Abstract
Chau Cuica was the name given by the regional government of Loreto in Peru for its school-based deworming program which was initiated in 2012 with a donation of mebendazole from an international non-governmental organization. Embedded in the program from the start was a sentinel surveillance component which consisted of 16 sentinel schools representing Loreto’s seven provinces. Coverage rates varied between 35% and 61% over the first two years of the program (and seven deworming cycles). Initial prevalences of soil-transmitted helminth infections were high, with 82.4% of schoolchildren having at least one infection and prevalences of both A. lumbricoides and T. trichiura infections both exceeding 60%. After two years, these prevalences had dropped to 56% for any STH infection, 38% for A. lumbricoides and 34% for T. trichiura. Importantly, the proportions of children with moderate and heavy infections also dropped. Both the regional Ministry of Health and the Ministry of Education were jointly charged to implement this deworming program. The program’s costs were estimated to be approximately 22 cents (USD) per child per deworming cycle. The responsibility for the surveillance component was initially undertaken by research partners from a local NGO and a Canadian university, which transferred gradually over the course of the deworming program to being entirely the responsibility of the Ministry of Health. This regional deworming program may serve as a model for other jurisdictions who are planning a school-based deworming program with an integrated surveillance component to monitor impact.
Keywords: Deworming, Peru, school-age children, soil-transmitted helminth infections, monitoring
Background
School-based deworming programs are widely recognized as a cost-effective way of reducing the disease burden caused by soil-transmitted helminth (STH) infections in endemic areas [1]. The prevalence and intensity of these infections usually peak in the school ages and cause both physical and cognitive impairments which can extend over the lifespan[2]. For these reasons, WHO has identified school-age children as a high risk group at increased morbidity from STH infections, and, consequently, for benefiting from deworming programs[3]. The urgency to initiate deworming programs in school-age children captured world attention in 2001 when the World Health Assembly unanimously ratified Resolution 54.19. This resolution set out the target of providing deworming to at least 75% of at-risk school-age children by 2010 [4]. While this target was only partially met, a subsequent road map was initiated and progress on a number of political, scientific and social fronts was realized[5].
School-based deworming programs are the most common way to reach school-age children even when some proportion of these children are not enrolled [6]. Depending on the level of endemicity, school-based deworming programs distribute anthelminthic drugs once, twice, or even three times per year [3]. Following the London Declaration in 2012, the two pharmaceutical companies producing single dose benzimidazole drugs (i.e. GlaxoSmithKline (albendazole) and Johnson & Johnson (mebendazole)) have pledged to meet the deworming drug requirements of all school-age children, in all endemic areas, every year, up to 2020 [7]. These two companies have partnered with WHO in directing the deworming drugs to those countries that meet the minimum eligibility and accountability requirements.
From data reported in the 2014 WHO PCT Databank, a total of 33,700, 059 school-age children living in 25 STH-endemic countries in the American Region of WHO (AMRO) (administered by the Pan American Health Organization) were estimated to be eligible for periodic deworming [8]. For 2014, of the 11 countries reporting coverage data, a mean regional coverage rate (in school-age children) was calculated to be 57%. Seven of these countries had rates exceeding 75% [8]. Mexico has had one of the longest running country-wide child deworming programs in the world (beginning in 1993) but most Latin American countries are only now developing national deworming policies [9 10].
Peru is a country that is developing its national deworming policy. Loreto is one of its most STH-endemic regions and, because of this, it has attracted parasite-focused research attention in the recent past [11–14]. In 2012, the Regional Government of Loreto received a donation of 1,386,000 tablets of single dose (500 mg) mebendazole from an international NGO (Ayuda Humanitaria Operación Benedición Internacional Perú (Operation Blessing International)) and decided to implement a regional school-based deworming program. The program was named Chau Cuica (meaning “Goodbye Worms” in the native Peruvian Quechua language). The program’s aim was to provide deworming to all school-age children in the region three times per year. This initiative created a unique opportunity for operational research to inform Peru’s rapidly evolving development of a national policy on deworming. This operational research was conducted by a research partnership between a Loreto-based NGO, the Asociación Civil Selva Amazónica; the Dirección Regional de Salud de Loreto and the Research Institute of the McGill University Health Centre (in Canada). Here we describe the governance and organization of the Loreto regional deworming program, provide results of its accountability (in terms of coverage and STH indicators after two years of operation) and comment on the sustainability of the program.
