the infectious agents and consequently lower intensity of infection) are excluded from PC or considered only a secondary target. The reduction in the number of individuals to be treated also allows containing the financial effort required for implementation of PC. The investment needed to control morbidity in a given population might be further reduced—in areas where the prevalence of infection is decreasing and appropriate logistics are available—by progressively restricting targeted chemotherapy to residual foci of transmission. Finally, should new evidence confirm that interruption of transmission of schistosomiasis can be achieved through PC, a reconsideration of the entire strategic approach would be needed; in this case, MDA – as in the case of LF and onchocerciasis – would appear as the most appropriate modality of implementation, for the reasons already exposed.
11. Potential target infections: perspectives and constraints
The requisites necessary to implement PC are not an exclusive characteristic of the four current target diseases. There are in fact other helminth infections such as clonorchiasis, opisthorchiasis and taeniasis/cysticercosis that possess epidemiological and biological characteristics that make them eligible for PC and therefore potential candidates for an expansion of this strategy’s target. There are then helminth infections, such as fascioliasis, that do not fully fit into the PC model: in this case, PC would still be an adequate public health measure in terms of attainment of the intended outcomes, but its implementation should be weighed against economic and financial considerations. Finally, there are infections such as strongyloidiasis that cannot be considered eligible for PC as its implementation would not produce any of the desired outcomes.
11.1. Clonorchiasis and opisthorchiasis
Clonorchiasis (due to Clonorchis sinensis) and opisthorchiasis (due to Opisthorchis spp.) are infections highly prevalent in limited areas of the world. They fulfil all the characteristics that indicate eligibility for PC: early stages of infection are often silent, particularly when of light intensity, whilst morbidity and associated symptoms tend to arise when the infection has attained advanced stages; this typically happens only after several years of exposure to re-infection episodes. The contribution of biological factors to EoT is typically modest because of the complexity of the life cycle, but the overall EoT is significant due to the fact that they are zoonotic infections whose transmission in the environment occurs mainly from animals to animals and is perpetuated by a significant animal reservoir. The considerations made above with regard to the zoonotic forms of schistosomiasis also apply to these infections, and therefore the outcomes of PC interventions directed against them would be mainly limited to control of morbidity. Both infections can be treated with PZQ 25 mg/kg three times daily for 2 consecutive days, or alternatively 40 mg/kg single administration31, with reasonably high cure and egg reduction rates. Age and gender patterns linked to food habits have been occasionally described in the epidemiology of both infections and might be useful in identifying the most appropriate PC intervention. For example, in rural communities in Vietnam and Korea, raw fish dishes are typically enjoyed by adult males over alcohol, and the epidemiology of clonorchiasis and opisthorchiasis reflects this fact: in this case the recommendation would be targeted treatment to this specific group. In contrast, where infection affects all age groups indistinctly, as is frequently the case in Lao PDR, MDA would be the most appropriate intervention. In any case, decrease and interruption of transmission should be considered as unlikely events whose achievement might be made possible only by a more comprehensive strategy that should possibly combine PC with veterinary public health measures. Selective chemotherapy is currently the least suitable option due to the low sensitivity and/or poor field applicability of parasitological examination techniques as well as of lack of availability and standardisation of other diagnostic tools.
