Abstract
Previous research into the prebiotic synthesis of the pyrimidine nucleotides has revealed a potential intermediate with remarkable properties – ribose aminooxazoline crystallises spontaneously from reaction mixtures, and with an enhanced enantiomeric excess if initially enantioenriched. This automatic chemical and chiral purification suggests that reservoirs of this compound in optically pure form might have accumulated on the early Earth. Studies have shown that ribose aminooxazoline can be efficiently converted to α-ribocytidine by way of 2,2'-anhydro-ribocytidine, though anomerization to β-ribocytidine by UV irradiation is extremely inefficient. Our previous work demonstrated the synthesis of pyrimidine β-ribonucleotides, but at the cost of ignoring ribose aminooxazoline and using arabinose aminooxazoline instead. Here, we describe a long sought route through ribose aminooxazoline to the pyrimidine β-ribonucleosides and their phosphate derivatives, that involves an extraordinarily efficient photoanomerisation of α-2-thioribocytidine. In addition to the canonical nucleosides, our synthesis accesses β-2-thioribouridine, a modified nucleoside found in tRNA and which enables both faster and more accurate nucleic acid template copying chemistry.
Introduction
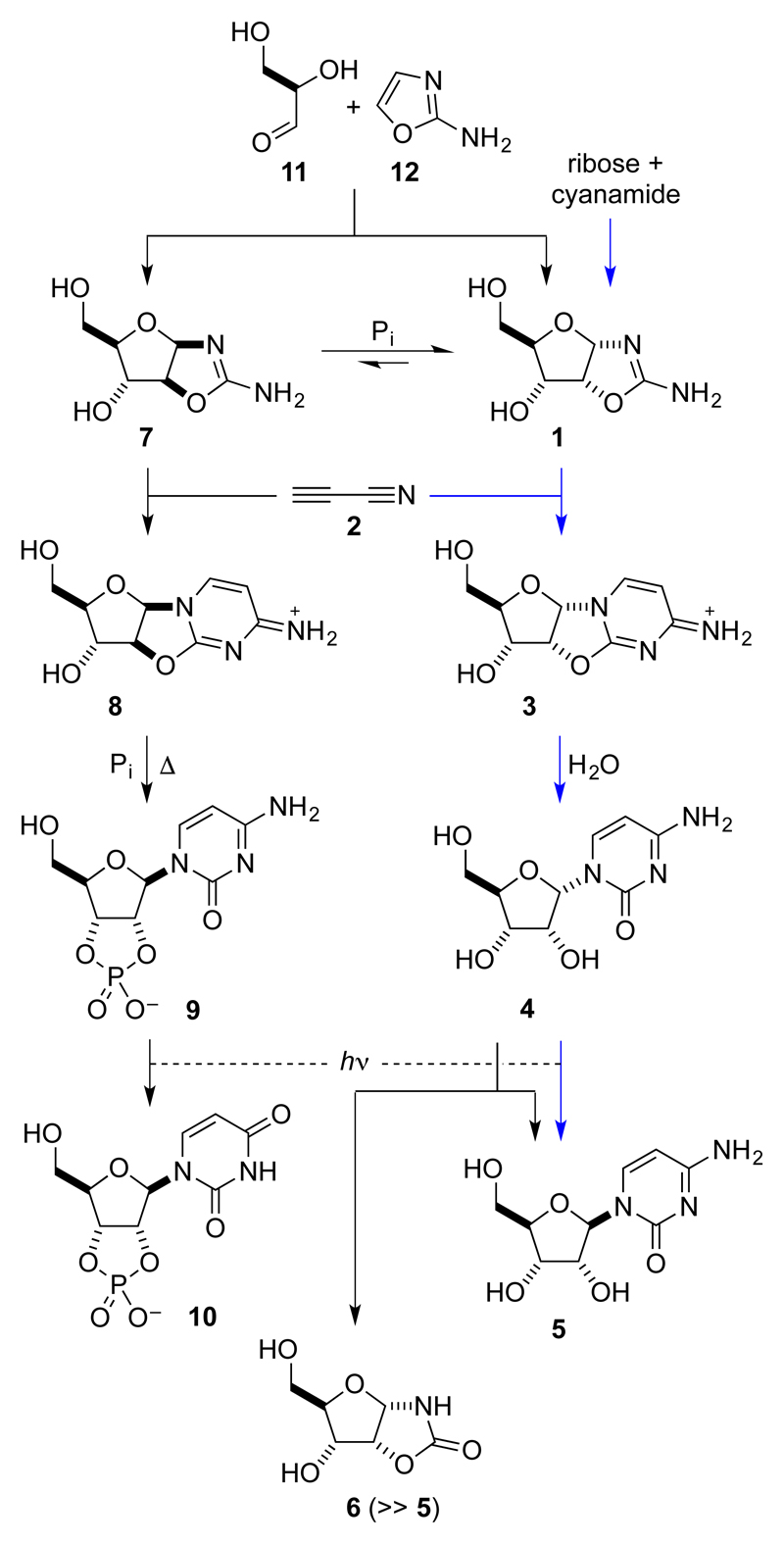
The first synthesis of pyrimidine ribonucleosides from aminooxazoline and anhydronucleoside intermediates dates back nearly fifty years, but suffers from an extremely low-yielding step and the questionable prebiotic availability of ribose.1 Orgel found that treatment of the cyanamide derivative of this sugar, ribose aminooxazoline 1 with cyanoacetylene 2 gave the anhydronucleoside 3 and thence α-ribocytidine 4 in good yield, but UV irradiation of the latter gave β-ribocytidine 5 in only 4% yield. Whilst the route had several attractive features, the inefficiency of this photoanomerization compounded concerns about the availability of ribose and made the route seem somewhat implausible from a prebiotic standpoint. (Fig. 1). Both the high crystallinity of ribose aminooxazoline 1 and its greater propensity to crystallise than its stereoisomers were noted by Orgel. In general, this behaviour is extremely attractive in prebiotic chemistry because it allows for spontaneous purification and accumulation of an intermediate such that subsequent synthetic steps in a sequence can occur without interference from the detritus of earlier steps. Accordingly, we were prompted to revisit this chemistry and, having found that nucleobase destruction giving ribose oxazolidinone 6 was the major photoreaction of α-ribocytidine 4,2 thus concluded that this route to the β-ribonucleosides from ribose aminooxazoline 1 was irredeemable. We then moved on to explore routes involving 2'-stereoinversion and the arabino-configured intermediates arabinose aminooxazoline 7 and 2,2'-anhydro-arabinocytidine 8.3 We found that phosphorylation of 8 occurred regioselectively on the 3'-OH group and was followed by intramolecular 2'-stereoinversion giving β-ribocytidine-2',3'-cyclic phosphate 9. Finally, we discovered that UV irradiation of 9 caused partial conversion to the corresponding ribouridine-2',3'-cyclic phosphate 10, rather than predominant nucleobase destruction, for subtle chemical reasons. However, although our synthesis provided both canonical pyrimidine ribonucleoside-2',3'-cyclic phosphates 9 and 10 in good yield, we had concerns about our reliance on arabinose aminooxazoline 7 as an intermediate.