Methods
Ethics Approval
This study received ethics approval in Perú from the “Comité Institucional de Bioética of the Asociación Civil Impacta Salud y Educación”, in Lima, and the local Ministry of Health office (Dirección Regional de Salud de Loreto) in Iquitos. Ethics approval was obtained in Canada from the Research Ethics Board of the Research Institute of the McGill University Health Centre in Montreal. Written informed consent was obtained from a parent or guardian of each child who participated in the sentinel surveillance component of the study.
Study design
Information regarding program implementation and coverage was obtained by reviewing the deworming program records at the Dirección Regional de Medicamentos Insumos y Drogas (Regional Drug Agency-DIREMID) and by key informant interviews at the DIREMID and the Regional Health Directorate (DIRESA). Schools administering the drug but not reporting were not considered as covered by the program. Impact assessment was measured using a repeated cross-sectional research design. All assessments immediately preceded drug administration and included a baseline assessment in July 2012 and every four months thereafter: in November 2012, March 2013, July 2013, November 2013, March 2014 and July 2014. In total, one baseline survey and six monitoring surveys were analyzed, over the first two years (and seven deworming cycles) of program implementation.
Study population for impact assessment
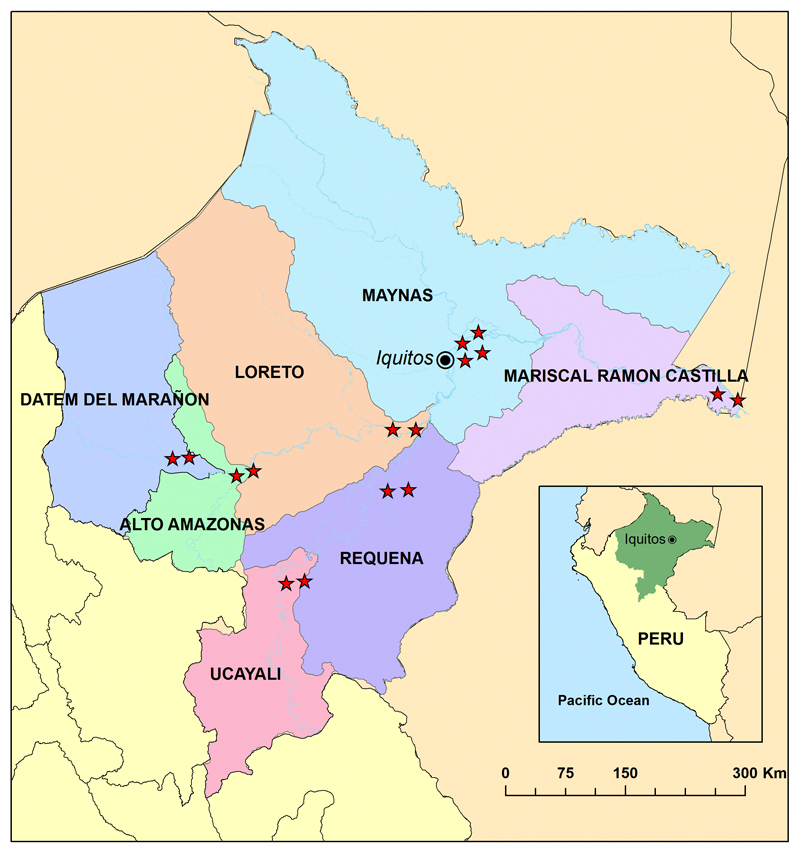
The impact assessment was carried out in 16 sentinel schools in the 7 provinces of the Loreto Region (Figure 1). The sentinel schools were randomly selected from all primary schools having a minimum enrolment of 50 students in grades 4 and 5 and that were located in the catchment area of the largest health centre serving peri-urban and rural communities surrounding the provincial capital. Two schools were chosen in each one of the provinces except in the case of Maynas where four schools were selected in the capital city of Iquitos. Before data collection for each deworming cycle, personnel from the local health centres held information meetings at each school. Subsequently, parents of grade 4 and grade 5 students were invited to provide their informed consent. The assent of each child in grades 4 and 5 in the sentinel schools was also obtained.
Figure 1.