11.2. Taeniasis/cysticercosis
Taeniasis/cysticercosis due to Taenia solium and taeniasis due to T. saginata are two other human helminthic diseases that can be considered eligible for PC. Humans are the only final hosts, and an obligatory passage in the intermediate hosts (pigs for T. solium and cattle for T. saginata) is needed to complete the life cycle.33,34 Transmission is very intense from humans to pigs/cattle because of the high number of eggs released in the environment by infected individuals;33 in addition, eggs can survive on pastures for weeks to months.34 On the other hand, transmission is much less intense from pigs/cattle to humans: consumption of cysticerci is an accidental event that occurs when they are not discarded during meat preparation or when meat is not properly cooked. This fact is reflected by the common finding of communities in which few human Taenia spp. carriers are responsible for a high prevalence of cysticercosis in their domestic animals.50,51
Parasite eggs dispersed with the faeces of individuals infected with T. solium are also responsible for human cysticercosis, the disease that is caught when such eggs are ingested by humans.34 This happens when eggs contaminate food or beverages or when external autoinfection occurs through the faecal–oral route. Such human-to-human transmission, in which humans act both as final and intermediate hosts of the parasite, is also intense, and clustering of cases of cysticercosis within families with a single T. solium carrier has been described.51 Both forms of taeniasis lack an early onset of clear symptomatology: most individuals who harbour adult T. saginata or T. solium in their intestine are actually either asymptomatic or only experience mild, non-distinct symptoms,34 which are unlikely to generate a health-seeking behaviour. In cysticercosis, neurological symptoms due to cysts in the brain may appear only 5–15 years after ingestion of eggs,33 whilst intramuscular cysts usually escape clinical attention;52 subcutaneous cysts might be more visible but are less common.33,52
These considerations make taeniasis/cysticercosis eligible for PC: single administration of PZQ 5–10 mg/kg or single-dose, single-administration niclosamide 2 g are highly effective against adult worms of both species.31 PC would result in killing of adult worms in the human host,53 thus immediately reducing morbidity associated with taeniasis. Most importantly, an additional outcome would be a reduction of the number of proglottids and eggs released in the environment, with an effect on the efficient part of the cycle (from human to intermediate host and, in T. solium infection, also from human to human), and therefore a reduction, both for humans and the intermediate hosts, of the risk of acquiring cysticercosis. The ΔEoT produced by PC on taeniasis/cysticercosis is potentially substantial because humans are the only final hosts and the sole source of infection for intermediate hosts.53 A more rapid and long-lasting impact would, however, be achieved by combining human and veterinary public health measures, such as concurrent treatment of humans and animal intermediate hosts.54,55
11.3. Fascioliasis
Fascioliasis is a zoonotic helminthiasis caused by Fasciola hepatica or F. gigantica. The disease has a clear correlation between number of worms within an individual, morbidity and transmission; its clinical picture is characterised by a short pre-patent period (not exceeding a few weeks after ingestion of metacercariae),33,56 followed by the onset of symptomatology (the so-called acute phase). In Vietnam, where fascioliasis results in early and severe acute symptoms, its eligibility for PC is limited by the fact that the infection is likely to trigger a health-seeking behaviour: passive case finding and management of individuals spontaneously reporting to health facilities would therefore be sufficient measures (triclabendazole has been shown to be highly effective against this disease; it is the current best therapeutic option and the only one recommended by the WHO 31,57,58). In addition, fascioliasis is widespread throughout the national territory with scarce geographical focalisation and low prevalence of infection: the higher costs per person of the individual case management approach are therefore justified by the low prevalence and are compensated by a simplified diagnostic protocol, which employs inexpensive techniques and is adapted to decentralised rural settings. In the Nile Delta region of Egypt, where symptoms appear to be less specific and pronounced, where prevalence of infection is higher and where the infection is geographically circumscribed, selective chemotherapy after regular screening in suspect villages located in endemic districts has proven a successful strategy.59 In highly endemic areas in the Andean highlands of Bolivia and Peru, where a high prevalence of infection has been documented,60 particularly in school-age children, targeted treatment of this age group or MDA to the entire population in the most affected communities has been identified as the most appropriate modality of implementation. In all cases, the outcomes of PC interventions against fascioliasis would be largely limited to control of morbidity. This is because the wide range of animals that can act as reservoirs of the infection results in a high EoT and consequently decreases the chances of achieving any impact on transmission through PC alone.
