Abstract
Background
The epidemiology of nasopharyngeal (NP) pneumococcal carriage varies with geography and has changed in response to pneumococcal conjugate vaccine (PCV): a low prevalence (3% or less of colonizing isolates) of colonization by vaccine-type (VT) pneumococcal serotypes after PCV introduction has been reported. The primary goal of this study was to determine the VT serotype prevalence of NP pneumococcal colonization of children residing in the St. Louis, MO, USA metropolitan area following introduction of the 13-valent PCV in 2010. The secondary goal of this study was to identify characteristics associated with NP pneumococcal carriage of any serotype.
Methods
Between July 2013 and April 2016, we enrolled 397 healthy children, aged 0 –17 years, who required sedation for procedures or minor surgeries at St. Louis Children’s Hospital. NP swabs were collected after sedation or anesthesia and cultured for pneumococcus. Vaccine records were obtained from primary care providers or from state immunization databases. Parents/guardians completed a questionnaire to provide demographics, past medical history and household characteristics.
Results
Of the 88 pneumococcal isolates recovered from 84 colonized subjects (21.2% of all enrolled subjects; 95% CI 17.2–25.2%), 16 were VT. Eleven isolates were serotype 19F (12.5%), four (4.5%) were 6A and one (1.1%) was 19A. Prevalence of VT among colonizing isolates was thus 18.2% (CI 10.1–26.1%) in our cohort, despite complete PCV vaccination in 87% of colonized children. Factors associated with pneumococcal colonization by any serotype included younger age and daycare attendance.
Conclusion
Children in St. Louis exhibit a higher prevalence of VT serotypes among pneumococcal carriage isolates than has been reported in other areas in the US, demonstrating the necessity of ongoing surveillance of local epidemiology and providing evidence that serotype 19F can remain prevalent in a pediatric population despite high vaccine uptake.
Keywords: epidemiology of pneumococcal carriage, pneumococcal conjugate vaccine, pneumococcal nasopharyngeal carriage
Introduction
Streptococcus pneumoniae infections cause over 500,000 deaths annually worldwide [1]. Invasive pneumococcal disease (IPD) affects primarily young children or adults over the age of 65 [1]. The thick, polysaccharide pneumococcal capsule serves as the pathogen’s primary virulence factor, protecting against immune recognition and phagocytosis. Thus, protection against all pneumococci has been hampered by the existence of over 90 capsular serotypes. Generation of specific anti-capsular antibodies via administration of pneumococcal conjugate vaccine (PCV) provides adaptive immunity that protects against IPD. PCV have been deployed since 2000 and have profoundly reduced the incidence of IPD [1].
Nasopharyngeal (NP) carriage by children is a major human reservoir of pathogenic pneumococci [2]. PCV implementation has not only decreased IPD incidence but also shifted the distribution of serotypes among pneumococcal carriage isolates [3]. NP carriage of vaccine-type (VT) serotypes in PCV7 (4, 6B, 9V, 14, 18C, 19F, and 23F) or PCV13 (additional serotypes 1, 3, 5, 6A, 7F, and 19A) has fallen dramatically in prevalence, while non-VT NP carriage has increased proportionally, such that the overall prevalence of NP pneumococcal carriage has changed little [4, 5].
The epidemiology of serotypes prevalent in a community varies geographically and demographically. Recent reports have described very low prevalence of NP colonization by VT pneumococci among children in Georgia (6–59 months of age) and in Massachusetts (< 7 years of age), US [4, 5]. While a 2006 study examined the prevalence of pneumococcal NP carriage and antibiotic resistance patterns of carriage isolates in St. Louis, Missouri, in children < 7 years of age prior to the introduction of PCV13 [6], these isolates were not serotyped. Thus, the local epidemiology of VT pneumococcal serotypes carried by children in the greater St. Louis area, and how this compares to other regions, remains unknown.