Map of the region of Loreto, Peru, showing the 16 sites for the school-based sentinel surveillance component of the deworming program in each province.
Data collection
All government-organized program activities were reported by the Director of the DIRESA (HRF). All data collection procedures were jointly planned by personnel from the DIRESA, the Asociación Civil Selva Amazónica and the Research Institute of McGill University Health Centre. Data pertaining to program organization and coverage were collected by Ministry of Health personnel working in each of the provincial capitals, supervised by the Regional Drug Monitoring Office. Child-specific variables were collected by local nurses working in the corresponding health centres and included date of birth, sex, hemoglobin levels, anthropometry and STH infection status. A fingerprick blood sample was obtained to measure hemoglobin levels using a Hemocue® device. Subsequently, height and weight were ascertained. Finally, a stool specimen was collected. Specimens were transported to the local provincial laboratory within 24 hours of collection and analyzed using the Kato-Katz technique to assess presence and intensity of STH infections.
Prior to the baseline assessment, a 3-day training workshop on laboratory and monitoring assessments was conducted in Iquitos for provincial teams (each composed of one nurse and one laboratory technologist). Nurses were trained on informed consent administration and on techniques for anthropometry, hemoglobin assessment and stool specimen collection and transport. Laboratory technologists were trained on the Kato-Katz technique and data recording.
Data were compiled using EXCEL (Microsoft) and analyzed using Stata 14 (StataCorp).
Sample size
A minimum of 50 grade 4 and grade 5 children from each of the 16 sentinel schools (a total of 800 schoolchildren) were planned to be recruited at each assessment timepoint. With this sample size it would be possible to detect a minimum difference of 7% in the prevalence of any STH (between baseline and follow-up time point) at a 5% significance level, with 80% power, assuming a baseline prevalence (of any STH) of 50%. The total sample size could exceed 800 as all children in a class would be included in an assessment, if the class had more than 50 eligible children.
Variables definition
Anemia was defined using different cut points for age and sex groups as suggested by WHO[15]. Stunting was calculated using the STATA WHO 2007 package for the “AnthroPlus” software provided by WHO for children between the ages of 5 and 19 years. Children were defined as being stunted if they were two standard deviations or more below the median height-for-age of the reference population.
Statistical analysis
To estimate the marginal population-average effect of the deworming program (comparing baseline data to the final monitoring timepoint data) on STH infections, anemia and stunting we used generalized estimating equation (GEE) computation techniques and adjusted for age, sex and province (when applicable). The outcomes were assumed to be correlated within schools with an exchangeable correlation structure. A t test was used to examine parasite-specific differences in mean intensity of infection between baseline and the end date. The GEE approach as described above was also used to estimate the percent change in the median parasite-specific intensity of infection by month. Intensities were log-transformed prior to analysis to normalize the variable. Results are expressed as percent change with 95% confidence intervals around the estimate.
Results
Program governance and organization
Chau Cuica was created as an initiative of the Regional Government of Loreto to improve the health of children between 3 years and 17 years of age. This was a political decision made by the President of the Regional Government of Loreto who mandated the region’s Ministry of Health and Ministry of Education to coordinate this deworming program. Because recent local research had confirmed an exceptionally high prevalence of STH infections among schoolchildren (86%)[16], and because there was sufficient deworming drugs in the form of a donation, in accordance with WHO guidelines, the frequency of administration was set at three deworming cycles per year. The Ministry of Health was charged with designing the distribution plan in the schools, making the drug available to the school treatment points in each of the seven provinces, collecting program distribution information and compiling and analyzing the data. The role of the Ministry of Education was to administer the deworming treatment to all children (in pre-primary, primary and secondary schools), to record the treatment administration information, and to submit the corresponding reports to the Ministry of Health within a reasonable time delay after every deworming cycle.
The monitoring component of the program was carried out jointly by the Ministry of Health Regional Drug Regulatory Office (DIREMID), in collaboration with the Ministry of Education and experts from the Research Institute of the McGill University Health Centre and the Asociación Civil Selva Amazónica.
Program coverage
In 2012, according to registries of the Regional Government of Loreto, the school population comprised 274,763 children between the ages of 3 and 17 years of age in a total of 4,746 schools (Table 1).
Table 1.
