Abstract
Activating mutations in the Kir6.2 (KCNJ11) subunit of the ATP-sensitive potassium channel cause neonatal diabetes. Many patients also suffer from neurological complications. By using mice carrying a human Kir6.2 mutation (Val59 to Met59; nV59M mice) targeted to neurones, we show that these mutations also result in altered anxiety behaviour. The light/dark box, successive alleys and elevated plus maze tasks revealed that nV59M mice have reduced anxiety related responses. Additionally, nV59M mice displayed enhanced basal locomotor activity and exploratory behaviour, as assessed by the low anxiety open-field test. These findings, in combination with previously reported hyperactivity of nV59M mice, appear to correlate with the increased impulsivity and inattentiveness reported in iDEND/DEND patients.
Keywords: KATP channels, neonatal diabetes, anxiety, hyperactivity
1. Introduction
The ATP-sensitive potassium (KATP) channel functions as a metabolic sensor, coupling cellular excitability to the metabolic state of the cell [1]. At low levels of metabolic activity, KATP channels are open, allowing the efflux of potassium ions, and thus maintaining the membrane potential at a hyperpolarized level and inhibiting electrical activity. By contrast, increased metabolic activity triggers the closure of KATP channels, leading to membrane depolarization, increased electrical activity, and stimulation of cellular responses [2,3]. Metabolic regulation of the channel is mediated by changes in the level of cytoplasmic adenine nucleotides, and involves both the pore-forming (Kir6.2) and regulatory (SUR1, or SUR2) subunits of the KATP channel. Closing of the channel is produced when ATP binds Kir6.2, whereas MgATP (and MgADP) binding and hydrolysis at the nucleotide-binding domains of SUR stimulates channel opening.
KATP channels are widely expressed throughout the body, being found in endocrine cells, cardiac and skeletal muscle, and in both the peripheral and central nervous system (CNS) [2]. They are widespread throughout the brain, including substantia nigra, hippocampus, hypothalamus, cerebral cortex, cerebellum and brain stem [4–8]. In most tissues, Kir6.2 forms the pore but the sulphonylurea receptor subunit varies, being SUR1 in endocrine cells and some neurones, and SUR2 in other types of neuron. The metabolic sensitivity of the channel varies with the SUR subunit: SUR2 subunits confer resistance to activation when metabolism falls [9].
Experimental evidence supports at least two roles for the KATP channel in the CNS. In glucose-responsive neurones, the KATP channel is a key player in the regulation of energy metabolism and glucose homeostasis and changes in extracellular glucose lead to changes in neuronal electrical activity [1,6,10–12]. In non-glucose-responsive neurones, the KATP channel appears to have a protective role in ischaemia, its activation reducing electrical activity and thereby metabolic demand [2,13–15]. KATP channel activity also appears to help prevent against seizures, by raising the threshold for epileptogenesis [2,3,14–19]. In non-glucose-responsive cells, it seems that the channel is almost completely closed at resting glucose levels, and opens only in response to a fall in metabolism. It is possible that this different metabolic response is, at least in part, determined by the type of SUR subunit [9].
Gain-of-function mutations in either the pore-forming (Kir6.2, encoded by KCNJ11) or regulatory (SUR1, ABCC8) subunit of the beta-cell plasma membrane ATP-sensitive potassium (KATP) channel are a common cause of neonatal diabetes [20–23]. These mutations impair the ability of ATP to close the KATP channel, and so increase the magnitude of the KATP current. More severe mutations, which cause a larger increase in KATP current, lead to intermediate DEND (iDEND) syndrome. This rare genetic disorder is characterised by diabetes within 6 months of birth, muscle weakness, delayed mental and motor development, balance and coordination problems [2,20] and hyperactivity [21,24]. In about 3% of cases, patients also have epilepsy (DEND syndrome): it is postulated that this arises due to excess activity of KATP channels in inhibitory neurones [20,25]. There is evidence that iDEND/DEND patients may also suffer from a range of other neurological symptoms, including attention deficit disorder, sleep disturbance and impulsive behaviour [21,24,26,27].
Previous work has shown that selective neuronal expression of the most common human iDEND mutation (Kir6.2-V59M) in mice recapitulates the muscle weakness, balance and coordination problems, and hyperactivity observed in human patients [8]. The effect of the Kir6.2-V59M mutation on other aspects of behaviour has not been assessed. However, it has previously been shown that mice with a global knockout of Kir6.2 have an increased anxiety phenotype [28] and that mice lacking KATP channels in their medial substantia nigra dopaminergic neurones show impaired novelty-induced exploration [29]. We now report that mice with selective neuronal expression of the activating Kir6.2-V59M mutation display a reduced anxiety phenotype and increased exploratory behaviour. Taken together these data suggest that the neuronal KATP channel is involved in the regulation of emotion.
2. Materials and Methods
2.1. Animal care
All work was conducted in accordance with the 1986 UK Animals (Scientific Procedures) Act and University of Oxford ethical guidelines. Animals were housed in same-sex littermate groups of 2-8 mice in a temperature and humidity controlled room on a 12h light-dark cycle (lights on at 7 am). They had ad libitum access to regular chow food and water at all times.
Mice heterozygous for the Kir6.2-V59M mutation were used in these studies. ROSA mice, expressing a floxed stop codon upstream of the Kir6.2-V59M gene, which was inserted into the ROSA locus and under the control of the ROSA promoter, were crossed with mice expressing Cre-recombinase under the control of the nestin promoter (Nes-cre+) to selectively express Kir6.2-V59M in neurons (nV59M), as previously described [8]. Male and female adult mice (11-20 weeks) were used for these studies. Littermates (ROSA+/-, Nes-cre+ and wild-type (WT)) were used as controls: as there was no difference in their responses, the control data have been pooled. Similarly, data from male and female mice (mutant and control) have been pooled. Genotypes were identified as previously described [30]. Both ROSA+/- and Nes-cre+ lines were backcrossed to C57Bl/6J mice for more than 3 generations. All experiments were performed blinded to genotype. One cohort of mice was used for the anxiety tests (light/dark box, successive alleys, plus maze) and a separate cohort for the low anxiety open field test. All mice were bred and maintained in the same facilities. All tests were performed during the animals’ light phase.