The primary goal of our study was to define the prevalence of pneumococcal colonization with VT serotypes in healthy children enrolled from an outpatient surgery center in the St. Louis metropolitan area [7]. We also aimed to identify factors associated with pneumococcal NP colonization, such as receipt of PCV, patient demographics, past medical history, and household characteristics. We tested the hypotheses that we would find associations of NP carriage with pre-school age, daycare attendance, recent symptoms of upper respiratory infection (URI), exposure to tobacco smoke, breast-feeding, and residence with a school-aged sibling, based on prior reports [4, 8–10].
Methods
Patient population
All study procedures were approved by the Washington University School of Medicine Institutional Review Board/Human Research Protection Office. Written informed consent for participation was obtained from the parents or legal guardians of healthy children aged 0 – 17 years requiring anesthesia for minor procedures (e.g., herniorrhaphy, eye muscle repair/release, or diagnostic upper endoscopy) in the St. Louis Children’s Hospital Same Day Surgery Center. As serotype distribution varies with age, we included children of all age groups [11]. Because we included subjects from the Urology service (e.g., infants undergoing hernia repairs and circumcision revisions), we enrolled more males than females. We obtained verbal assent from school-aged children (7 – 11 years old) and written assent from subjects ≥ 12 years of age. Exclusion criteria were: presence of oropharyngeal anatomical abnormalities; undergoing tonsillectomy/adenoidectomy; presence of chronic illness such as immunodeficiency, autoimmune disorder, cystic fibrosis, heart disease, or cancer. We also excluded subjects who had URI symptoms at the time of the procedure, fever or hospital admission within the preceding 24 hours, were previously enrolled in the study, or were on antibiotics (defined as receiving an antibiotic within the 24 hours preceding the procedure other than a single pre-operative dose; most minor surgical procedures did not necessitate pre-operative antibiotics). Children were enrolled year-round from July, 2013 until April, 2016.
Data collection
Parents or legal guardians completed a questionnaire at the time of enrollment, with self-report of race, ethnicity, insurance coverage, vaccine receipt, past medical history, prescription medicines, and household characteristics. Our questionnaire included elements previously associated (either positively or negatively) with pneumococcal NP carriage, such as symptoms of recent URI within the preceding month, daycare attendance, exposure to tobacco smoke, presence of school-aged siblings in the home, and breastfeeding [4, 5, 8, 9]. Past medical history included specific questions regarding recent (preceding three months) receipt of antibiotics; diagnosis of acute otitis media, sinusitis, pneumonia, or other infection in the prior month; or any past diagnosis of acute otitis media, sinusitis, pneumonia, or other infections. We included source of insurance (private, public, or self-pay) as an indicator of socioeconomic status. We also assessed characteristics previously associated with nasal carriage of Staphylococcus aureus, such as frequent contact with the health care system [12].
Vaccine records were obtained with consent for 371 (93.5%) subjects from either primary care provider offices or from state databases. Subjects were considered to be completely vaccinated if they had received all recommended PCV for their age. PCV7 was first introduced in the US in 2000, with subsequent release of PCV13 in the US in 2010. Routine PCV recommended for all children in the US follows a 3 + 1 schedule, with doses given at 2, 4 and 6 months of age and a fourth (booster) dose given between 12–15 months of age. At the time of PCV13 introduction, a single dose of PCV13 was recommended for all children aged 24–59 months with any incomplete PCV7 or PCV13 schedule [13].
Sample collection and isolation of pneumococci
Samples were obtained and processed as previously described, in accordance with World Health Organization (WHO) guidelines for pneumococcal identification [14, 15]. In brief, one NP swab (E-swab Minitip, Copan) was introduced into one nostril of each subject immediately after sedation. Eluent (100 μl) was directly inoculated to sheep’s blood agar plates (BAP; BD Biosciences) and into 3 ml Todd Hewitt broth containing 0.5% yeast (THY) extract and 0.5 ml rabbit serum for enrichment. After incubation, inoculated broth (100 μl) was also directly streaked onto BAP. After overnight incubation (35°C in 5% CO2), up to 12 individual α-hemolytic colonies per subject were selected from either plate for further analysis. Pneumococci were identified as catalase-negative, optochin-susceptible, bile-soluble colonies of Gram-positive cocci in pairs and chains [6, 14]. Isolates were frozen (−80°C) for future use.