11.4. Strongyloidiasis
Despite its unclear progression of symptoms and the direct correlation existing between intensity of infection, morbidity and transmission, strongyloidiasis is not a suitable candidate for PC because it does not fulfil the second criterion of eligibility for PC, that is the slow increase in the likelihood of an infected host to develop morbidity and to transmit the infection. This happens because in strongyloidiasis human autoinfection can occur, especially, but not exclusively, in the immunocompromised host.61 This fact, very unusual among human helminth infections, means that the parasite can replicate within the human host without the need to undergo non-human phases.34 Considering that the cure rate of the single-administration regimen of choice against Strongyloides stercoralis (IVM 200 μg/kg)31 is estimated at 83%,62 surviving worms would immediately re-infect the individual. As such, the semiannual or annual interval of re-treatment typical of most PC interventions appears unlikely to keep the number of worms within an individual below the threshold of high intensity and achieve an effective control of morbidity: a higher frequency would rather be required. The most appropriate intervention would therefore be individual case management with IVM, an effective, safe and inexpensive drug,31,62 together with an adequate follow-up of infected individuals aimed at ensuring that they are fully cured and therefore not subject to auto-re-infection. Whilst PC interventions distributing IVM and/or ALB, such as those carried out against LF, onchocerciasis or STH, are likely to have some effect on the burden of strongyloidiasis, they cannot be considered an effective means to reduce morbidity and transmission associated with the infection.
12. Conclusions
The concepts exposed in this paper are the result of an effort aimed at systematising empirical observations made by the authors or taken from the scientific literature on the outcomes of PC interventions applied to different diseases, in different epidemiological settings and with different modalities of implementation. Further observations on the impact produced by PC on morbidity and transmission of those helminth infections for which this is already the recommended public health strategy, as well as of those for which this is not the case yet, are expected to generate new evidence and enable to refine and expand the field of applicability of this public health tool.
Funding
None.
Footnotes
Authors’ contributions: A-FG and AM contributed equally to this paper. All of the authors discussed the need for the article and outlined the different parts; A-FG and AM developed together the first draft, which was then discussed and reviewed collegially. AM is guarantor of the paper.
Conflicts of interest: None declared.
Ethical approval: Not required.
References
- 1.WHO. Geneva: World Health Organization; 2006. Preventive chemotherapy in human helminthiasis. Coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. [Google Scholar]
- 2.WHO. Neglected tropical diseases. PCT databank. Geneva: World Health Organization; 2010. [Google Scholar]
- 3.Fenwick A. Host–parasite relations and implications for control. Adv Parasitol. 2009;68:247–61. doi: 10.1016/S0065-308X(08)00610-6. [DOI] [PubMed] [Google Scholar]
- 4.de Carneri Parassitologia Generale e Umana. 1st ed. Milan: Casa Editrice Ambrosiana; 1961. [Google Scholar]
- 5.Crompton DW, Savioli L. Handbook of helminthiasis for public health. Boca Raton, FL: CRC Press; 2006. [Google Scholar]
- 6.Anderson RM, May RM. Infectious diseases of humans: dynamics and control. New York, NY: Oxford University Press; 1991. [Google Scholar]
- 7.WHO. Report of the WHO of the Informal Consultation on the use of chemotherapy for the control of morbidity due to soil-transmitted nematodes in humans. Geneva: World Health Organization; 1996. [Google Scholar]