2.2. Molecular biology
2.2.1. RNA extraction and cDNA synthesis
Total RNA was extracted from brain tissue and processed using the procedure outlined in [8]. Briefly, the RNeasy Lipid mini kit (Qiagen, Manchester, UK) with an on-column DNase digestion step was used for the RNA extraction. The High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Warrington, UK) was used for the cDNA synthesis. A second aliquot of RNA was processed identically, but with no reverse transcriptase (Non-RT control).
2.2.2. Semi-quantitative PCR
Mouse Kcnj11 transcript was amplified by PCR using cDNA prepared from brain tissue of n-V59M and control mice, following the procedure outlined in [8]. The following primers were used: forward (5’-ATGCTGTCCCGAAAGGGCATT-3’) and reverse (5’-GGCGATGAGCCACCAGACCAT-3’). PCR products were digested with BtsCI restriction enzyme (New England Biolabs, Hitchin, UK) for 2h at 50°C. Digested PCR products were visualized using a 2% agarose gel with GelRed Nucleic Acid Stain (Biotium, California, USA).
2.2.3. Real-time quantitative PCR
Real-time quantitative PCR was preformed using an ABI Prism 7000 sequence detection system (Applied Biosystems, Warrington, UK) and SYBR green reagents (PowerSYBR green, Applied Biosystems, Warrington, UK). The reaction cycle comprised: 10min at 95°C followed by 40 cycles (95°C/15sec, 60°C/60sec). All reactions were performed in triplicate in a final volume of 25μl. Non-RT controls were included to confirm the absence of genomic DNA contamination. Primers were designed using Geneious Pro software (Biomatters Inc, Auckland, New Zealand) based on NCBI sequence data. Sequences of primers used were as follows:
| Hprt1: | fwd: 5’-CCGGCAGCGTTTCTGAGCCA-3’ |
| rev: 5’-GCTCGCGGCAAAAAGCGGTC-3’ | |
| Hspa8: | fwd: 5’-CAGCGCAGCTGGGCCTACAC-3’ |
| rev: 5’-TAGCTTGGCGTGGTGCGGTT-3’ | |
| Actb: | fwd: 5’-GCAGCTCCTTCGTTGCCGGT-3’ |
| rev: 5’-TACAGCCCGGGGAGCATCGT-3’ | |
| Kcnj11: | fwd: 5’-CGGGCGCATGGTGACAGAGG-3’ |
| rev: 5’-CGATGGGCCTGGGCCGTTTT-3’ | |
| Abcc8: | fwd: 5’- ACAGCCTTCGCAGACCGCAC-3’ |
| rev: 5’- GCCCGAGCCAGGCAGAACAG-3’ | |
| Gfp: | fwd: 5’-CGGCGACGTAAACGGCCACA-3’ |
| rev: 5’-CAGCTTGCCGGTGGTGCAGA-3’ | |
The ABI 7000 SDS software (Applied Biosystems, Warrington, UK) was used to measure threshold cycle (Ct) values. Gene expression was determined using the Pfaffl method [31] and normalized to three house-keeping genes: Actb, Hprt1 and Hspa8.
2.3. Behavioural tests
2.3.1. Light/dark box
The light/dark box is an anxiogenic task, which tests the tendency of a mouse to explore new environments versus the aversiveness of a brightly lit area [32]. The test was carried out using the equipment and procedure described in [28]. Briefly, the test was started by placing the mouse in the middle of the light side, facing away from the door separating the two compartments. The latency to cross (with all four paws) to the dark side, the amount of time spent in the dark side, and the number of transitions between the two compartments, were measured over the next 5 minutes.
2.3.2. Successive alleys
The apparatus consisted of four linearly connected wooden alleys of increasing anxiogenic character, as described in [28,33]. Mice were initially placed at the back of Alley 1 (the least anxiogenic), facing away from the open end. They were observed for 5 minutes and the latency to enter each alley (with all four paws), the number of entries into each alley, and the time spent on each section were recorded.
2.3.3. Elevated plus maze
The plus maze consisted of four arms (35cm x 6cm) arranged in a cross formation: two non-anxiogenic closed arms with 20cm-high walls and two anxiogenic open arms without walls [34]. It was elevated 70cm above the ground and placed in a brightly lit room with a digital video camera mounted overhead. Individual mice were placed at the junction of the open and closed arms, in a 6cm x 6cm centre square, facing the open arm opposite the entrance to the testing room. Their movements were recorded for 5 minutes. An automated tracking system (Any-maze, Stoelting, Wisconsin, USA) was used to record the time spent in each region, the number of entries to each region, the latency to enter an open arm, and the total distance covered.
2.3.4. Low anxiety open field
The open field test measures basal activity (squares crossed) and direct exploration (rears). The open field was a grey PVC arena with no additional illumination, as described in [28]. Mice were initially placed in a corner square, facing the walls, and observed for 3 minutes. The total number of squares crossed, the latency to first rear, and the total number of rears, were measured. The number of faecal boli and the presence of urine was noted, as an indication of anxiety [28].
2.4. Data analysis
All statistical analyses were performed with Prism 5 (Graphpad Software, La Jolla, California, USA). Statistical testing (Two-way ANOVA with gender and genotype as the two factors) indicated there was no statistically significant effect of gender on the results obtained. Thus data from female and male mice were pooled. When the data distribution permitted, a Student’s t-test was performed. Otherwise, data were analysed using the Mann-Whitney U-test. P<0.05 was considered statistically significant.