Students enrolled in schools in the region of Loreto, Peru, by province, in 2012
| Province | Pre-School (# schools) |
Primary School (# schools) |
Secondary School (# schools) |
Total Students (# schools) |
|---|---|---|---|---|
| Alto Amazonas | 7892 (259) | 23816 (330) | 8588 (49) | 40296 (638) |
| Datem Del Marañon | 4138 (211) | 13401 (319) | 3833 (57) | 21372 (587) |
| Loreto | 4324 (199) | 11954 (287) | 4108 (73) | 20386 (559) |
| Maynas | 28041 (652) | 69718 (836) | 32278 (213) | 130037 (1701) |
| Mariscal Ramon Castilla | 4024 (143) | 12445 (203) | 3443 (29) | 19912 (375) |
| Requena | 4781 (178) | 11788 (222) | 4327 (52) | 20896 (452) |
| Ucayali | 5324 (171) | 12185 (207) | 4355 (56) | 21864 (434) |
| Total (Region) | 58524 (1813) | 155307 (2404) | 60932 (529) | 274763 (4746) |
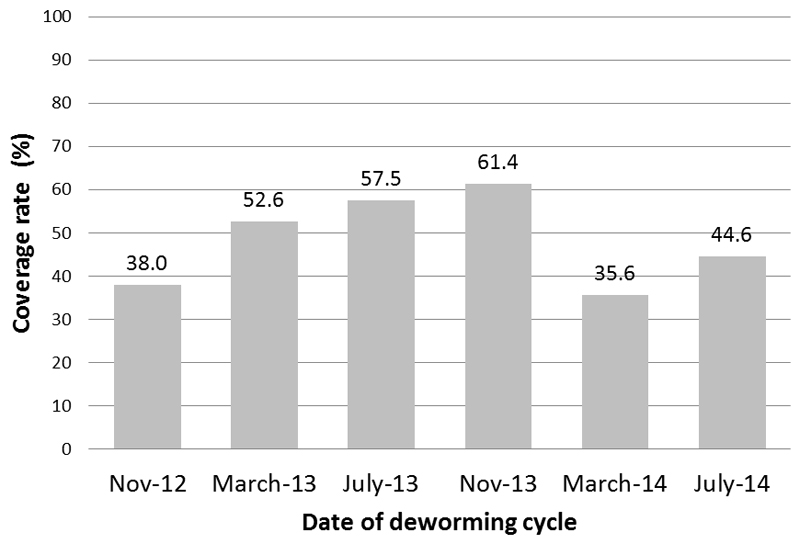
This number represents 94% of the total population of this age range in Loreto. The coverage of the deworming program, by deworming cycle, starting at the second cycle, is shown in Figure 2. (Preparations for obtaining coverage for the first deworming cycle could not be completed in time.) Coverage rates ranged from a low of 32% in November 2012 to a high of 61% in July 2013, with lower rates at the two-year anniversary month (i.e. July 2014). Although data on underreporting were not collected systematically, Ministry of Health officials noted that reporting was more frequently provided by schools located closer to the provincial capitals than those located farther away. The overall coverage rate conceals important variation in coverage rates in each province and over the two years of the deworming program (data not shown). These provincial-level data also show that coverage was similar in girls and boys.
Figure 2.
Coverage rates of the deworming program in Loreto, Peru, July 2012-July 2014.
Program Impact
Over 800 children from the 16 sentinel schools participated in the periodic assessments of each cycle (Table 2).
Table 2.
Number of children participating in the sentinel surveillance component of each deworming cycle, Loreto, Peru, 2012-2014.