Serotype analysis
Serotypes were determined through a combination of genotyping and phenotyping. DNA was isolated from cultures using mutanolysin and hyaluronidase (Sigma) or BiOstic Bacteremia DNA isolation kit (MO Bio Laboratories, Inc.). First, one isolate from each colonized subject was serotyped using multiplex PCR (QIAGEN) [16]. Next, to determine if subjects were colonized with multiple serotypes, all additional isolates from each subject were tested for identity with the initial isolate by PCR. Non-identical isolates then underwent multiplex PCR serotyping until all isolates that could be typed by PCR were assigned a serotype according to CDC guidelines (2014, http://www.cdc.gov/streplab/pcr.html). Serotypes determined by PCR were confirmed in a subset of isolates through phenotypic analysis by Quellung reaction (Statens Serum Institut, Denmark). Phenotypic serotyping was also performed on eight isolates that could not be serotyped by PCR. Of note, five isolates that are described as “serotype unknown” could not be serotyped by either phenotype or genotype.
Sample size determination
The primary objective of this study was to define the prevalence of VT pneumococcal serotypes isolated from the NP of colonized children in St. Louis following the 2010 introduction of PCV13. Using the prevalence of VT pneumococcal colonization pre- and post-vaccine introduction reported in other studies, we calculated that a minimum of 62 serotyped isolates would provide sufficient power to detect a change from 25% to 7% in VT serotypes [11]. Based upon an overall pneumococcal carriage prevalence of 18%, established after our first year of enrollment [15], we calculated that enrollment of at least 344 children would provide 80% power at a 5% significance level to compare our prevalence of VT serotypes to that in other US geographic regions.
Statistics
REDCap database software was used to maintain subject and pneumococcal isolate data [17]. Pearson chi-square was used to determine association between likelihood of pneumococcal NP colonization and characteristics of subjects. The multivariable model was constructed using backwards stepwise logistic regression, including all variables that met statistical significance (p < 0.05) in the univariate analysis. Intervals between last dose of PCV and sample collection is given in years (mean ± S.D.) and comparison between two groups made using Student’s t-test. All statistics were performed using SPSS Statistics 23 for Windows (IBM SPSS).
Results
We enrolled 397 children from July, 2013 to April, 2016 in the St. Louis Children’s Hospital Same Day Surgery Center. Of the 397 subjects, 9 (2.3%) underwent surgical procedures requiring a pre-operative antibiotic dose. Subjects ranged in age from 0 – 17 years (median age 4.5 years). Of the 371 (93.5%) subjects for whom we were able to obtain documentation, 81.9% had received complete PCV series, including the fourth “booster” dose if the subject was ≥ 15 months of age. Of those enrolled, 84 (21.2%; 95% CI 17.2%–25.2%) subjects were colonized with pneumococcus, with subjects aged 0–2 years having the highest prevalence (30.8%; 95% CI 22.4%–39.2%). The prevalence of colonization of all children ≥ 6 years old was 29.8% (71/238 subjects; 95% CI 24%–35.6%). Of the colonized subjects, 86.7% had received all recommended doses of PCV for age (Table 1); 94% of colonized subjects had received either all recommended doses of PCV for age (including the booster dose) or ≥ 3 doses of PCV, indicating high PCV uptake in this community. The intervals between the last dose of PCV given to subjects and the day of sample collection did not vary significantly between colonized and non-colonized subjects in children aged 0–2 years, 2–6 years, or > 12 years. In subjects aged 6–12 years, colonized children had significantly shorter intervals (4.5 ± 1.7 years, n = 10) between last PCV doses and sample collection than non-colonized subjects (6.8 ± 2.6 years, n = 70; p = 0.002 by Student’s t-test). None of the nine subjects who received a pre-operative antibiotic dose were colonized with pneumococcus. In our univariate analysis, NP carriage was inversely associated with age (p < 0.001), and also associated with African-American race (p = 0.014). Sex, ethnicity, source of insurance coverage and receipt of PCV were not associated with pneumococcal colonization (Table 1).