3. Results
3.1. Expression of Kir6.2 in nV59M mice
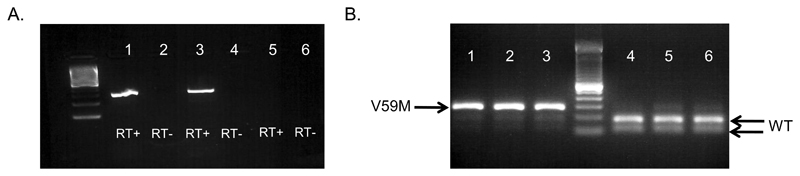
We used a Cre-lox approach to target expression selectively to neurones (nV59M mice). This approach is preferable to global expression of Kir6.2, as it avoids any confounding effects due to the high blood glucose concentration and severe diabetes observed when Kir6.2-V59M is targeted to pancreatic beta cells [30]. Mice expressing a floxed Kir6.2-V59M transcript (ROSA+/- mice) [30] were crossed with mice expressing the nestin promoter (Nes-cre+) to generate nV59M mice and littermate controls. Expression of wild-type and V59M Kir6.2 mRNA in control and nV59M mice was verified using semi-quantitative PCR (Fig. 1). To distinguish between WT and mutant mRNA we exploited the fact that introduction of the V59M mutation removes a unique BtsCI restriction site in the Kcnj11 cDNA, which prevents its cleavage. Thus, the presence of two bands on the gel indicates WT Kcnj11 mRNA alone whereas three bands indicates the presence of both WT and mutant (V59M) Kcnj11 mRNAs. As Figure 1B shows, V59M mRNA was expressed in brain tissue from nV59M but not that of control mice.
Figure 1. Kir6.2 expression in adult nV59M mice.
(A) Identical RNA aliquots of brain tissue from control (lanes 1-2) or nV59M (lanes 3-4) mice were processed in a reverse transcription reaction with reverse transcriptase included (RT+) or omitted (RT-). Amplification of the Kir6.2 transcript was only seen in RT+ samples, indicating there was little genomic DNA contaminating the RNA samples. Lanes 5-6 are nuclease-free water controls. (B) Kir6.2 RNA expression in brain tissue isolated from nV59M (lanes 1-3) or control (lanes 4-6) mice. Wild-type (WT), but not mutant (V59M), Kir6.2 cDNA was digested by restriction enzyme BtsCI, hence two bands indicates the presence of WT Kir6.2 only while three bands indicates both WT and V59M mutant Kir6.2 are present. Data are representative of experiments on 5 control and 5 nV59M mice.
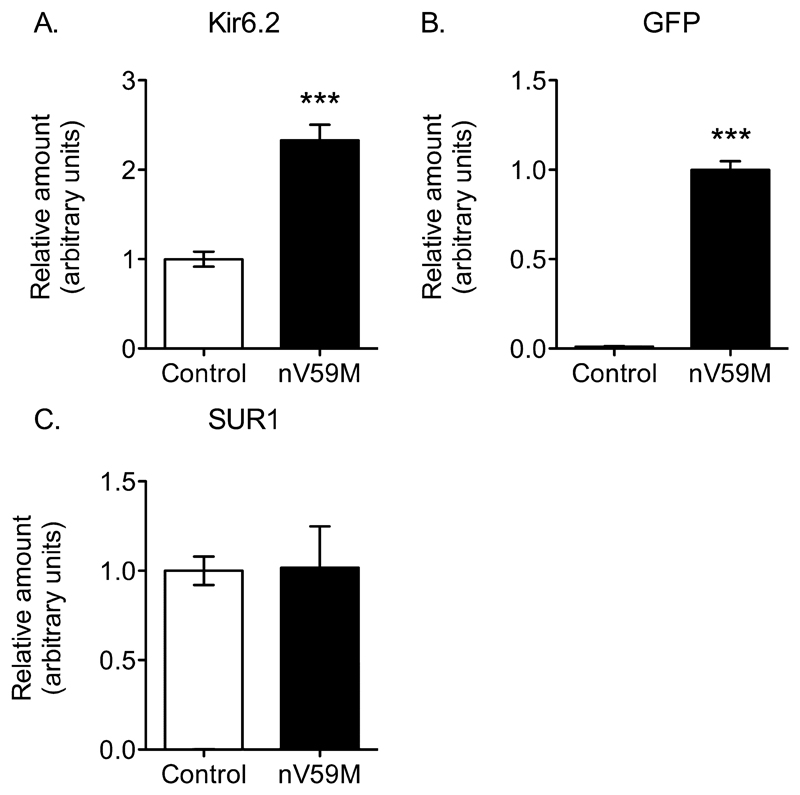
To determine the relative amount of Kcnj11 and Abcc8 expression in control and nV59M mice we performed quantitative PCR. Expression of Kcnj11, Abcc8 and Gfp (encoding green fluorescent protein) mRNAs was expressed relative to a panel of housekeeping genes (Actb, Hprt1 and Hspa8). Figure 2A shows that Kir6.2 expression was two-fold higher in brains of nV59M mice compared to control mice. This increase in Kir6.2 expression correlates with the amount of GFP expression (Fig. 2B). The Kir6.2-V59M transcript contains an internal ribosome re-entry site followed by the GFP sequence, which lies downstream of Kir6.2-V59M and is transcribed from the same promoter. The similarity between GFP expression and the increase in Kir6.2 expression suggests the latter reflects expression of Kir6.2-V59M. There was no difference in SUR1 expression in nV59M and control mice (Fig. 2C).
Figure 2. Relative expression of Kir6.2, GFP and SUR in brain.