| Province | Jul-12 | Nov-12 | Mar-13 | Jul-13 | Nov-13 | Mar-14 | Jul-14 |
|---|---|---|---|---|---|---|---|
| Alto Amazonas | 101 | 110 | 100 | 118 | 126 | 115 | 113 |
| Requena | 105 | 115 | 109 | 111 | 107 | 102 | 108 |
| Ramon Castilla | 100 | 100 | 101 | 101 | 102 | 102 | 105 |
| Loreto | 100 | 100 | 100 | 100 | 100 | 100 | 101 |
| Ucayali | 101 | 104 | 100 | 105 | 101 | 105 | 103 |
| Datem del Marañon | 98 | 92 | 94 | 103 | 90 | 103 | 102 |
| Maynas | 211 | 214 | 203 | 214 | 205 | 208 | 209 |
| Total (Region) | 816 | 835 | 807 | 852 | 831 | 835 | 841 |
Baseline data
Of the 816 children assessed at the July 2012 baseline, the mean age was 10.8 years and there were more girls than boys (55% vs 45%). STH infections were highly prevalent in all seven provinces with 82.4% of the children infected with at least one STH infection. The prevalence of Ascaris lumbricoides infection was the highest, at 65.2%, followed by Trichuris trichiura infections at 64.83%. Hookworm infections were less frequent with only 4.2% of the children infected. The intensity of infection was also high at baseline where 40.0% and 25.3% of the children had a heavy or moderate infection for Ascaris and Trichuris, respectively. There were no heavy or moderate hookworm infections. One of every five children (i.e. 20.9%) were anemic although the mean hemoglobin level was over 12 g/dl (12.35; 95% CI: 12.27 – 12.43). The prevalence of stunting was 23.4%. Prevalences of STH infections, anemia and stunting were not statistically significantly different between girls and boys.
Prevalences of infection, intensity of infection and anemia varied greatly between provinces (Table 3). Ascaris infection dominated the STH infection profile with prevalences higher than 50% in all provinces except Maynas. In addition, the proportion of children having moderate or heavy intensity Ascaris infection exceeded 20% in all provinces except Alto Amazonas. Trichuris infection was also highly prevalent, ranging from a low of 24% in Alto Amazonas to a high of 87% in Datem del Maranon. The occurrence of moderate and heavy Trichuris infections was more focal in nature with only four provinces recording proportions over 20%. The prevalence of hookworm was quite low, registering 10% in Alto Amazonas, 5% in Maynas and 2% or less in the remaining provinces.
Table 3.
Baseline prevalence of STH infections, proportion of moderate and heavy intensity infections, and prevalence of stunting and anemia, by province, in July 2012, Loreto, Peru.
| Province | Prevalence (%) of Ascaris Infections | Prevalence (%) of moderate and heavy Ascaris infections | Prevalence (%) of Trichuris Infections | Prevalence (%) of moderate and heavy Trichuris infections | Prevalence (%) of hookworm Infections | Prevalence (%)of moderate and heavy hookworm infections | Prevalence (%) of any STH Infection | Prevalence (%) of any moderate and heavy STH infections | Stunting | Anemia |
|---|---|---|---|---|---|---|---|---|---|---|
| Alto Amazonas | 53.5 | 4.0 | 23.8 | 1.0 | 9.9 | 0 | 77.2 | 5.0 | 18.8 | 7.9 |
| Datem del Marañon | 75.5 | 38.8 | 86.7 | 68.4 | 2.0 | 0 | 96.9 | 75.5 | 30.6 | 18.3 |
| Loreto | 82.0 | 59 | 77 | 23 | 8 | 0 | 100 | 61 | 24 | 24 |
| Maynas | 37.4 | 22.2 | 49.3 | 9.4 | 5.2 | 0 | 60.6 | 27.0 | 25.4 | 19.4 |
| Ramon Castilla | 76 | 40.0 | 79 | 42 | 0 | 0 | 81 | 55 | 20 | 13 |
| Requena | 72.4 | 55.2 | 69.5 | 9.5 | 1.0 | 0 | 90.5 | 60 | 30.4 | 35.2 |
| Ucayali | 90.1 | 79.2 | 86.1 | 42.6 | 2.0 | 0 | 94.1 | 84.2 | 29.7 | 29.7 |
An important proportion of children in every province was stunted, with all province-specific prevalences over 18%. Two provinces had stunting prevalence levels greater than 30%. The prevalence of anemia ranged from 8% in Alto Amazonas to 35% in Requena.
Regional Impact of Chau Cuica over the first two years
Table 4 shows the impact of the deworming program over time on the prevalence and intensity of STH infections, anemia and stunting. Statistically significant reductions in prevalences were observed between baseline and two years later. The most important reduction, in both prevalences and intensities of infection, occurred immediately after the first deworming cycle. Over the two years of the deworming program (i.e. between July 2012 and July 2014), the odds of infection by any STH were reduced by 73%. The odds of infection by Ascaris, Trichuris and hookworm were reduced by 73%, 77% and 27%, respectively. There was no change in the prevalence of stunting over the two years of the deworming program. Anemia levels remained at levels similar to baseline values for the first 16 months but increased in the last 8 months.