- 8.Warren KS. The control of helminths: nonreplicating infectious agents of man. Annu Rev Public Health. 1981;2:101–5. doi: 10.1146/annurev.pu.02.050181.000533. [DOI] [PubMed] [Google Scholar]
- 9.May RM. Ecology and population biology. In: Warren KS, Mahmoud AAF, editors. Tropical and geographical medicine. 2nd ed. New York, NY: McGraw Hill Book Company; 1990. pp. 130–45. [Google Scholar]
- 10.Hotez PJ. Stigma: the stealth weapon of the NTD. PLoS Negl Trop Dis. 2008;2:e230. doi: 10.1371/journal.pntd.0000230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Molyneux DH, Hopkins DR, Zagaria N. Disease eradication, elimination and control: the need for accurate and consistent usage. Trends Parasitol. 2004;20:347–51. doi: 10.1016/j.pt.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Remme J, De Sole G, Dadzie KY, Alley ES, Baker RH, Habbema JD, et al. Large scale ivermectin distribution and its epidemiological consequences. Acta Leiden. 1990;59:177–91. [PubMed] [Google Scholar]
- 13.Gyapong JO, Kumaraswami V, Biswas G, Ottesen EA. Treatment strategies underpinning the global programme to eliminate lymphatic filariasis. Expert Opin Pharmacother. 2005;6:179–200. doi: 10.1517/14656566.6.2.179. [DOI] [PubMed] [Google Scholar]
- 14.Gryseels B, Polman K, Clerinx J, Kestens L. Human schistosomiasis. Lancet. 2006;368:1106–18. doi: 10.1016/S0140-6736(06)69440-3. [DOI] [PubMed] [Google Scholar]
- 15.Albonico M, Montresor A, Crompton DWT, Savioli L. Intervention for the control of soil-transmitted helminthiasis in the community. Adv Parasitol. 2006;61:311–48. doi: 10.1016/S0065-308X(05)61008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boatin BA, Richards FO., Jr Control of onchocerciasis. Adv Parasitol. 2006;61:349–94. doi: 10.1016/S0065-308X(05)61009-3. [DOI] [PubMed] [Google Scholar]
- 17.Ottesen EA. Lymphatic filariasis: treatment, control and elimination. Adv Parasitol. 2006;61:395–441. doi: 10.1016/S0065-308X(05)61010-X. [DOI] [PubMed] [Google Scholar]
- 18.Albonico M, Allen H, Chitsulo L, Engels D, Gabrielli AF, Savioli L. Controlling soil-transmitted helminthiasis in pre-school-age children through preventive chemotherapy. PLoS Negl Trop Dis. 2008;2:e126. doi: 10.1371/journal.pntd.0000126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Satayathum SA, Muchiri EM, Ouma JH, Whalen CC, King CH. Factors affecting infection or reinfection with Schistosoma haematobium in coastal Kenya: survival analysis during a nine-year, school-based treatment program. Am J Trop Med Hyg. 2006;75:83–92. [PMC free article] [PubMed] [Google Scholar]
- 20.Keang H, Odermatt P, Odermatt-Biays S, Cheam S, Degremont A, Hatz C. Liver morbidity due to Schistosoma mekongi in Cambodia after seven rounds of mass drug administration. Trans R Soc Trop Med Hyg. 2007;101:759–65. doi: 10.1016/j.trstmh.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Urbani C, Sinoun M, Socheat D, Pholsena K, Strandgaard H, Odermatt P, et al. Epidemiology and control of mekongi schistosomiasis. Acta Trop. 2002;82:157–68. doi: 10.1016/s0001-706x(02)00047-5. [DOI] [PubMed] [Google Scholar]
- 22.Dowdle WR. The principles of disease elimination and eradication. Bull World Health Organ. 1998;76(Suppl 2):22–5. [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrari MJ, Bansal S, Meyers LA, Bjornstad ON. Network frailty and the geometry of herd immunity. Proc Biol Sci. 2006;273:2743–8. doi: 10.1098/rspb.2006.3636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacDonald G. The dynamics of helminth infections, with special reference to schistosomes. Trans R Soc Trop Med Hyg. 1965;59:489–506. doi: 10.1016/0035-9203(65)90152-5. [DOI] [PubMed] [Google Scholar]
- 25.Ross R. Report on the prevention of malaria in Mauritius. London: J. and A. Churchill; 1909. [Google Scholar]