Table 1.
Characteristics of carriers and non-carriers of S. pneumoniae recruited from St. Louis Children’s Hospital.
| Total n (%) n=397 |
S. pneumoniae carriers n (%) n=84 |
S. pneumoniae non-carriers n (%) n=313 |
p-value | |
|---|---|---|---|---|
| Age at time of sampling | < 0.001 | |||
| 0 – 2 years | 117 (29.5%) | 36 (42.9%) | 81 (25.9%) | |
| > 2 years – 6 years | 121 (30.5%) | 35 (41.7%) | 86 (27.5%) | |
| > 6 years – 12 years | 93 (23.4%) | 10 (11.9%) | 83 (26.5%) | |
| > 12 years | 66 (16.6%) | 3 (3.6%) | 63 (20.1%) | |
| Sex | 0.071 | |||
| Female | 142 (35.8%) | 23 (27.4%) | 119 (38.0%) | |
| Male | 255 (64.2%) | 61 (72.6%) | 194 (62.0%) | |
| Race†‡ | 0.014 | |||
| Black or African American | 53 (13.5%) | 18 (21.7%) | 35 (11.3%) | |
| Other | 340 (86.5%) | 65 (78.3%) | 275 (88.7%) | |
| Ethnicity‡ | 0.266 | |||
| Hispanic | 12 (3.0%) | 1 (1.3%) | 11 (3.5%) | |
| Not Hispanic | 383 (97.0%) | 83 (98.8%) | 300 (96.5%) | |
| Insurance Coverage‡ | 0.721 | |||
| None or self-pay | 8 (2.2%) | 1 (1.3%) | 7 (2.5%) | |
| Private | 217 (59.8%) | 49 (62.8%) | 168 (58.9%) | |
| Public | 138 (38.0%) | 28 (35.9%) | 110 (38.6%) | |
| PCV Vaccine Status‡ | 0.192 | |||
| Complete Series | 303 (81.9%) | 72 (86.7%) | 231 (80.5%) | |
| Incomplete Series^ | 67 (18.1%) | 11 (13.3%) | 56 (19.5%) |
Black or African American race includes biracial (Black/White). Other races include White/Caucasian, Asian, Alaskan Native, American Indian, and/or Pacific Islander.
Some participants chose not to answer certain questions, resulting in a different number included in indicated analyses: race (n = 393), ethnicity (n = 395); and insurance coverage (n = 363). Vaccine records could not be located for 27 participants (n = 370).
Incomplete vaccination was defined as incomplete series for age. Six of the 11 colonized subjects had received 3 PCV doses, while 5 subjects received < 3 doses of PCV.
We next determined the elements of past medical history or household characteristics that were associated with pneumococcal carriage (Table 2). A recent history (defined as within the prior month) of URI symptoms (p = 0.007), residence in a household with another young child (p = 0.004), and daycare attendance in children < 7 years of age (p < 0.002) were associated with pneumococcal carriage in a univariate analysis. We found no other associations among the other elements queried (Table 2). We then constructed a multivariable model including age, race, recent URI symptoms, age of siblings, and daycare attendance. In our multivariable analysis, pneumococcal NP colonization remained significantly associated (p < 0.05) with younger age (for each decrease in year of age, OR for colonization was 1.17; 95% CI 1.08–1.26), and daycare attendance (OR 2.54; 95% CI 1.48–4.37).
Table 2.