Quantitative PCR showing expression of Kir6.2 (A), GFP (B), and SUR1 (C) mRNAs in the brain of control (n=8) and nV59M mice (n=7), relative to a panel of house-keeping genes. Data are mean ± SEM. *** P<0.0001 (Unpaired Student’s t-test)
3.2. Behavioural Testing
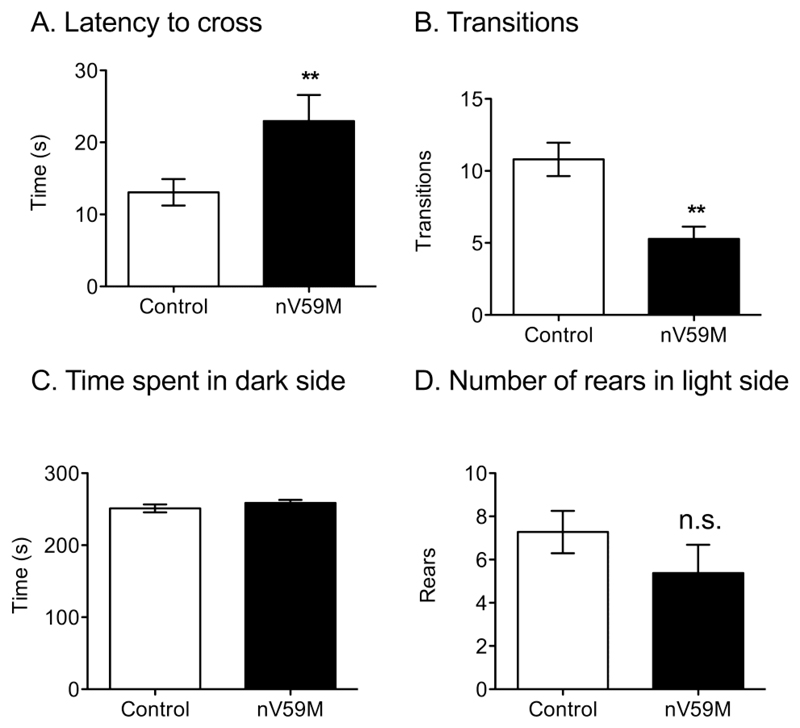
In the light/dark box test, nV59M mice took significantly longer to cross into the dark compartment than control littermates (Fig. 3A). However, once they had entered the dark compartment, they made fewer transitions between the two compartments than control mice (Fig. 3B). They also, like control mice, spent most of their time on the dark side (~250s in a 300s time period; Fig. 3C). There appeared to be a trend towards fewer rears by nV59M mice on the light side, but statistical significance was not reached (Fig. 3D). Neither nV59M nor control mice showed a tendency to freeze when first placed in the light compartment.
Figure 3. Light-dark box test.
(A) Latency to cross to the dark side of the light-dark box, (B) number of transitions between the light and dark compartments, (C) amount of time spent in the dark compartment of the light-dark box, and (D) number of rears in light compartment for adult (11-20 week-old) control (wild-type: n=1; ROSA+/-: n=12; Nes-Cre+: n=13) and nV59M (n=14) littermates over a 300s period. Data are mean ± SEM. ** P<0.01 (Mann-Whitney U-test).
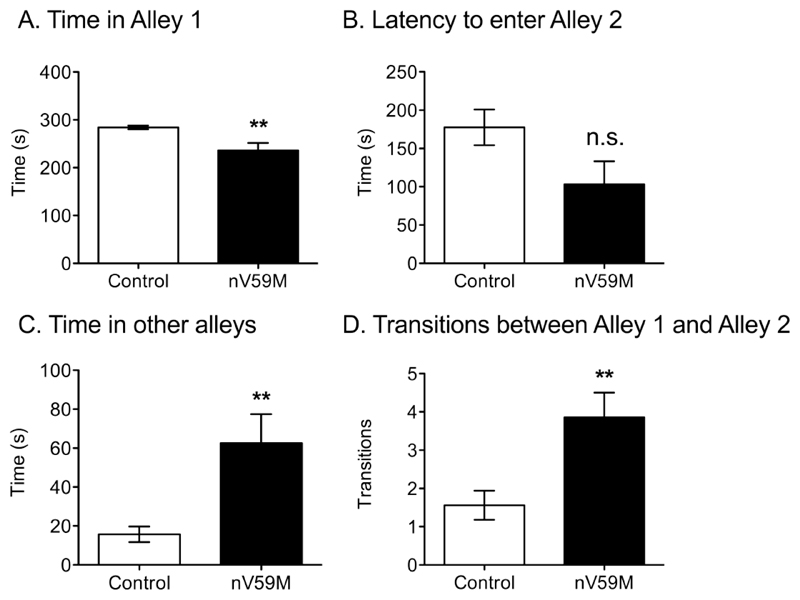
The nV59M mice spent significantly less time than control littermates in Alley 1 of the successive alleys task (Fig. 4A). They also took less time to enter Alley 2 initially, although statistical significance was not reached (Fig. 4B). Furthermore, nV59M mice spent significantly longer than control littermates in Alleys 2, 3 and 4, which are more anxiogenic (Fig. 4C), and they made a greater number of transitions between Alleys 1 and 2 (Fig. 4D). Few nV59M (n=2) or control mice (n=1) ventured into Alley 3. No mice ventured into Alley 4.
Figure 4. Successive alleys test.
(A) Amount of time spent in the first alley, (B) latency to enter the second alley, (C) amount of time spent in the second, third and fourth alleys, and (D) number of transitions between the first and second alleys for adult (11-20 week-old) control (wild-type: n=1; ROSA+/-: n=12; Nes-Cre+: n=13) and nV59M (n=14) littermates over a 300s period. Data are mean ± SEM. ** P<0.01; n.s. not statistically significant (Mann-Whitney U-test).