- 26.Stolk WA, de Vlas SJ, Habbema JD. Advances and challenges in predicting the impact of lymphatic filariasis elimination programmes by mathematical modelling. Filaria J. 2006;5:5. doi: 10.1186/1475-2883-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.WHO/GPELF. Lymphatic filariasis. Monitoring and epidemiological assessment of mass drug administration. A manual for national elimination programmes. Geneva: World Health Organization; 2011. [Google Scholar]
- 28.WHO. Onchocerciasis and its control. Report of a WHO Expert Committee. Geneva: World Health Organization; 1995. [PubMed] [Google Scholar]
- 29.WHO. The schistosomiasis manual. Geneva: World Health Organization; 1995. [Google Scholar]
- 30.WHO. Helminth control in school-age children. A guide for managers of control programmes. Geneva: World Health Organization; 2002. [Google Scholar]
- 31.WHO. WHO model formulary 2008. Geneva: World Health Organization; 2008. [Google Scholar]
- 32.Hotez PJ, Molyneux DH, Fenwick A, Kumaresan J, Sachs SE, Sachs JD, et al. Control of neglected tropical diseases. N Engl J Med. 2007;357:1018–27. doi: 10.1056/NEJMra064142. [DOI] [PubMed] [Google Scholar]
- 33.Muller R. Worms and human diseases. 2nd ed. Wallingford: CABI Publishing; 2001. [Google Scholar]
- 34.Cook G, Zumla A. Manson’s tropical diseases. 21st ed. London: Saunders Ltd; 2003. [Google Scholar]
- 35.Hopkins AD. Ivermectin and onchocerciasis: is it all solved? Eye (Lond) 2005;19:1057–66. doi: 10.1038/sj.eye.6701962. [DOI] [PubMed] [Google Scholar]
- 36.Lindblade KA, Arana B, Zea-Flores G, Rizzo N, Porter CH, Dominguez A, et al. Elimination of Onchocercia volvulus transmission in the Santa Rosa focus of Guatemala. Am J Trop Med Hyg. 2007;77:334–41. [PubMed] [Google Scholar]
- 37.Diawara L, Traoré MO, Badji A, Bissan Y, Doumbia K, Goita SF, et al. Feasibility of onchocerciasis elimination with ivermectin treatment in endemic foci in Africa: first evidence from studies in Mali and Senegal. PLoS Negl Trop Dis. 2009;3:e497. doi: 10.1371/journal.pntd.0000497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ottesen EA, Duke BO, Karam M, Behbehani K. Strategies and tools for the control/elimination of lymphatic filariasis. Bull World Health Organ. 1997;75:491–503. [PMC free article] [PubMed] [Google Scholar]
- 39.Onchocerciasis (river blindness). Report from the sixteenth InterAmerican Conference on Onchocerciasis, Antigua Guatemala, Guatemala. Wkly Epidemiol Rec. 2007;82:314–6. [PubMed] [Google Scholar]
- 40.Bockarie MJ, Molyneux DH. The end of lymphatic filariasis? BMJ. 2009;338:b1686. doi: 10.1136/bmj.b1686. [DOI] [PubMed] [Google Scholar]
- 41.WHO/GPELF. Lymphatic filariasis. Progress Report 2000-2009 and Strategic Plan 2010-2020. Geneva: World Health Organization; 2010. [Google Scholar]
- 42.Fenwick A, Rollinson D, Southgate V. Implementation of human schistosomiasis control: challenges and prospects. Adv Parasitol. 2006;61:567–622. doi: 10.1016/S0065-308X(05)61013-5. [DOI] [PubMed] [Google Scholar]
- 43.Bethony J, Brooker S, Albonico M, Geiger SM, Loukas A, Diemert D, et al. Soil-transmitted helminth infections: ascariasis, trichuriasis, and hookworm. Lancet. 2006;367:1521–32. doi: 10.1016/S0140-6736(06)68653-4. [DOI] [PubMed] [Google Scholar]
- 44.de Silva NR, Brooker S, Hotez PJ, Montresor A, Engels D, Savioli L. Soil-transmitted helminth infections: updating the global picture. Trends Parasitol. 2003;19:547–51. doi: 10.1016/j.pt.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Hall A, Anwar KS, Tomkins AM. Intensity of reinfection with Ascaris lumbricoides and its implications for parasite control. Lancet. 1992;339:1253–57. doi: 10.1016/0140-6736(92)91593-w. [DOI] [PubMed] [Google Scholar]