Figure 1. Previous prebiotic syntheses of pyrimidine β-ribonucleotides via 2,2'-anhydronucleoside intermediates.
The route initially described by Orgel is indicated by the blue arrows.1 Thus treatment of ribose with cyanamide gave a beautifully crystalline aminooxazoline 1 which underwent reaction with cyanoacetylene 2 to give the anhydronucleoside 3 and thence, by hydrolysis, α-ribocytidine 4. However, anomerization to β-ribocytidine 5 by irradiation of 4 with UV light was very inefficient and this, along with doubts about the prebiotic provenance of ribose, lessened the attractiveness of the route. We determined that one of the reasons for the low photoanomerization yield was competing formation of the oxazolidinone 6 and then found an alternate route to the pyrimidine ribonucleotides via the arabinose aminooxazoline 7 and anhydronucleoside 8. The key steps were a regioselective phosphorylation and 2'-inversion reaction of 8 giving β-ribocytidine-2',3'-cyclic phosphate 9 and the partial photochemical conversion of 9 to the corrsponding uridine analogue 10. We also found that pentose aminooxazolines, predominantly 1 and 7, can be produced in an indirect way from glyceraldehyde 11 and 2-aminooxazole 12. Although our route marked the first high-yielding synthesis of the pyrimidine ribonucleotides under prebiotically plausible conditions, it did not exploit the crystallinity of 1.
We were further motivated to explore this area of chemistry in more detail because we had also found that pentose cyanamide derivatives can be formed in an indirect way that avoids the requirement for the free pentoses, which are unstable and difficult to access. Thus, all four pentose aminooxazolines are formed by reaction of glyceraldehyde 11 with 2 aminooxazole 12, another compound that has desirable purification capabilities, subliming readily.4 Although ribose aminooxazoline 1 (44% yield) and arabinose aminooxazoline 7 (30% yield) greatly predominate, and are thus on the face of it equally plausible as ribonucleotide precursors, 1 selectively crystallises from the product solution. Augmenting earlier observations that this crystallisation provides a potential means whereby ribose aminooxazoline 1 might be chemically purified,1 we also found that it allows enantiomeric enrichment if the input glyceraldehyde is non-racemic. This is because 1 behaves as a true conglomerate (different enantiomers forming separate crystals or crystalline domains such that solid phase enantiomeric excesses are enhanced over those in residual material in solution).4 Although we also found that 1 can be partly epimerized to arabinose aminooxazoline 7 in phosphate buffer – presumably, on the basis of the mechanism, without loss of enantiomeric purity – the latter is the minor stereoisomer at equilibrium.5 Accordingly, we felt that enantiomerically pure ribonucleotides would most plausibly derive from ribose aminooxazoline 1 if a route therefrom that avoided arabinose aminooxazoline 7 could be found.
Results and discussion
We recently discovered a synthetic route from hydrogen cyanide to the sugars needed for assembly of ribose aminooxazoline 1 based on reductive homologation with photochemically generated hydrated electrons.6,7 Hydrosulfide, the conjugate base of hydrogen sulfide (pKa = 7.2), turned out to be the ideal source of these electrons.7 Hydrogen sulfide is a constituent of volcanic exhalations and hydrosulfide can be produced by dissolution of metal sulfides in cyanide solution.7 Given their availability and their involvement in the photochemical synthesis of the constituents of ribose aminooxazoline 1, we wondered if these sulfur species and irradiation might also play a role in the further conversion of 1 to ribonucleotides. For the sake of clarity, we present our findings before we discuss the mechanisms and implication of the reactions we uncovered.