Elements of past medical and social history of carriers and non-carriers of S. pneumoniae.
| Total n(%) n=397 |
S. pneumoniae carriers n (%) n=84 |
S. pneumoniae non-carriers n (%) n=313 |
p-value | |
|---|---|---|---|---|
| Underlying condition | ||||
| Asthma | 44 (11.1%) | 10 (11.9%) | 34 (10.9%) | 0.787 |
| Allergies | 78 (19.6%) | 12 (14.3%) | 66 (21.1%) | 0.164 |
| Ear infections | 119 (30.0%) | 27 (32.1%) | 92 (29.4%) | 0.625 |
| Other† | 80 (20.2%) | 16 (19.0%) | 64 (20.4%) | 0.776 |
| Minor illness (prior month)‡ | 150 (38.7%) | 38 (45.8%) | 112 (36.7) | 0.133 |
| Cold/URI (cough, runny nose) | 92 (23.7%) | 29 (34.9%) | 63 (20.7%) | 0.007 |
| Ear infection | 22 (5.7%) | 5 (6.0%) | 17 (5.6%) | 0.875 |
| Sinus infection | 10 (2.6%) | 1 (1.2%) | 9 (3.0%) | 0.373 |
| Other | 59 (15.2%) | 8 (9.6%) | 51 (16.7%) | 0.111 |
| Taken antibiotics in prior three months‡ | 89 (23.2%) | 15 (18.5%) | 74 (24.4%) | 0.263 |
| Age of youngest child in home (other than subject) | 0.004 | |||
| 0 – 2 years | 57 (14.4%) | 16 (19.0%) | 41 (13.1%) | |
| > 2 years 6 years | 60 (15.1%) | 19 (22.6%) | 41 (13.1%) | |
| > 6 years 12 years | 94 (23.7%) | 13 (15.5%) | 81 (25.9%) | |
| > 12 years | 39 (9.8%) | 3 (3.6%) | 36 (11.5%) | |
| No siblings reported | 147 (37.0%) | 33 (39.3%) | 114 (36.4%) | |
| Child attends daycare, limited to children < 7 (n = 250)‡ | 125 (50.0%) | 49 (64.5%) | 76 (43.7%) | 0.002 |
| In school, limited to children ≤ 5 years of age (n=185)‡ | 170 (93.9%) | 14 (93.3%) | 156 (94.0%) | 0.921 |
| Child was breastfed (duration)‡ | 0.558 | |||
| Reported no breastfeeding | 125 (47.2%) | 23 (41.8%) | 102 (48.6%) | |
| < 3 months | 62 (23.4%) | 14 (25.5%) | 48 (22.9%) | |
| 3 – 9 months | 39 (14.7%) | 11 (20.0%) | 28 (13.3%) | |
| > 9 months | 39 (14.7%) | 7 (12.7%) | 32 (15.2%) | |
| Exposure to tobacco smoke‡ | 91 (23.6%) | 14 (16.9%) | 77 (25.5%) | 0.101 |
Other underlying conditions included meningitis (n=1), bone or joint infection (n=2), heart disease (n=1), birth defect (n=11 ), GI related (n=7), liver or kidney disease (n=2), UTI (n=1), seizures (n=13), eczema (n=39), other (n=13)
Some participants chose not to answer certain questions, resulting in a different number included in indicated analyses: minor illness in preceding month (n = 388), taken antibiotics in preceding three months (n = 384); daycare attendance (n = 250 of 259 children < 7 years of age); school attendance (n = 181 of 185 children ≤ 5 years of age); child was breastfed (n = 265); exposure to tobacco smoke (n = 385).