Table 4.
Prevalence and intensity of STH infections, by deworming cycle, between July 2012 and July 2014, in Loreto, Peru.
| Jul-12 | Nov-12 | Mar-13 | Jul-13 | Nov-13 | Mar-14 | Jul-14 | aOR* | OR 95% CI | |
|---|---|---|---|---|---|---|---|---|---|
|
Ascaris (%) (moderate and heavy) |
65.2 (40.0) |
43.1 (6.5) |
39.4 (8.0) |
37.0 (3.0) |
39.6 (6.8) |
41.3 (3.5) |
37.5 (2.9) |
0.27 0.04 |
(0.22-0.34) (0.03-0.08) |
|
Trichuris (%) (moderate and heavy) |
64.8 (25.2) |
40.4 (10.0) |
38.4 (9.4) |
36.0 (8.2) |
34.5 (10.0) |
34.0 (7.9) |
33.8 (6.7) |
0.23 0.22 |
(0.19-0.30) (0.14-0.33) |
| Hookworm (%) (moderate and heavy) |
4.2 (0) |
3.8 (0.2) |
3.5 (0.3) |
5.9 (1.7) |
4.9 (1.2) |
2.9 (0) |
3.09 (0) |
0.73 (--) |
(0.43-1.25) (--) |
| Any STH (%) (moderate and heavy) |
82.4 (49.0) |
61.7 (13.9) |
58.4 (16.2) |
58.5 (12.0) |
56.0 (16.3) |
57.5 (10.7) |
55.8 (9.5) |
0.27 0.11 |
(0.21-0.34) (0.08-1.56) |
| Stunting (%) | 23.4 | 24.0 | 23.0 | 22.2 | 21.9 | 20.4 | 24.0 | 0.89 | (0.86-1.86) |
| Anemia (%) | 21.0 | 22.6 | 21.7 | 24.2 | 23.3 | 32.6 | 33.1 | 1.96 | (1.56-2.46) |
aOR= adjusted odds ratio comparing prevalences between July 2012 and July 2014 (obtained from a GEE model, conditional on age, sex, province (when applicable) and adjusting for clustering by schools, assuming an exchangeable correlation structure)
The intensity of Ascaris and Trichuris infections decreased significantly over the two years of the deworming program (Table 5). The values for hookworm infections show high variability due to its low prevalence in this population.
Table 5.
Intensity (arithmetic mean epg*) of STH infection over the two years of the deworming program Chau Cuica, Loreto, 2012-2014, by parasite species.
| Jul-12 | Nov-12 | Mar-13 | Jul-13 | Nov-13 | Mar-14 | Jul-14 | p-value** | |
|---|---|---|---|---|---|---|---|---|
| Ascaris | 9504 | 1312 | 1403 | 889 | 1368 | 1222 | 812 | < 0.05 |
| Trichuris | 1125 | 426 | 368 | 701 | 410 | 234 | 238 | < 0.05 |
| Hookworm | 13 | 23 | 29 | 98 | 69 | 11 | 13 | NS |
* Epg= eggs per gram of feces (values are rounded to the nearest integer); ** p-value from t-test of the difference between values at baseline and the end date; NS = not statistically significant
On average, over the two years of the program, and controlling for age, sex and province of residence, each month was associated with a 4.7% decrease (95% CI: 3.2, 6.1%) in median Ascaris intensity and with a 2.1% decrease (95% CI:1.0%, 3.3%) in median Trichuris intensity.
Cost of the deworming program
Operating costs for each deworming cycle were assessed and categorized into: human resources needed to direct the program; personnel to re-package the medication (from bottles containing 1000 tablets into bottles of 100 tablets); personnel to prepare the bottles for transport to the different health centres in the region; and, teachers and other school personnel to administer and record the deworming activity in each school (Table 6).
Table 6.