Formation, photoanomerization and hydrolysis of thionucleosides
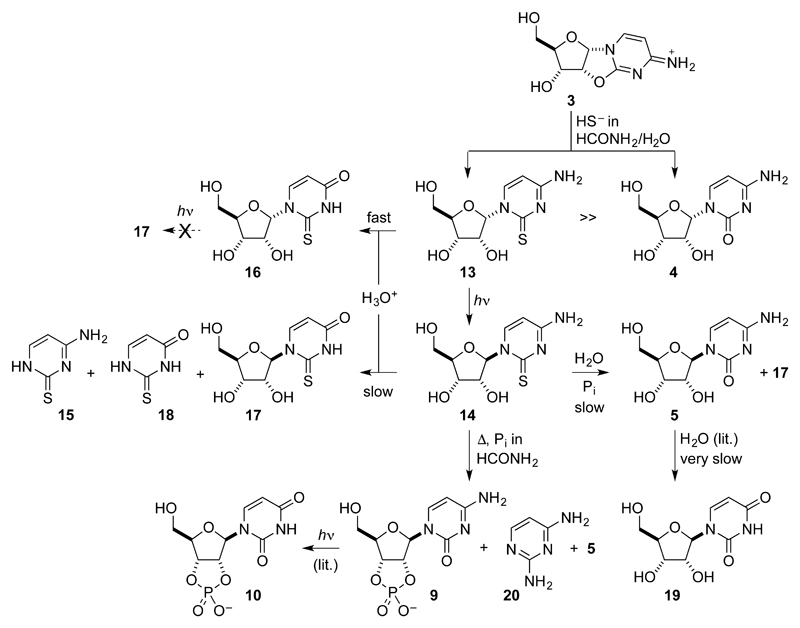
We first sought an inherently favoured reaction of ribose aminooxazoline 1, or one of its derivatives, with hydrosulfide and, in this way, discovered that the anhydronucleoside 3 undergoes smooth thiolysis8 in aqueous formamide giving α-2-thioribocytidine 13 in 84% yield along with the hydrolysis product α-ribocytidine 4 in 16% yield (Figs. 2 & 3a–c, Supplementary Figs. 30 & 31). We then investigated the UV photoanomerization of α-2-thioribocytidine 13 and found that it took place extremely efficiently to give β-2-thioribocytidine 14 in 76% yield (Figs. 2 & 3c–e, Supplementary Figs. 32–34). Small amounts of starting material (11%) remained after irradiation at 254nm for 2.5 days (in aqueous solution containing hydrogen sulfide to minimize photooxidation by adventitious oxygen) and there was also low level nucleobase loss (thiocytosine 15 detected in 4% yield) and hydrolysis to α-ribocytidine 4 (9%).
Figure 2. Synthesis of pyrimidine β-ribonucleosides and β-ribonucleotides involving photoanomerization of α-2-thioribocytidine 13.
The reaction scheme incorporates the new synthesis and the known hydrolysis of β-ribocytidine 5 to β-ribouridine 19.9 Thiolysis of the ribose anhydronucleoside 3 gives α-2-thioribocytidine 13 in high yield along with a small amount of α-ribocytidine 4. In marked contrast to 4, 13 undergoes remarkably efficient photoanomerization, giving β-2-thioribocytidine 14 in excellent yield. The canonical pyrimidine ribonucleosides 5 and 19 can be produced from 14 by hydrolysis. Phosphorylation of 14 is accompanied by conversion of the 2-thiocytosine nucleobase to cytosine providing a direct route to the canonical pyrimidine ribonucleoside-2',3'-cyclic phosphates 9 and thence 10 and an alternative route to the ribonucleoside 5. 2-Thioribouridines 16 and 17 can also be produced by hydrolysis of 13 and 14 respectively under acidic conditions.
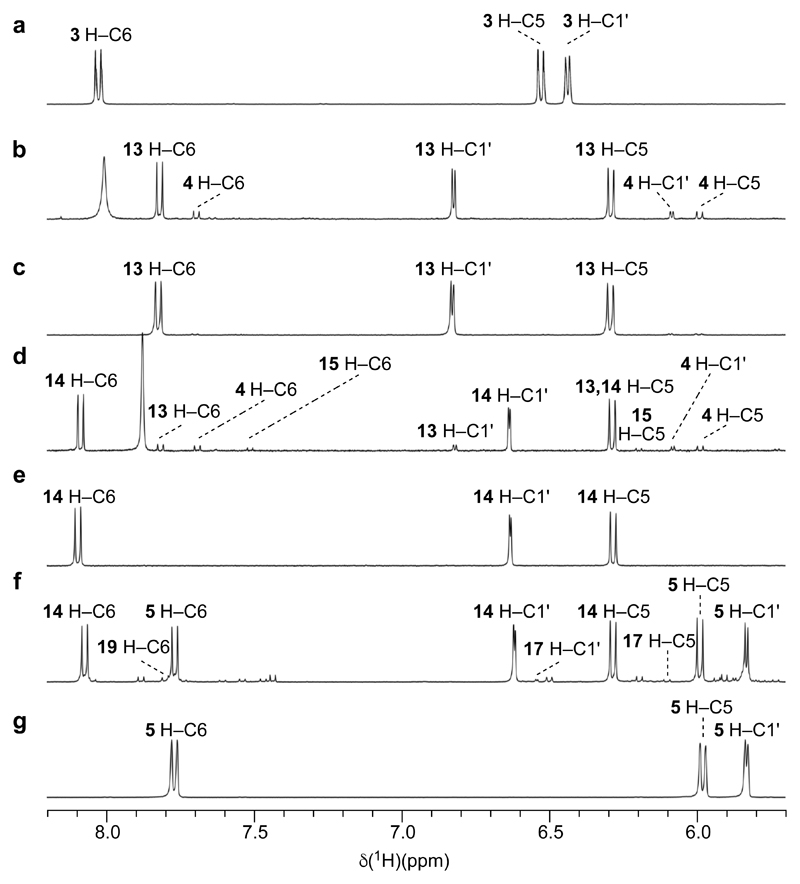
Figure 3. 1H NMR spectra demonstrating the inherently favoured nature of the individual reactions of the newly discovered synthetic sequence.