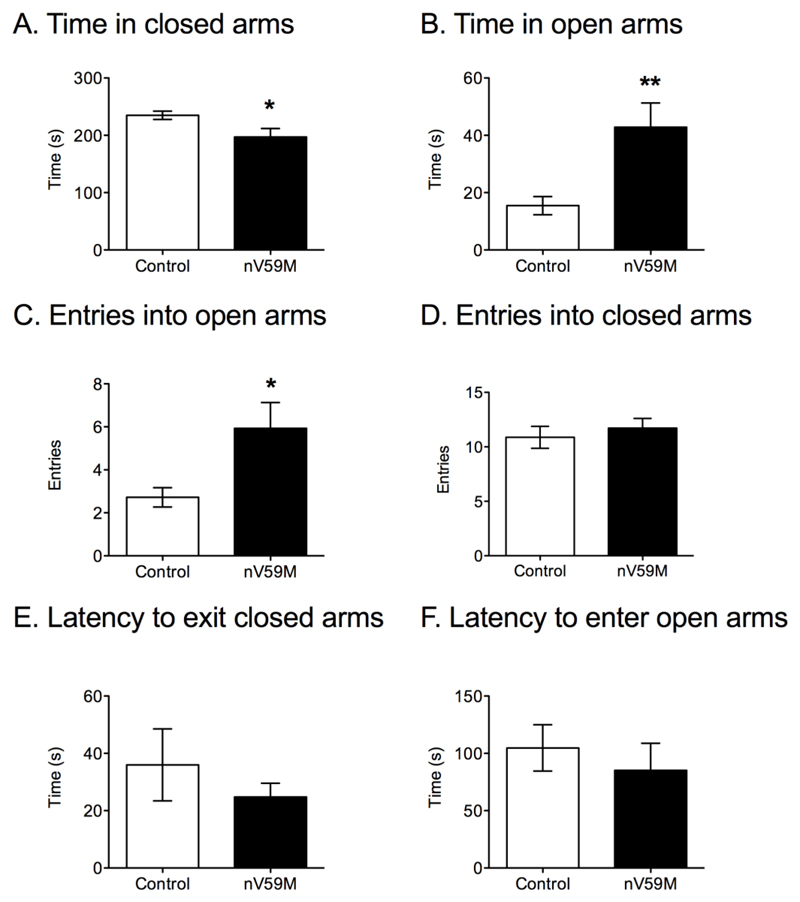
The plus maze is an anxiety task that measures the tendency of mice to remain in closed, protected spaces, such as the closed arms of the maze. nV59M mice spent significantly less time in the closed arms than control mice (Fig. 5A) and spent longer exploring the open arms of the maze (Fig. 5B). This was also reflected in an increased number of entries into the open arms (Fig. 5C). There was no difference in the number of entries into the closed arms between nV59M and control mice (Fig. 5D), indicating the overall activity levels were similar for both groups. In addition, there was a tendency for nV59M mice to take less time to exit the closed arms, although statistical significance was not reached (Fig. 5E). No significant difference in the latency to enter the open arms (Fig. 5F), the number of entries into the centre square or the time spent in the central square was observed (Supplementary Fig. 1).
Figure 5. Plus maze test.
(A) Amount of time spent in the closed arms, (B) amount of time spent in the open arms (C) number of entries into the open arms, (D) number of entries into the closed arms, (E) latency to exit the closed arms, and (F) latency to enter the open arms for adult (11-20 week-old) control (wild-type: n=1; ROSA+/-: n=12; Nes-Cre+: n=13) and nV59M (n=14) littermates over a 300s period. Data are mean ± SEM. * P<0.05; ** P<0.01 (Mann-Whitney U-test).
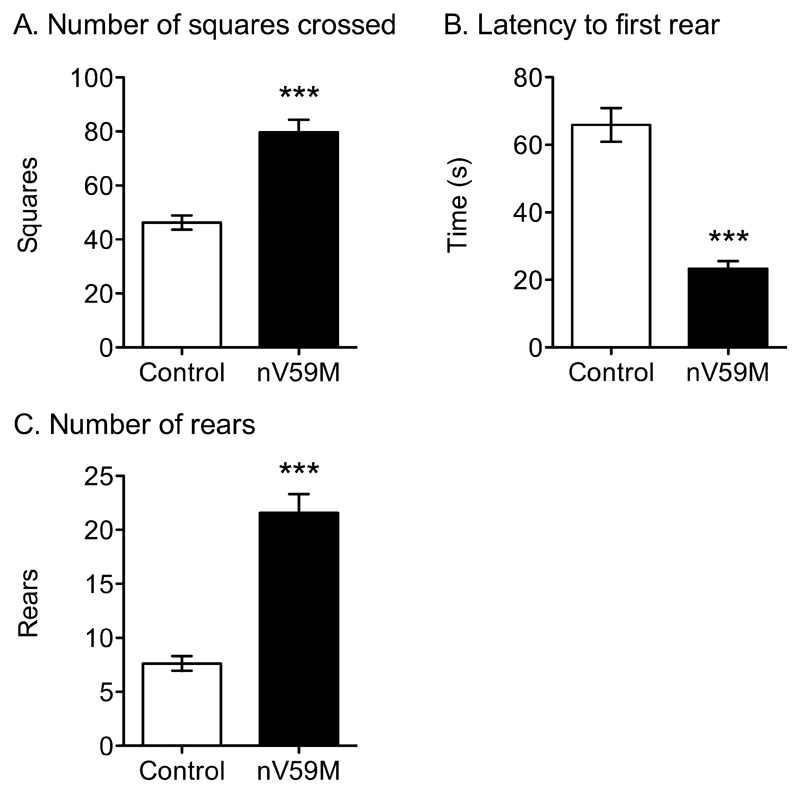
A separate cohort of nV59M mice and control littermates were tested on the non-axiogenic open field task to assess their basal locomotor activity and their exploratory behaviour [28]. Open field activity, as measured by the number of squares crossed (Fig. 6A) was significantly increased in nV59M mice compared to control littermates. Furthermore, nV59M mice reared earlier and significantly more often than control mice (Fig. 6B-C). No differences were observed in terms of faecal boli and urination (data not shown).
Figure 6. Low anxiety open field test.
(A) Number of squares crossed, (B) latency to first rear, and (C) number of rears for adult (11-14 week-old) control (wild-type: n=6; ROSA+/-: n=24; Nes-Cre+: n=23) and nV59M (n=14) littermates over a 300s period. Data are mean ± SEM. *** p<0.0001 (Mann-Whitney U-test).
4. Discussion
Neuronal expression of KATP channels containing Kir6.2-V59M subunits in mice results in muscle weakness, balance and coordination problems, and hyperactivity [8]. We now show that neuronal expression of this gain-of-function mutation also results in altered emotionality.