- 46.French MD, Churcher TS, Gambhir M, Fenwick A, Webster JP, Kabatereine N, et al. Observed reductions in Schistosoma mansoni transmission from large-scale administration of praziquantel in Uganda: a mathematical modelling study. PLoS Negl Trop Dis. 2010;4:e897. doi: 10.1371/journal.pntd.0000897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sinuon M, Tsuyuoka R, Socheat D, Odermatt P, Ohmae H, Matsuda H, et al. Control of Schistosoma mekongi in Cambodia: results of eight years of control activities in the two endemic provinces. Trans R Soc Trop Med Hyg. 2007;101:34–9. doi: 10.1016/j.trstmh.2006.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Touré S, Zhang Y, Bosqué-Oliva E, Ky C, Ouedraogo A, Koukounari A, et al. Two-year impact of single praziquantel treatment on infection in the national control programme on schistosomiasis in Burkina Faso. Bull World Health Organ. 2008;86:780–7. doi: 10.2471/BLT.07.048694. A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huyse T, Webster BL, Geldof S, Stothard JR, Diaw OT, Polman K, et al. Bidirectional introgressive hybridization between a cattle and human schistosome species. PLoS Pathog. 2009;5:e1000571. doi: 10.1371/journal.ppat.1000571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schantz PM, Cruz M, Sarti E, Pawlowski Z. Potential eradicability of taeniasis and cysticercosis. Bull Pan Am Health Organ. 1993;27:397–403. [PubMed] [Google Scholar]
- 51.Garcia HH, Gilman RH, Gonzalez AE, Varastegui E. Epidemiology of Taenia solium infection in Peru. In: Garcia HH, Martinez S, editors. Taenia solium taeniosis/cysticercosis. Lima: Editorial Universo; 1999. pp. 297–306. [Google Scholar]
- 52.Strickland GT. Hunter's tropical medicine. 7th ed. Philadelphia, PA: W.B. Saunders Co.; 1991. [Google Scholar]
- 53.Schantz PM. Taenia solium cysticercosis/taeniosis is a potentially eradicable disease: developing a strategy for action and obstacles to overcome. In: Garcia H, Martinez S, editors. Taenia solium taeniosis/cysticercosis. Lima: Editorial Universo; 1999. pp. 215–8. [Google Scholar]
- 54.Gonzalez AE, Garcia HH, Gilman RH, Tsang VC, Cisticercosis Working Group in Peru Control of Taenia solium. Acta Trop. 2003;87:103–9. doi: 10.1016/s0001-706x(03)00025-1. [DOI] [PubMed] [Google Scholar]
- 55.Pawlowski ZS. Role of chemotherapy of taeniasis in prevention of neurocysticercosis. Parasitol Int. 2006;55(Suppl):S105–9. doi: 10.1016/j.parint.2005.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mas-Coma S, Bargues MD, Esteban JC. Human fascioliasis. In: Dalton JP, editor. Fascioliasis. Wallingford: CABI Publishing; 1999. pp. 411–34. [Google Scholar]
- 57.Savioli L, Chitsulo L, Montresor A. New opportunities for the control of fascioliasis. Bull World Health Organ. 1999;77:300. [PMC free article] [PubMed] [Google Scholar]
- 58.Keiser J, Engels D, Buscher G, Utzinger J. Triclabendazole for the treatment of fascioliasis and paragonimiasis. Expert Opin Investig Drugs. 2005;14:1513–26. doi: 10.1517/13543784.14.12.1513. [DOI] [PubMed] [Google Scholar]
- 59.Curtale F, Hassanein YA, Savioli L. Control of human fascioliasis by selective chemotherapy: design, cost and effect of the first public health, school-based intervention implemented in endemic areas of the Nile Delta, Egypt. Trans R Soc Trop Med Hyg. 2005;99:599–609. doi: 10.1016/j.trstmh.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 60.Mas-Coma S. Epidemiology of fascioliasis in human endemic areas. J Helminthol. 2005;79:207–16. doi: 10.1079/joh2005296. [DOI] [PubMed] [Google Scholar]
- 61.Eberhard M, Gabrielli AF, Savioli L. Strongyloidiasis. In: Heymann DL, editor. Control of communicable diseases manual. Washington, DC: American Public Health Association; 2008. pp. 588–90. [Google Scholar]
- 62.Marti H, Haji HJ, Savioli L, Chwaya HM, Mgeni AF, Ameir JS, et al. A comparative trial of a single-dose ivermectin versus three days of albendazole for treatment of Strongyloides stercoralis and other soil-transmitted helminth infections in children. Am J Trop Med Hyg. 1996;55:477–81. doi: 10.4269/ajtmh.1996.55.477. [DOI] [PubMed] [Google Scholar]