1H NMR spectra were recorded at 400 MHz with samples of reaction products (after removal of solvents) and synthetic standard dissolved in H2O-D2O a, Spectrum of the starting material, 2,2'-anhydro-ribocytidine 3. b, Spectrum of the products of thiolysis of 3 by HS– in wet formamide (singlet at δ ~ 8.0 ppm is due to residual formamide). Note the quantitative transformation of 3 and the predominance of α-2-thioribocytidine 13 over α-ribocytidine 4 in the products. c, Spectrum of an authentic sample of α-2-thioribocytidine 13. d, Spectrum of the products of irradiation of 13 in aqueous solution (singlet at δ ~ 7.9 ppm is due to residual dimethylformamide from the conventional synthesis of 13). Note the remarkably clean conversion of 13 to the anomer β-2-thioribocytidine 14. e, Spectrum of an authentic sample of β-2-thioribocytidine 14. f, Spectrum of the products of hydrolysis of 14 in pH = 7 phosphate buffer after ~ one half life. Despite the conversion of 14 being only partial, note the clean production of β-ribocytidine 5 and the appearance of its hydrolysis product, β-ribouridine 19 as well as β-2-thioribouridine 17, the product of alternate hydrolysis of 14. g, Spectrum of an authentic sample of β-ribocytidine 5.
We then investigated hydrolysis of the 2-thioribocytidine nucleosides 13 and 14 and further chemistry of the hydrolysis products. Mild acid hydrolysis of α-2-thioribocytidine 13 in dilute formic acid (40 mM, pH = 3, 60°C) gave α-2-thioribouridine 16 in 93% yield after 14 days (Supplementary Figs. 36 & 37), whereas the hydrolysis of β-2-thioribocytidine 14 under the same conditions was slower and less selective giving β-2-thioribouridine 17 in 38% yield after 54 days along with products of nucleobase loss (2-thiocytosine 15, 34%; 2-thiouracil 18, 18% and ribose) (Supplementary Figs. 39 & 40). In contrast to what we found in the 2-thiocytidine series, photoanomerization of α-2-thioribouridine 16 to β-2-thioribouridine 17 was not observed, rather there was extensive nucleobase destruction resulting in the liberation of ribose (Supplementary Fig. 35). Hydrolysis of β-2-thioribocytidine 14 at pH = 7 in the presence of phosphate buffer (100 mM, 60°C) was slow, but after 84 days, cleanly furnished the canonical nucleoside β-ribocytidine 5 (41%, Figs. 2 & 3e–g, Supplementary Figs. 41 & 42) along with small amounts of β-2-thioribouridine 17 (2%) and β-ribouridine 19 (3%) produced by the very slow hydrolysis of 5 itself.9 Hydrolysis at pH = 5 in the presence of phosphate buffer (100 mM, 60°C) for a similar length of time gave more β-2-thioribouridine 17 (8%) and less β-ribocytidine 5 (33%). Although we only monitored the hydrolysis of β-2-thioribocytidine 14 for about one half-life at pH = 7 and pH = 5, more β-ribocytidine 5, β-ribouridine 19 and β-2-thioribouridine 17 would be produced after longer periods.
Phosphorylation
Prebiotically plausible phosphorylation of nucleosides has been demonstrated in hot formamide solution and in urea melts.10,11 Although the 5'-monophosphates or the 2',3'-cyclic phosphates of the β-ribopyrimidine nucleosides 5 and 19 can be formed this way depending on the conditions,11 we also investigated the direct phosphorylation of β-2-thioribocytidine 14. Somewhat surprisingly, the 2',3'-cyclic phosphate of the 2-thiocytidine nucleoside was not detected when 14 was allowed to react with inorganic phosphate in hot formamide. Instead, β-ribocytidine 5 (48%) and its 2',3'-cyclic-phosphate derivative 9 (24%) were detected along with 2,4-diaminopyrimidine 20 (21%) (Supplementary Figs. 43–46). Since it has previously been shown that UV irradiation partly converts 9 to its uridine analogue 10,3 the direct phosphorylation of β-2-thioribocytidine 14 can therefore lead to both canonical β-ribopyrimidine nucleoside 2',3'-cyclic phosphates 9 and 10.
Thiolysis mechanism
Mechanistically, thiolysis of the anhydronucleoside 3 presumably involves repeated reversible nucleophilic attack at C-2 by hydrosulfide followed by occasional extrusion of O-2' from the tetrahedral intermediate thus formed giving α-2-thioribocytidine 13. Although water or hydroxide are less nucleophilic than hydrosulfide, extrusion of O-2' from the tetrahedral intermediate formed by addition of these oxygenous nucleophiles to the anhydronucleoside 3 must be a common event and this is probably one of the reasons why thiolysis of 3 is accompanied by hydrolysis to α-ribocytidine 4. The hydrolysis might also be subject to general base catalysis by hydrosulfide. As solvent for subsequent phosphorylation chemistry, we envisioned using formamide – the hydration product of hydrogen cyanide. We therefore also evaluated aqueous formamide for the thiolysis step and found it to allow smooth conversion of the anhydronucleoside 3 to α-2-thioribocytidine 13 with minimal competing hydrolysis. In the phosphorylation step, the formamide solvent has to be essentially anhydrous and this can easily be plausibly achieved simply by heating.
The pH dependence of the hydrolysis of β-2-thioribocytidine 14 is presumably due to the relative electrophilicities of C-2 and C-4 depending on the protonation state of 14, and relative leaving group abilities. At low pH, ammonia can be lost from a tetrahedral intermediate formed by nitrogen protonation and attack of water at C-4 of 14 (or the reversibly formed 5,6-double bond hydration adduct thereof), whereas at neutral pH, hydrosulfide can be lost from a more rarely accessed tetrahedral intermediate formed by attack of water or hydroxide at C-2. The more rapid dilute acid hydrolysis of α-2-thioribocytidine 13 is due to neighbouring group participation by the 2'-OH and a tricyclic intermediate could be detected by 1H-NMR spectroscopy during the hydrolysis (Supplementary Figs. 37 & 38).