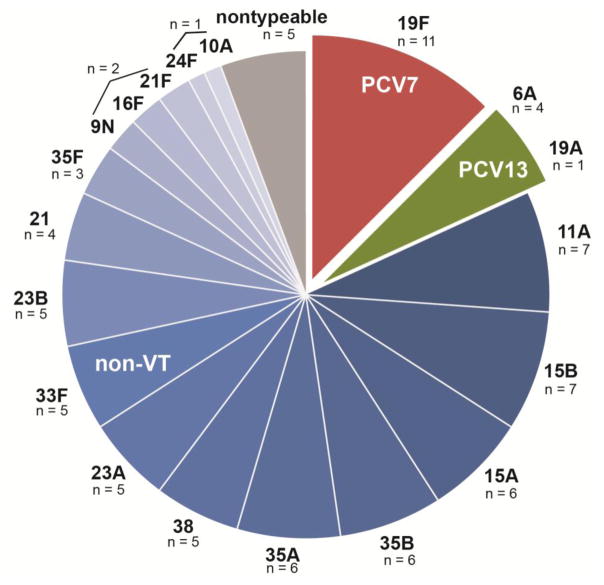
We then analyzed the serotype prevalence of the colonizing pneumococcal isolates (Figure). Four (4.8%) subjects were colonized with two different serotypes, such that 88 isolates from 84 subjects are shown. One subject was co-colonized with serotypes 21F and 23B, one with serotypes 35A and 23A, one with serotypes 15A and 23B, and one with serotypes 15B and 33F, none of which are VT. Overall, the most commonly identified serotype was 19F, included in both PCV7 and PCV13 (n=11/88, 12.5%; 95% CI 5.6 – 19.4%). We also found five isolates (5.7%; CI 0.9 – 10.5%) with serotypes included in PCV13: 19A (n = 1) and 6A (n = 4). Thus, three VT serotypes comprised 18.1% (CI 10.1 – 26.1%) of pneumococcal isolates recovered from healthy children in St. Louis.
Figure 1.
Serotype distribution of 88 pneumococcal isolates recovered from the nasopharynx of 84 healthy pediatric subjects. Red represents a serotype included in PCV7; green represents a serotype included in PCV13; blue represents non-VT; gray represents non-typeable S. pneumoniae.
We focused on VT pneumococci, to determine if age, lack of vaccination, or other factor correlated with the likelihood of colonization with VT pneumococci (Table 3). The ages of the 16 subjects colonized with VT serotypes ranged from 7.5 months to 6.5 years (median age 3 years), and age did not associate with likelihood of carriage of VT versus non-VT pneumococcus. The 16 subjects colonized with VT serotypes resembled the demographics of the overall subject population (12 males, 4 females; 14 non-black, 2 African-American/biracial). All subjects colonized with VT serotypes were insured (11 private, 5 public) and none had histories of serious infections such as pneumonia, bacteremia, or meningitis. Of the 16 subjects with VT isolated, 6 (37.5%) had recent URI symptoms, similar to the 34.9% of all carriers reported with recent URI symptoms (Table 2). VT serotypes were isolated in all seasons and years of sampling (7 winter, 9 summer; 5 in 2014, 8 in 2015, 3 in 2016). Of the 11 children colonized with 19F, seven had received complete PCV13 series and two had complete vaccine series that were combinations of PCV7 and PCV13 (Table 3). One subject was documented as having received fewer than three immunizations, and the vaccination records for one subject colonized with 19F could not be located. Of the five children colonized with PCV13 types (6A and 19A), two had complete PCV13 series, two had complete series that were combinations of PCV7 and PCV13 and one had a documented incomplete series. The intervals between receipt of last PCV dose and sample collection did not differ significantly between subjects colonized with VT and non-VT pneumococci, nor did they differ significantly between subjects colonized with serotype 19F and non-19F serotypes. We were therefore unable to find an association between carriage of VT pneumococci and age, vaccine receipt, or other surveyed factor.
Table 3.