Operational costs for one school-based deworming cycle, Loreto, Peru (2012-2014).
| Activity | No. persons | Monthly cost (Nuevos soles) | Time devoted to program (months) | Cost per person per deworming cycle (Nuevos soles) |
Total cost (Nuevos soles) |
|---|---|---|---|---|---|
| Program administration | 1 | 2,000 | 4.00 | 8,000.00 | 8,000 |
| Re-packaging the mebendazole | 10 | 1,000 | 0.50 | 500.00 | 5,000 |
| Preparation for shipping to each health centre | 2 | 1,000 | 0.50 | 500.00 | 1,000 |
| Personnel in DIREMID | 4 | 1,000 | 2.00 | 2,000.00 | 8,000 |
| Health personnel in each province | 6 | 1,000 | 2.00 | 2,000.00 | 12,000 |
| Teachers | 4,400 | 1,500 | 0.01 | 15.00 | 66,000 |
| TOTAL (nuevos soles) | 100,000 | ||||
Source: Dr. Yuri Cabello Quispe, Director DIREMID at the time of the 2012-2014 deworming program.
Over the seven deworming cycles, a mean of 154,016 children were reached in each cycle. Teachers` time devoted to the program was calculated based on having a mean of 35 children and using 2 hours of their time for each deworming cycle. The total cost for each cycle was estimated to be 100,000 nuevos soles or approximately $33,333.30 USD. This translates into approximately 65 nuevos soles (22 cents USD) per child per cycle. The cost of the mebendazole was not included in these calculations as it was a donation. Transport to the health centres was not included as the mebendazole was transported with other medications routinely sent out by the Ministry of Health to all health centres in the region. Costs associated with the embedded monitoring component included the cost of materials, transportation and the yearly training of personnel involved in the monitoring. This was estimated at approximately 12, 000 nuevos soles or $4,068 USD per cycle.
Discussion
Several unique features of this deworming program should be highlighted. First, Chau Cuica began with the seminal event of a large donation of deworming drugs by an international non-governmental organization to the regional government of Loreto. Second, the regional government acted quickly to plan a region-wide deworming program by authorizing a unique joint initiative between its Ministry of Health and its Ministry of Education. Third, because of the close collaboration between the Ministry of Health and a research team in Iquitos (a long-standing research partnership between a local NGO, the Asociacion Civil Selva Amazonica (ACSA), and McGill University), a surveillance component (externally funded by the Canadian Institutes of Health Research) was integrated into the program from the start. Lastly, to optimize the sustainability of the deworming program, the lead responsibility for the surveillance component transferred, over the course of the program’s two years, from the ACSA-McGill partnership to the Ministry of Health. One, or more, of these features would be expected to occur in other settings planning and implementing a large-scale deworming program.
Chau Cuica has had a positive impact in the region in terms of parasitological indicators. Over the two years of the program, even with coverage rates below optimal, the initially high prevalences of Ascaris and Trichuris infections dropped significantly in all provinces. More importantly, the proportion of children with moderate and heavy Ascaris and Trichuris infections decreased dramatically. Of note, in this region, hookworm infections were less common and were likely not an important determinant of anemia in our school population. Other factors associated with anemia require investigation. The decreasing infection parameters will ultimately transform into improved health not only for the children participating in the school-based deworming program, but also for non-enrolled children and for other population sub-groups, through reduced environmental contamination [17]. Recognizing that provincial STH prevalence rates can differ, the regional government may benefit from strategic planning in terms of both the organization of the overall deworming program, and the implementation of the surveillance component, in continuing the program.
It should be noted that, over the two years of the deworming program, the prevalences of stunting and anemia did not decrease. These chronic manifestations of underlying malnutrition are less likely to decrease over a short period of time and in response to a single intervention like deworming. Instead, a multi-pronged approach is required, which would include, as a minimum, improved nutrition interventions and improved sanitation and waste management interventions (to reduce environmental contamination).
The impact of this program goes beyond the parasitological results presented here. Chau Cuica raised awareness of STH as a neglected tropical disease, not only in the government health and education ministries themselves, but also among schoolchildren, parents, teachers, other NGOs, and in the general public. With a confluence of NGO, government and university good will, this program was able to be implemented within a relatively short period of time. Key to its success was the partnership between the ministries of health and education, and the recognition that, to demonstrate impact, a baseline assessment with periodic monitoring needed to be embedded in the program from the start. As conditions for continuing the deworming program are realized in the region of Loreto, this first two-year experience will provide a solid base for ensuring its sustainability. It may also serve to inform the planning and implementation of deworming programs in other endemic regions of Peru, and in other endemic countries of Latin America.