Phosphorylation mechanism
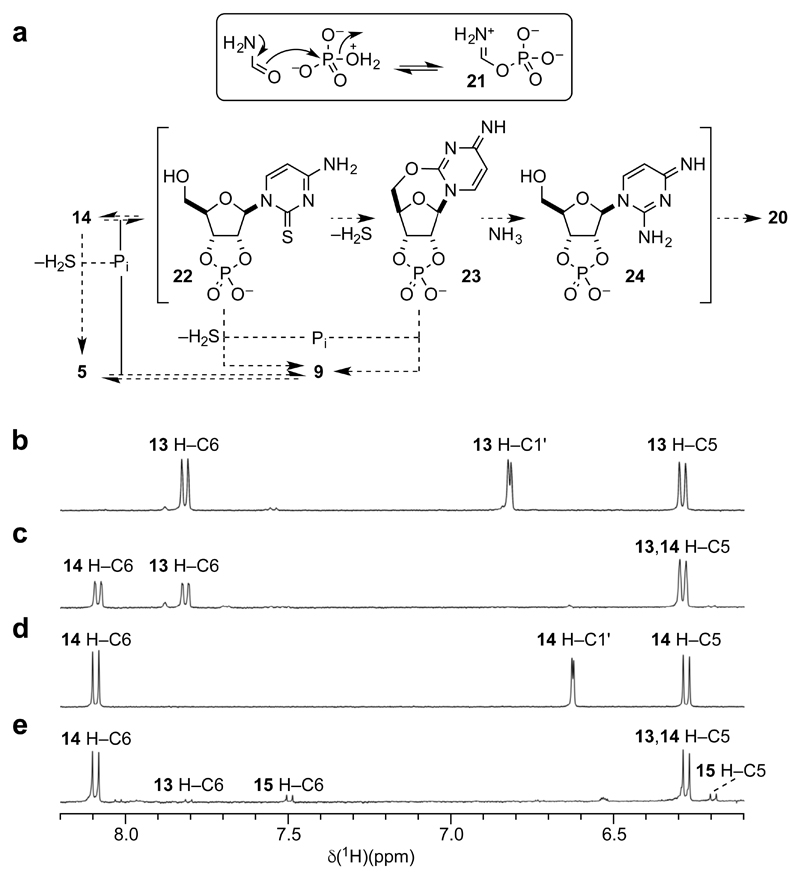
A mechanism for the photochemical conversion of α-2-thioribocytidine 13 to the β-anomer 14 was not immediately apparent, and further experimental and computational investigations of this key reaction were carried out as described below. However, conventional organic reasoning did explain the behaviour of 14 under phosphorylation conditions (Fig. 4a). Reversible phosphorylation in hot formamide is thought to involve formidoylphosphate 21 formed by dissociative nucleophilic displacement of water from a rare tautomer of the phosphate monoanion (Fig. 4a, inset box). The ability of formamide to displace an oxygenous leaving group from a phosphorus atom in such a molecular context means that if phosphate adds to an electrophilic carbon and then undergoes attack by formamide, an oxygen atom is delivered to the electrophilic carbon. Thus, on heating with inorganic phosphate in formamide, β-2-thioribocytidine 14 can either undergo phosphorolytic loss of hydrogen sulfide to give β-ribocytidine 5 – which can then undergo reversible phosphorylation to give β-ribocytidine-2',3'-cyclic phosphate 9 – or be directly phosphorylated to the thermodynamically favoured β-2-thioribocytidine-2',3'-cyclic phosphate 22. Intermolecular phosphorolytic loss of hydrogen sulfide from 22 giving β-ribocytidine-2',3'-cyclic phosphate 9 is then possible. However, the 2',3'-cyclic phosphate ring of 22 constrains the sugar's conformational preferences12 such that intramolecular addition of the 5'-OH group to C-2 of the nucleobase with loss of hydrogen sulfide additionally becomes possible. The 2,5'-anhydronucleotide 23 that results is susceptible to phosphorolytic cleavage to give β-ribocytidine-2',3'-cyclic phosphate 9, and to aminolysis – by ammonia produced by α-elimination from formamide – giving the 2,4-diaminopyrimidine cyclic nucleotide 24. Glycosidic bond cleavage of 24 is then presumed to be facile because of the basicity of the nucleobase,13 and the enhanced leaving group ability of the protonated nucleobase. This cleavage would generate the free 2,4-diaminopyrimidine 20 that we observed, and an unstable sugar moiety prone to decompose to a slew of products that we did not observe – presumably because of the low yield of each individual product.
Figure 4. Reaction mechanisms.
a, Mechanisms of phosphorylation and the degradation of β-2-thioribocytidine-2',3'-cyclic phosphate 22. The generation of the active phosphorylating agent, formidoyl phosphate 21, in the phosphorylation of β-2-thioribocytidine 14 is shown in the box – the phosphorylation of 14 itself involves a hydroxyl group thereof attacking the imidoyl phosphate in a manner analogous to the reverse of the electron movements indicated by the arrows. Outside of the box, the mechanisms of the reactions taking place during the phosphorylation of β-2-thioribocytidine 14 are shown. b–e, 1H NMR spectra (400 MHz, H2O-D2O) demonstrating incorporation of deuterium at at C-1' of α/β-2-thioribocytidine 13/14 during photoanomerization. b, Expansion of the spectrum of α-2-thioribocytidine 13 showing signals for H-6, H-1' and H-5. c, Spectrum of the products of irradiation of α-2-thioribocytidine 13 in D2O for 12 hours showing signals for H-6 and H-5 of both 13 and β-2-thioribocytidine 14 but lacking signals for H-1' of either anomer. d, Spectrum of 14 prior to irradiation. e, Spectrum of the products of irradiation of 14 in D2O for 36 hours. The deuteration data shown indicate a mechanism of photoanomerization involving exchange of H-1' with solvent.