Location of the transgene
It is predicted that the mutant KATP channel exerts its effect by reducing neuronal electrical activity. The nV59M mouse model was generated by crossing the ROSA-Kir6.2-V59M line [30] with the Nes-cre line [8]. It has previously been shown, using using three different reporter lines, that Cre-mediated recombination occurs in virtually all nervous tissue cells [35]. Thus the Kir6.2-V59M transgene is expected to be expressed in all brain areas. Nevertheless, mutant KATP channels will only occur in those cells that also express SUR, because Kir6.2 is unable to traffic to the plasma membrane in the absence of SUR [36]. Our quantitative PCR results show that SUR1 levels in brain tissue of nV59M mice are similar to those in control littermates, suggesting that the overall KATP channel density should remain relatively constant.
Previous in situ hybridization and glibenclamide binding studies have shown that Kir6.2 and SUR1 are widely expressed throughout the brain, with particular high levels observed in the hippocampus, cerebellum and substantia nigra [4,37]. Strong expression was also observed in the hippocampus, which has been implicated in affective behaviours [38]. Relatively weak KATP channel expression was observed in the nucleus accumbens and amygdala [4,37]. While this provides some support for the idea that the hippocampus may be involved in the differences in affective behaviour we observed in nV59M mice, it is impossible to be certain that this is the only, or indeed the most important, location.
Behavioural testing
The light/dark box, successive alleys and plus maze are designed to assess anxiety-like behaviour in mice. These tasks produce an approach-avoidance conflict in which the tendency of a mouse to explore a novel environment is in conflict with its desire to avoid brightly lit and exposed places [33,39]. In the successive alleys test, nV59M mice spent significantly longer exploring the more anxiogenic alleys and transitioned more between the alleys. They also spent significantly longer than control mice exploring the more anxiogenic arms of the plus maze. This suggests they experience less anxiety than controls.
In contrast, the results of the light/dark box experiments tend to favour the opposite idea. A decrease in transitions between the dark and light compartments of the light/dark box, and a reduced number of rears in the light compartment, are normally considered an indicator of anxiogenic activity [39,40]. We found that nV59M mice took significantly longer to cross into the dark compartment than control mice and transitioned significantly less between the two compartments. It is therefore possible that nV59M mice have a particular aversion to light but do not find exposure threatening.
Limitations
We cannot rule out the possibility that there may be cumulative effects of repeated behavioural testing such that the results on the later tests may have been influenced by the prior experience of the animals on earlier tests. However, it is important to point out that we got essentially the same result across all three tests. The nV59M mice were slower to the leave the brightly lit/anxiogenic section of the light dark box, spent more time both in the more exposed, anxiogenic sections of the successive alleys, and in the more anxiogenic, open arms of the EPM. Therefore, we saw the same phenotype consistently across all three tests, irrespective of any cumulative effect of testing. Additionally, we cannot completely rule out the possibility that the effects we see on our anxiety tests are driven, in part, by differences in locomotor activity rather than by direct effects on anxiety. It is notoriously difficult to disentangle effects on anxiety from effects on locomotor activity. At the behavioural level, performance measures used to assess anxiety very often depend on activity levels. Conversely, it is difficult to assess exploration/activity levels in the complete absence of anxiety. Furthermore, it is important to point out that psychological definitions of anxiety emphasise a motoric component (or more precisely an inhibition of ongoing motor behaviour; see [41,42]).
Anxiety is said to occur when there is competition between concurrently available goals or response choices. Anxiety arises when there are situations of conflict, uncertainty or ambiguity. In most ethological, unconditioned tests of anxiety that are used with rodents (e.g. elevated plus maze, successive alleys), there is an approach/avoidance conflict and the animal must choose whether to explore the open arms or stay safe in the enclosed sections. When situations of conflict and uncertainty arise, a constellation of responses are evoked, including the behavioural inhibition of on-going motor activity. Thus, the phenotype of reduced anxiety, in part, reflects inappropriate motor activity (i.e. the experimental animal demonstrates inappropriate motor activity in situations where the control animal would exhibit inhibition of motor activity). Thus, the relationship between activity and anxiety is complex.
In the context of the present data, the key observation is that the nV59M mice are not hyperactive in every experimental setting. They took longer to cross from the light to the dark sections of the light/dark box, and made less crossings altogether. Furthermore, there were no genotype differences in terms of entries into closed arms of the elevated plus maze. This measure is often used as a control for more general changes in activity levels during this anxiety test. Therefore, the nV59M mice are not always more active than the controls. Thus, the present data cannot be explained by a global change in activity levels.
Conclusions
Knockout of the Kir6.2 subunit results in increased anxiety as evaluated by the light/dark box, successive alleys and elevated plus maze tests [28]. In contrast, the results of our successive alleys and elevated plus maze tests suggest that enhanced activity of the KATP channel leads to less anxiogenesis. Taken together, these findings argue for a role of the neuronal KATP channel in the regulation of emotion: loss of KATP channel activity leads to increased anxiety levels while increased KATP channel activity results in decreased anxiety levels.
Furthermore, the results from the low anxiety open field suggest not only that nV59M mice are more active, consistent with previous findings [8], but that they also display increased exploratory behaviour. Knockout of the Kir6.2 subunit has previously been shown to result in the opposite phenotype - a selective decrease of initial locomotor activation in the open field as well as a significant reduction in the number of rears [29]. Selective knockout of Kir6.2 in substantia nigra dopaminergic neurones replicates this phenotype [29]. Our results, in conjunction with those of Schiemann and colleagues [29], argue for the involvement of the neuronal KATP channel in the initiation and regulation of exploratory behaviour.
The reduced anxiety phenotype and increased exploratory behaviour, in combination with the previously reported hyperactivity of nV59M mice [8], appears to correlate with the increased impulsivity and inattentiveness reported in iDEND/DEND patients [21,24,26,27].