References
- 1.WHO. eLibrary of Evidence for Nutrition Actions (eLENA) Geneva: World Health Organization; 2012. Deworming to combat the health and nutritional impact of helminth infections. [Google Scholar]
- 2.Albonico M, Montresor A, Crompton DW, et al. Intervention for the control of soil-transmitted helminthiasis in the community. Advances in Parasitology. 2006;61:311–48. doi: 10.1016/S0065-308X(05)61008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO. Preventive chemotherapy in human helminthiasis - Coordinated use of anthelminthic drugs in control interventions: a manual for health professionals and programme managers. Geneva: World Health Organization; 2006. [Google Scholar]
- 4.WHO. Resolution WHA54.19. Geneva: World Health Organization; 2001. Schistosomiasis and soil-transmitted helminth infections. [Google Scholar]
- 5.WHO. Accelerating Work to Overcome the Global Impact of Neglected Tropical Diseases: a Roadmap for Implementation. Geneva: World Health Organization; 2012. [Google Scholar]
- 6.Montresor A, Ramsan M, Chwaya HM, et al. Extending anthelminthic coverage to non-enrolled school-age children using a simple and low-cost method. Tropical Medicine & International Health. 2001;6(7):535–37. doi: 10.1046/j.1365-3156.2001.00750.x. [DOI] [PubMed] [Google Scholar]
- 7.WHO. Uniting to Combat Neglected Tropical Diseases. London Declaration on Neglected Tropical Diseases Geneva: World Health Organization; 2012. [Google Scholar]
- 8.WHO. Soil transmitted helminthiases:number of children treated in 2014. Weekly Epidemiological Record. 2015;90(51):701–12. [PubMed] [Google Scholar]
- 9.Flisser A, Valdespino J, García-García L, et al. Using national health weeks to deliver deworming to children: lessons from Mexico. Journal of Epidemiology and Community Health. 2008;62(4):314–17. doi: 10.1136/jech.2007.066423. [DOI] [PubMed] [Google Scholar]
- 10.Inter-American Development Bank, PAHO, Sabin Vaccine Institute. A Call to Action: Addressing Soil-transmitted Helminths in Latin America & the Caribbean. Washington DC: Inter-American Development Bank, PAHO, Sabin Vaccine Institute; 2011. [Google Scholar]
- 11.Gyorkos TW, Maheu-Giroux M, Blouin B, et al. Impact of Health Education on Soil-Transmitted Helminth Infections in Schoolchildren of the Peruvian Amazon: A Cluster-Randomized Controlled Trial. PLoS Negl Trop Dis. 2013;7(9):e2397. doi: 10.1371/journal.pntd.0002397. [published Online First: Epub Date] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mofid LS, Casapía M, Montresor A, et al. Maternal Deworming Research Study (MADRES) protocol: a double-blind, placebo-controlled randomised trial to determine the effectiveness of deworming in the immediate postpartum period. BMJ Open. 2015;5(6):e008560. doi: 10.1136/bmjopen-2015-008560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joseph SA, Casapía M, Lazarte F, et al. The effect of deworming on early childhood development in Peru: A randomized controlled trial. SSM - Population Health. 2015;1:32–39. doi: 10.1016/j.ssmph.2015.10.001. [published Online First: Epub Date] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thériault FL, Maheu-Giroux M, Blouin B, et al. Effects of a Post-Deworming Health Hygiene Education Intervention on Absenteeism in School-Age Children of the Peruvian Amazon. PLoS Negl Trop Dis. 2014;8(8):e3007. doi: 10.1371/journal.pntd.0003007. [published Online First: Epub Date] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.WHO. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Geneva: World Health Organization; 2011. WHO/NMH/NHD/MNM/11.1, 2014. [Google Scholar]
- 16.Casapía M, Joseph SA, Núñez C, et al. Parasite risk factors for stunting in grade 5 students in a community of extreme poverty in Peru. International Journal for Parasitology. 2006;36:741–47. doi: 10.1016/j.ijpara.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Bundy DA, Wong MS, Lewis LL, et al. Control of geohelminths by delivery of targeted chemotherapy through schools. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1990;84(1):115–20. doi: 10.1016/0035-9203(90)90399-y. [DOI] [PubMed] [Google Scholar]