Photoanomerization mechanism
We then turned our attention to the mechanism of photoanomerization of α-2-thioribocytidine 13, and further sought an explanation as to why α-2-thioribouridine 16 did not undergo similar epimerization upon irradiation. Suspecting an interrupted Norrish type II reaction similar to that we observed for the photoanomerization of 2'-deoxycytidine,14 we irradiated α-2-thioribocytidine 13 in D2O since anomerization by this mechanism would (presumably) be associated with deuterium incorporation at C-1'. Gratifyingly, after 12 hours irradiation we observed complete deuterium incorporation at C-1' of the product, β-2-thioribocytidine 14 and of the residual starting material 13 demonstrating that exchange is faster than anomerization (Fig. 4b,c). Furthermore, by 36 hours the reaction appeared to reach equilibrium with the β-and α-isomers in a ratio of 85:15 – as calculated using relative integrations of signals due to H-6 of both anomers in the 1H-NMR spectrum of the crude reaction products (Supplementary Fig. 47). To confirm the equilibrium, the β-isomer was irradiated in D2O whereupon conversion to the α-isomer and C-1' deuteration were observed and, after 36 hours equilibrium had again been reached ((Fig. 4d,e, Supplementary Fig. 48) β-and α-isomers in a ratio of 88:12). In further support of the proposed mechanism, the deuterated anomers were purified from a preparative scale UV reaction and characterised by NMR and LCMS (See Supplementary Information).
Photoanomerization theory
Deuterium incorporation at the C-1' carbon atom is consistent with 2-thioribocytidine 13/14 undergoing photoanomerization via excited state C1'–H atom abstraction by the C=S group (Fig. 5a,b). The intermediate α-/β-25 formed after the hydrogen abstraction possesses an SH group which can readily exchange the proton with a deuteron from surrounding D2O molecules. The SD group thus formed can subsequently deliver this deuteron to the C-1' position before or after anomerization through C1' radical inversion. In the case of 2'-deoxycytidine, the excited-state H-atom abstraction reaction occurs on the hypersurface of the lowest-lying nπ* singlet state (S1). The minima of the S1 states of both 2-thioribocytidine 13/14 and 2-thioribouridine 16/17 are of nπ* character and according to our calculations the S1/S0 state crossing related to this process is energetically accessible in both molecules. However, it is worth noting that the presence of sulfur significantly enhances spin-orbit couplings (SOCs) in organic molecules, and in consequence may enable very efficient non-radiative transitions to the triplet manifold. 15–17 In particular, it was shown that thiothymidines, 6-thioguanine and 2-thiouracil undergo ultrafast intersystem crossing (ISC) resulting in near unity triplet yields.17,18 Our estimates of intersystem crossing (ISC) rates suggest that the photoanomerization of 2-thioribocytidine 13/14 may occur predominantly on the triplet hypersurface.
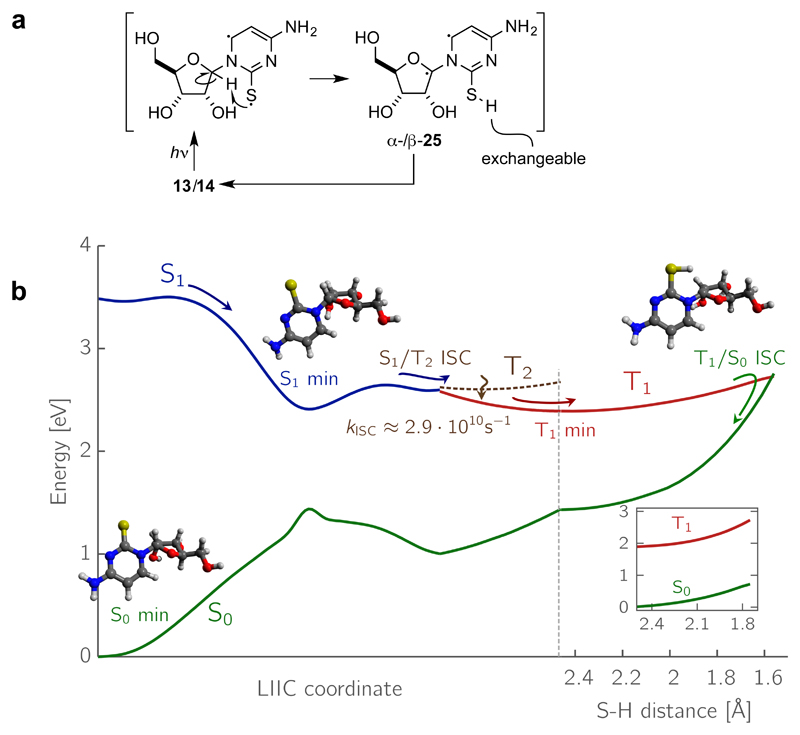
Figure 5. Mechanism of the key photoanomerization reaction.
a, Conventional organic chemical depiction of the mechanism of the photoanomerization of α/β-2-thioribocytidine 13/14 via the diradical 25. b, The same mechanism but analysed at a deeper level to explain the niceties of the reaction. Potential energy (PE) profiles presenting the photoanomerization reaction of α-2-thioribocytidine. The PE cuts on the left-hand side were obtained by interpolations between the Franck-Condon region, S1 minimum, S1/T2 minimum energy crossing point and the T1 minimum. The PE profile on the right was obtained from a relaxed scan along the S–H distance. The inset box on the right corresponds to the relaxed scan along the S–H distance of α-2-thioribouridine, for which the T1(ππ*) and S0 states do not cross. The energies were calculated using the ADC(2)/cc-pVTZ method and the computations of the SOC matrix elements were performed at the CASPT2/CASSCF level (employing cc-pVTZ-DK basis set). Details of the S1/T2 intersystem crossing rate constant computations can be found in the Supplementary Information.