Age and vaccine status of carriers of VT vs. non-VT S. pneumoniae.‡
| PCV7 Types n (%) n=11 |
PCV13 Types n (%) n=5 |
Non-VT n (%) n=63 |
p-value | |
|---|---|---|---|---|
| Age at enrollment | 0.888 | |||
| 0 – 2 years | 4 (36.4%) | 2 (40.0%) | 27 (42.9%) | |
| > 2 years 6 years | 6 (54.5%) | 3 (60.0%) | 25 (39.7%) | |
| > 6 years 12 years | 1 (9.1%) | 0 | 9 (14.3%) | |
| > 12 years | 0 | 0 | 2 (3.2%) | |
| †Vaccine status | 0.68 | |||
| Complete PCV7 series | 0 | 0 | 4 (6.3%) | |
| Complete PCV13 series | 7 (70.0%) | 2 (40.0%) | 44 (69.8%) | |
| Complete PCV7 and 13 series | 2 (20.0%) | 2 (40.0%) | 9 (14.3%) | |
| Incomplete | 1 (10.0%) | 1 (20.0%) | 6 (9.5%) | |
| Unable to obtain vaccine records | 1 | 0 | 0 |
Defined complete coverage as having recommended number of doses for age at time of enrollment.
Carriers with isolates that could not be serotyped (n = 5) were excluded from analysis.
Discussion
First administered in the US in 2000, PCV7 has been highly effective in preventing IPD [1, 18]. With the recent introduction of 10- and 13-valent PCV, IPD incidence is continuing to decline [18]. Much of the effect of PCV appears to be related to reduced NP colonization by serotypes most likely to cause IPD [3]. While the overall prevalence of pneumococcal NP carriage has been relatively constant since PCV introduction, the prevalence of VT serotypes has declined dramatically in most areas, including in the US [4, 5, 11, 19]. For instance, in a recent study performed in the metropolitan area surrounding Atlanta, Georgia, prevalence of PCV13 VT colonization declined from 29% (36/124 isolates) in 2010 to 3% (3/99 isolates) in 2013, following the 2010 introduction of PCV13 [5].
Our overall prevalence of pneumococcal colonization, 21.2%, was lower than that reported for other studies in the US, likely due to our inclusion of older children. Colonization prevalence of children ≤ 6 years old in St. Louis was 29.8%, comparable to the reported prevalence of 31% of children < 7 years old in Massachusetts [4] and 32% of children aged 6 – 59 months in Georgia [5]. It is concerning that the proportion of VT serotypes among NP isolates in our cohort (18.1%) greatly exceeds the corresponding 3% and 5% prevalence in Georgia (2013) and Massachusetts (2011), respectively [4, 5]. The proportion of isolates that were 19F in our St. Louis cohort, 12.5%, was also higher than in Georgia (3.8%; 27/711). In Alaska, the proportion of VT serotypes colonizing children ≤ 5 years of age was also lower at 9.9% (93/938) [11]. Thus, certain VT serotypes appear to persist in our region, even among vaccinated children.
The CDC performs active bacterial surveillance of selected sites in the US. The most recently available data indicate incidences of IPD from VT of 2/100,000 among children < 5 years of age and of 6/100,000 cases in adults > 65 years and older in 2015, demonstrating the persistence of VT pneumococci in US children [20]. However, the sites selected for active bacterial surveillance do not include Missouri, or any midwestern state other than Minnesota. As invasive pneumococcal infections are not reportable to the CDC, clinical pneumococcal isolates in St. Louis are no longer routinely serotyped, and the prevalence of colonization and disease by VT serotypes prior to PCV13 introduction and the incidence of IPD due to VT serotype in the metropolitan St. Louis are therefore currently indeterminable. We were not able to discern any characteristics of children who carried VT pneumococci distinct from those colonized with non-VT pneumococci in our cohort. We also did not find any difference in the interval between last administered dose of PCV and sample collection in children colonized with VT vs those colonized with non-VT pneumococci, arguing against the possibility that PCV efficacy may decline over time. Also, we did not find any difference in interval between PCV dose and sample collection in colonized and non-colonized children 0 – 6 years of age. We did find a significantly shorter interval since the last dose of PCV and sample collection in colonized children aged 6–12 years than non-colonized children in the same age group. This is likely explained by the fact that younger children are more likely to be colonized with pneumococcus and are also closer in time to their last scheduled dose of PCV. Finally, our cohort does not differ significantly in demographics from other urban areas, such as Atlanta, Georgia, or Boston, Massachusetts, that would explain increased colonization with VT pneumococci. Our higher prevalence of VT serotypes suggests the importance of a follow-up study to define the incidence of IPD due to VT serotypes, especially 19F, in pediatric and adult populations in St. Louis.