Supplementary Material
5. Acknowledgements
We thank David Bannerman and Amy Taylor for helping design the behavioural experiments. We thank the Wellcome Trust (grant no: 089795/Z/09/Z), the Royal Society and the EU (LSHM-CT-2006-518153 and 332620) for support. CL holds Wellcome Trust Prize (OXION) and Clarendon Fund Studentships. FMA holds a Royal Society-Wolfson Research Merit Award.
References
- [1].Ashcroft SJ, Ashcroft FM. Properties and functions of ATP-sensitive K-channels. Cell Signal. 1990;2:197–214. doi: 10.1016/0898-6568(90)90048-f. [DOI] [PubMed] [Google Scholar]
- [2].Seino S, Miki T. Physiological and pathophysiological roles of ATP-sensitive K+ channels. Prog Biophys Mol Biol. 2003;81:133–76. doi: 10.1016/s0079-6107(02)00053-6. [DOI] [PubMed] [Google Scholar]
- [3].Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–6. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- [4].Karschin C, Ecke C, Ashcroft FM, Karschin A. Overlapping distribution of K(ATP) channel-forming Kir6.2 subunit and the sulfonylurea receptor SUR1 in rodent brain. FEBS Lett. 1997;401:59–64. doi: 10.1016/s0014-5793(96)01438-x. [DOI] [PubMed] [Google Scholar]
- [5].Trapp S, Ballanyi K. KATP channel mediation of anoxia-induced outward current in rat dorsal vagal neurons in vitro. J Physiol (Lond) 1995;487(Pt 1):37–50. doi: 10.1113/jphysiol.1995.sp020859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pocai A, Lam TKT, Gutierrez-Juarez R, Obici S, Schwartz GJ, Bryan J, et al. Hypothalamic K(ATP) channels control hepatic glucose production. Nature. 2005;434:1026–31. doi: 10.1038/nature03439. [DOI] [PubMed] [Google Scholar]
- [7].Griesemer D, Zawar C, Neumcke B. Cell-type specific depression of neuronal excitability in rat hippocampus by activation of ATP-sensitive potassium channels. Eur Biophys J. 2002;31:467–77. doi: 10.1007/s00249-002-0241-3. [DOI] [PubMed] [Google Scholar]
- [8].Clark RH, McTaggart JS, Webster R, Mannikko R, Iberl M, Sim XL, et al. Muscle dysfunction caused by a KATP channel mutation in neonatal diabetes is neuronal in origin. Science. 2010;329:458–61. doi: 10.1126/science.1186146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Liss B, Bruns R, Roeper J. Alternative sulfonylurea receptor expression defines metabolic sensitivity of K-ATP channels in dopaminergic midbrain neurons. Embo J. 1999;18:833–46. doi: 10.1093/emboj/18.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Steffens AB, Strubbe JH, Balkan B, Scheurink JW. Neuroendocrine mechanisms involved in regulation of body weight, food intake and metabolism. Neurosci Biobehav Rev. 1990;14:305–13. doi: 10.1016/s0149-7634(05)80040-5. [DOI] [PubMed] [Google Scholar]
- [11].Levin BE, Dunn-Meynell AA, Routh VH. Brain glucose sensing and body energy homeostasis: role in obesity and diabetes. Am J Physiol. 1999;276:R1223–31. doi: 10.1152/ajpregu.1999.276.5.R1223. [DOI] [PubMed] [Google Scholar]
- [12].Miki T, Liss B, Minami K, Shiuchi T, Saraya A, Kashima Y, et al. ATP-sensitive K+ channels in the hypothalamus are essential for the maintenance of glucose homeostasis. Nat Neurosci. 2001;4:507–12. doi: 10.1038/87455. [DOI] [PubMed] [Google Scholar]
- [13].Nichols CG, Shyng SL, Nestorowicz A, Glaser B, Clement JP, Gonzalez G, et al. Adenosine diphosphate as an intracellular regulator of insulin secretion. Science. 1996;272:1785–7. doi: 10.1126/science.272.5269.1785. [DOI] [PubMed] [Google Scholar]
- [14].Muñoz A, Nakazaki M, Goodman JC, Barrios R, Onetti CG, Bryan J, et al. Ischemic preconditioning in the hippocampus of a knockout mouse lacking SUR1-based K(ATP) channels. Stroke. 2003;34:164–70. doi: 10.1161/01.str.0000048215.36747.d1. [DOI] [PubMed] [Google Scholar]
- [15].Bancila V, Nikonenko I, Dunant Y, Bloc A. Zinc inhibits glutamate release via activation of pre-synaptic K channels and reduces ischaemic damage in rat hippocampus. J Neurochem. 2004;90:1243–50. doi: 10.1111/j.1471-4159.2004.02587.x. [DOI] [PubMed] [Google Scholar]
- [16].Liss B, Roeper J. A role for neuronal K(ATP) channels in metabolic control of the seizure gate. Trends Pharmacol Sci. 2001;22:599–601. doi: 10.1016/s0165-6147(00)01861-7. discussion601-2. [DOI] [PubMed] [Google Scholar]
- [17].Yamada K, Ji JJ, Yuan H, Miki T, Sato S, Horimoto N, et al. Protective role of ATP-sensitive potassium channels in hypoxia-induced generalized seizure. Science. 2001;292:1543–6. doi: 10.1126/science.1059829. [DOI] [PubMed] [Google Scholar]
- [18].Tanner GR, Lutas A, Martínez-François JR, Yellen G. Single K ATP channel opening in response to action potential firing in mouse dentate granule neurons. Journal of Neuroscience. 2011;31:8689–96. doi: 10.1523/JNEUROSCI.5951-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Giménez-Cassina A, Martínez-François JR, Fisher JK, Szlyk B, Polak K, Wiwczar J, et al. BAD-dependent regulation of fuel metabolism and K(ATP) channel activity confers resistance to epileptic seizures. Neuron. 2012;74:719–30. doi: 10.1016/j.neuron.2012.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Hattersley AT, Ashcroft FM. Activating mutations in Kir6.2 and neonatal diabetes: new clinical syndromes, new scientific insights, and new therapy. Diabetes. 2005;54:2503–13. doi: 10.2337/diabetes.54.9.2503. [DOI] [PubMed] [Google Scholar]