The initially populated excited state of 2-thioribocytidine 13/14 may undergo barrierless vibrational relaxation towards the minimum on the corresponding S1 potential energy surface (Fig. 5b). Since the minimum-energy structure of the T1 state of 2-thioribocytidine is also of nπ* character, the corresponding 1nπ*(S1)→3nπ*(T1) intersystem crossing (ISC) operates without molecular orbital change and should be rather slow according to the El-Sayed rules and the results of our calculations. Therefore we suggest (based on reasoning given in the Supplementary Information) that the primary ISC channel is most likely related to the 1nπ*(S1)→3ππ*(T2) transition. The T1 state can then be accessed almost immediately after the S1→T2 ISC since at the S1/T2 minimum energy crossing point the T2 and T1 states are almost degenerate (yielding a S1/T2/T1 three-state crossing). The H-atom abstraction can then take place in the T1 state and when the S–H distance approaches 1.55 Å, the T1 and S0 (ground) electronic states cross.
Similarly, as found for 2-thiouracil,17,19,20 the T1 state of 2-thioribouridine 16/17 has a ππ* character and it can be more efficiently accessed from the S1 state due to large SOCs between these two states. However, H-abstraction is not possible on the surface of the 3ππ* state and consequently, the S0 and T1 surfaces correlate adiabatically and do not cross (Fig. 3g, inset box). The T1/S0 state crossing can be accessed along the H-abstraction coordinate only after the change of the T1 character from 3ππ* to 3nπ*, which is related to an energy barrier of 0.84 eV in the gas phase. This energy barrier is presumably even higher in bulk water due to destabilization of nπ* states in aqueous environment (see the Supplementary Information).21 Furthermore, the C=S....H–C1' distance is significantly larger in the T1 minimum of 2-thioribouridine 16/17 (2.65 Å) than in the respective minimum of 2-thioribocytidine 13/14 (2.43 Å), which should significantly affect the accessibility of this reaction pathway. Therefore, we conclude that photoanomerization via an interrupted Norrish type II reaction in the triplet manifold is clearly hindered in the case of 2-thiouridine. Unable to lose energy by T1/S0 state crossing along the H-abstraction coordinate and subsequent vibrational relaxation, the 3ππ* state undergoes other, as yet undefined, photochemistry that results in nucleobase destruction and liberation of ribose.
Conclusions
The route to the canonical pyrimidine β-ribonucleosides 5 and 19 that we have uncovered in this work is short and high-yielding. The 5'-phosphates of 5 and 19 can be produced easily under plausible conditions, as can their 2',3'-cyclic phosphates 9 and 10.11 The latter can alternatively be produced by irradiation of the phosphorylation products of β-2-thioribocytidine 14. The β-ribonucleosides and nucleotides all derive from ribose aminooxazoline 1, crystallization of which provides an opportunity for enantioenrichment because of its conglomerate behaviour. Enantiomerically pure pyrimidine β-ribonucleotides can thus be considered prebiotically plausible if a means of producing glyceraldehyde 11 with sufficient enantiomeric excess for the subsequent crystallization of 1 to give a single enantiomer is demonstrated. Having enantiomerically pure β-ribonucleotides is crucial to producing RNA by oligomerization since racemic (or even scalemic) mixtures can either be expected to produce vast numbers of diastereoisomerically isomeric oligonucleotides, or result in inhibition of oligomerization through enantiomeric cross-inhibition.22 Because different phosphorylated β-ribonucleosides can be produced, oligomerisation could result in 5'- or 2',(/)3'-phosphorylated RNA.
Finally, we note that a side product of the chemistry documented in this paper, β-2-thioribouridine 17, and its derivatives are found in the anticodon stem loop of certain tRNAs where they are involved in split-box decoding.23 Furthermore, replacing β-ribouridine 19 with β-2-thioribouridine 17 leads to both faster and more accurate nonenzymatic template copying chemistry suggesting that 17 might have played a role in chemical RNA replication at an early stage in the origin of life.24 Taken with the foregoing, the formation of β-2-thioribocytidine 14 reported herein suggests that its role in RNA replication chemistry should also be investigated.
Supplementary Material
Supplementary information and chemical compound information are available in the online version of the paper.
Acknowledgements
This work was supported by the Medical Research Council (No. MC_UP_A024_1009), a grant from the Simons Foundation (No. 290362 to J.D.S.), grant 14-12010S from the Grant Agency of the Czech Republic and by the project CEITEC 2020 (LQ1601) with financial support from the Ministry of Education, Youth and Sports of the Czech Republic under the National Sustainability Programme II. Support from a statutory activity subsidy from the Polish Ministry of Science and Higher Education for the Faculty of Chemistry of Wrocław University of Technology is gratefully acknowledged. Theoretical calculations were partly performed at the Wrocław Center for Networking and Supercomputing (WCSS) and Interdisciplinary Centre for Mathematical and Computational Modelling in Warsaw (ICM).
Footnotes
Author contributions
J.D.S. supervised the experimental research, and J.X., M.T. and C.J.M. performed the experiments. J.E.S, J.S. and R.W.G. oversaw the theoretical work which was carried out by R.S. All authors contributed intellectually as the project unfolded. J.D.S. wrote most of the paper and J.X., M.T., C.J.M. and R.S. further contributed and assembled the Supplementary Information.
Additional information
Reprints and permissions information is available online at www.nature.com/reprints.
Competing financial interests
The authors declare no competing financial interests.
References
- 1.Sanchez RA, Orgel LE. Studies in prebiotic synthesis. Synthesis and photoanomerization of pyrimidine nucleosides. J Mol Biol. 1970;47:531–543. doi: 10.1016/0022-2836(70)90320-7. [DOI] [PubMed] [Google Scholar]
- 2.Powner MW, et al. On the prebiotic synthesis of ribonucleotides: photoanomerisation of cytosine nucleosides and nucleotides revisited. ChemBioChem. 2007;8:1170–1179. doi: 10.1002/cbic.200700098. [DOI] [PubMed] [Google Scholar]
- 3.Powner MW, Gerland B, Sutherland JD. Synthesis of activated pyrimidine ribonucleotides in prebiotically plausible conditions. Nature. 2009;459:239–242. doi: 10.1038/nature08013. [DOI] [PubMed] [Google Scholar]
- 4.Anastasi C, Crowe MA, Powner MW, Sutherland JD. Direct assembly of nucleoside precursors from two- and three-carbon units. Angew Chem Int Ed. 2006;45:6176–6179. doi: 10.1002/anie.200601267. [DOI] [PubMed] [Google Scholar]
- 5.Powner MW, Sutherland JD. Phosphate-mediated interconversion of ribo- and arabino-configured prebiotic nucleotide intermediates. Angew Chem Int Ed. 2010;49:4641–4643. doi: 10.1002/anie.201001662. [DOI] [PubMed] [Google Scholar]
- 6.Ritson D, Sutherland JD. Prebiotic synthesis of simple sugars by photoredox systems chemistry. Nature Chem. 2012;4:895–899. doi: 10.1038/nchem.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ritson DJ, Sutherland JD. Synthesis of aldehydic ribonucleotide and amino acid precursors by photoredox chemistry. Angew Chem Int Ed. 2013;52:5845–5847. doi: 10.1002/anie.201300321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawaguchi T, et al. Enzymatic reactivity and anti-tumor activity of 1-(β-D-arabinofuranosyl)-2-thiocytosine derivatives. Chem Pharm Bull. 2000;48:454–457. doi: 10.1248/cpb.48.454. [DOI] [PubMed] [Google Scholar]
- 9.Frick L, Mac Neela JP, Wolfenden R. Transition state stabilization by deaminases: rates of nonenzymatic hydrolysis of adenosine and cytidine. Bioorg Chem. 1987;15:100–108. [Google Scholar]
- 10.Schoffstall AM. Prebiotic phosphorylation of nucleosides in formamide. Orig Life. 1976;7:399–412. doi: 10.1007/BF00927935. [DOI] [PubMed] [Google Scholar]
- 11.Lohrmann R, Orgel LE. Urea-inorganic phosphate mixtures as prebiotic phosphorylating agents. Science. 1971;171:490–494. doi: 10.1126/science.171.3970.490. [DOI] [PubMed] [Google Scholar]
- 12.Saenger W. Principles of Nucleic Acid Structure. Springer-Verlag; New York: 1984. [Google Scholar]
- 13.Brown DJ, Jacobsen NW. 612. Pyrimidine reactions. Part IV. The methylation of 2,4- and 4,5-diaminopyrimidine and related compounds. J Chem Soc (Resumed) 1962:3172–3179. [Google Scholar]
- 14.Szabla R, et al. Excited-state hydrogen atom abstraction initiates the photochemistry of β-2′-deoxycytidine. Chem Sci. 2015;6:2035–2043. doi: 10.1039/c4sc03761h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mai S, Marquetand P, González L. Intersystem crossing pathways in the noncanonical nucleobase 2-thiouracil: A time-dependent picture. J Phys Chem Lett. 2016;7:1978–1983. doi: 10.1021/acs.jpclett.6b00616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martínez-Fernández L, Corral I, Granucci G, Persico M. Competing ultrafast intersystem crossing and internal conversion: a time resolved picture for the deactivation of 6-thioguanine. Chem Sci. 2014;5:1336–1347. [Google Scholar]
- 17.Pollum M, Crespo-Hernández CE. The dark singlet state as a doorway state in the ultrafast and efficient intersystem crossing dynamics in 2-thiothymine and 2 thiouracil. J Chem Phys. 2014;140:071101. doi: 10.1063/1.4866447. [DOI] [PubMed] [Google Scholar]
- 18.Taras-Goślińska K, Burdziński G, Wenska G. Relaxation of the T1 excited state of 2-thiothymine, its riboside and deoxyriboside-enhanced nonradiative decay rate induced by sugar substituent. J Photochem Photobiol Chem. 2014;275:89–95. [Google Scholar]
- 19.Gobbo JP, Borin AC. 2-Thiouracil deactivation pathways and triplet states population. Comput Theor Chem. 2014;1040:195–201. [Google Scholar]
- 20.Mai S, Marquetand P, González L. A static picture of the relaxation and intersystem crossing mechanisms of photoexcited 2-thiouracil. J Phys Chem A. 2015;119:9524–9533. doi: 10.1021/acs.jpca.5b06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Besley NA, Hirst JD. Ab initio study of the electronic spectrum of formamide with explicit solvent. J Am Chem Soc. 1999;121:8559–8566. [Google Scholar]
- 22.Joyce GF, Schwartz AW, Miller SL, Orgel LE. The case for an ancestral genetic system involving simple analogues of the nucleotides. Proc Natl Acad Sci USA. 1987;84:4398–4402. doi: 10.1073/pnas.84.13.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grosjean H, de Crécy-Lagard V, Marck C. Deciphering synonymous codons in the three domains of life: co-evolution with specific tRNA modification enzymes. FEBS Lett. 2010;584:252–264. doi: 10.1016/j.febslet.2009.11.052. [DOI] [PubMed] [Google Scholar]
- 24.Heuberger BD, Pal A, Del Frate F, Topkar VV, Szostak JW. Replacing uridine with 2-thiouridine enhances the rate and fidelity of nonenzymatic RNA primer extension. J Am Chem Soc. 2015;137:2769–2775. doi: 10.1021/jacs.5b00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.