- [21].Mlynarski W, Tarasov AI, Gach A, Girard CA, Pietrzak I, Zubcevic L, et al. Sulfonylurea improves CNS function in a case of intermediate DEND syndrome caused by a mutation in KCNJ11. Nat Clin Pract Neurol. 2007;3:640–5. doi: 10.1038/ncpneuro0640. [DOI] [PubMed] [Google Scholar]
- [22].Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350:1838–49. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- [23].Edghill EL, Flanagan SE, Ellard S. Permanent neonatal diabetes due to activating mutations in ABCC8 and KCNJ11. Rev Endocr Metab Disord. 2010;11:193–8. doi: 10.1007/s11154-010-9149-x. [DOI] [PubMed] [Google Scholar]
- [24].Slingerland AS, Hurkx W, Noordam K, Flanagan SE, Jukema JW, Meiners LC, et al. Sulphonylurea therapy improves cognition in a patient with the V59M KCNJ11 mutation. Diabet Med. 2008;25:277–81. doi: 10.1111/j.1464-5491.2007.02373.x. [DOI] [PubMed] [Google Scholar]
- [25].Pearl PL. Inherited Metabolic Epilepsies. 1st ed. New York: Demos Medical Publishing; 2012. [Google Scholar]
- [26].Mohamadi A, Clark LM, Lipkin PH, Mahone EM, Wodka EL, Plotnick LP. Medical and developmental impact of transition from subcutaneous insulin to oral glyburide in a 15-yr-old boy with neonatal diabetes mellitus and intermediate DEND syndrome: extending the age of KCNJ11 mutation testing in neonatal DM. Pediatr Diabetes. 2010;11:203–7. doi: 10.1111/j.1399-5448.2009.00548.x. [DOI] [PubMed] [Google Scholar]
- [27].Battaglia D, Lin Y-W, Brogna C, Crinò A, Grasso V, Mozzi AF, et al. Glyburide ameliorates motor coordination and glucose homeostasis in a child with diabetes associated with the KCNJ11/S225T, del226-232 mutation. Pediatr Diabetes. 2012;13:656–60. doi: 10.1111/j.1399-5448.2012.00874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Deacon RMJ, Brook RC, Meyer D, Haeckel O, Ashcroft FM, Miki T, et al. Behavioral phenotyping of mice lacking the K ATP channel subunit Kir6.2. Physiol Behav. 2006;87:723–33. doi: 10.1016/j.physbeh.2006.01.013. [DOI] [PubMed] [Google Scholar]
- [29].Schiemann J, Schlaudraff F, Klose V, Bingmer M, Seino S, Magill PJ, et al. K-ATP channels in dopamine substantia nigra neurons control bursting and novelty-induced exploration. Nat Neurosci. 2012;15:1272–80. doi: 10.1038/nn.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Girard CA, Wunderlich FT, Shimomura K, Collins S, Kaizik S, Proks P, et al. Expression of an activating mutation in the gene encoding the KATP channel subunit Kir6.2 in mouse pancreatic beta cells recapitulates neonatal diabetes. J Clin Invest. 2009;119:80–90. doi: 10.1172/JCI35772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Crawley JN. Exploratory behavior models of anxiety in mice. Neurosci Biobehav Rev. 1985;9:37–44. doi: 10.1016/0149-7634(85)90030-2. [DOI] [PubMed] [Google Scholar]
- [33].Contet C, Rawlins JN, Deacon RM. A comparison of 129S2/SvHsd and C57BL/6JOlaHsd mice on a test battery assessing sensorimotor, affective and cognitive behaviours: implications for the study of genetically modified mice. Behav Brain Res. 2001;124:33–46. doi: 10.1016/s0166-4328(01)00231-5. [DOI] [PubMed] [Google Scholar]
- [34].Lister RG. The use of a plus-maze to measure anxiety in the mouse. Psychopharmacology (Berl) 1987;92:180–5. doi: 10.1007/BF00177912. [DOI] [PubMed] [Google Scholar]
- [35].Dubois NC, Hofmann D, Kaloulis K, Bishop JM, Trumpp A. Nestin-Cre transgenic mouse line Nes-Cre1 mediates highly efficient Cre/loxP mediated recombination in the nervous system, kidney, and somite-derived tissues. Genesis. 2006;44:355–60. doi: 10.1002/dvg.20226. [DOI] [PubMed] [Google Scholar]
- [36].Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron. 1999;22:537–48. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- [37].Mourre C, Widmann C, Lazdunski M. Sulfonylurea binding sites associated with ATP-regulated K+ channels in the central nervous system: autoradiographic analysis of their distribution and ontogenesis, and of their localization in mutant mice cerebellum. Brain Res. 1990;519:29–43. doi: 10.1016/0006-8993(90)90057-i. [DOI] [PubMed] [Google Scholar]
- [38].Bannerman DM, Rawlins JNP, McHugh SB, Deacon RMJ, Yee BK, Bast T, et al. Regional dissociations within the hippocampus--memory and anxiety. Neurosci Biobehav Rev. 2004;28:273–83. doi: 10.1016/j.neubiorev.2004.03.004. [DOI] [PubMed] [Google Scholar]
- [39].Buccafusco JJ, Bailey KR, Crawley JN. Anxiety-Related Behaviors in Mice. 2nd ed. Boca Raton (FL): CRC Press; 2009. [PubMed] [Google Scholar]
- [40].Bourin M, Hascoët M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- [41].Gray JA. A theory of anxiety: the role of the limbic system. Encephale. 1983;9:161B–166B. [PubMed] [Google Scholar]
- [42].McNaughton N, Gray JA. Anxiolytic action on the behavioural inhibition system implies multiple types of arousal contribute to anxiety. J Affect Disord. 2000;61:161–76. doi: 10.1016/s0165-0327(00)00344-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.