A relatively high prevalence of serotype 19F in our study is in accordance with other recent findings. Gounder et al. also noted an unexplained persistence or resurgence of colonization with serotype 19F after PCV13 introduction [11]. Serotype 19F was the most persistent VT found in PCV7 in a large longitudinal study that monitored pneumococcal serotype prevalence at 45 U.S. medical centers from 1999 – 2011 [21]. Serotype 19F was also recently noted to be the most commonly identified serotype in cases of IPD associated with vaccine failure [22] and was associated with breakthrough pneumococcal infection in a separate study [23]. Although PCV13 was clearly shown to reduce acquisition of 19F [24], our new results, combined with others [11, 22], warrant ongoing surveillance to determine if the carriage of 19F persists in some areas despite widespread vaccination uptake. This is important, as increased prevalence of serotype 19F could lead to an increased incidence of IPD [22, 23]. Furthermore, as serotype 19F can be resistant to penicillin [21], serotype analysis of isolates obtained in clinical microbiology laboratories from all patients presenting with IPD could be routinely performed to monitor for potential changes in antimicrobial resistance.
We confirmed increased likelihood of pneumococcal colonization in younger children and in children attending daycare in our multivariable model, as has been previously reported [4, 5, 8, 9]. We did not find associations between colonization and breast-feeding, exposure to tobacco smoke, or presence of other young children in the home in our multivariable model, as we had hypothesized based on published reports [4, 5, 8–10]. We also found no statistical association between likelihood of pneumococcal colonization and receipt of antibiotics within the 3 months preceding enrollment, or a diagnosis of acute otitis media or sinusitis within the preceding month. Finally, while we did not specifically exclude subjects who were undergoing surgical procedures that prompted a pre-operative antibiotic dose, only 9 subjects who had hardware placement or removal during orthopedic procedures required a pre-operative antibiotic dose. These subjects tended to be in the older age groups, and none were colonized with pneumococcus. Excluding them from analysis did not significantly alter our reported prevalence of pneumococcal NP colonization (Supplemental Table).
The strengths of our study include the recruitment of children who did not have concurrent symptoms of URI or acute otitis media, as these symptoms have been associated with increased likelihood of carriage. Our serotype prevalence thus represents a cross-section of healthy children in the metropolitan St. Louis area, which differentiates it from other US studies in which children presenting with URI symptoms were included [4–6, 11]. Another strength of the study is documentation of vaccine records from 93.5% of subjects, including all but one colonized subject. The limitations of our study included a sample drawn from a single center and reliance on parent self-report for several patient characteristics, such as past medical history, tobacco exposure and recent receipt of antibiotics.
In summary, our analysis of the serotype distribution of pneumococcal isolates from pediatric carriers in the St. Louis area demonstrates a higher prevalence of VT serotypes than in other metropolitan areas in the US [4, 5, 11, 18, 21], despite equivalent vaccine coverage in our region. Our results thus provide additional evidence that PCV may not reduce NP carriage of serotype 19F to as great an extent as other VT serotypes, and highlight the need for ongoing surveillance of local epidemiology of pneumococcal NP colonization.
Supplementary Material
Highlights.
Prevalence of pneumococcal nasopharyngeal colonization of children 0–17 years old was 21.2%.
Of 88 isolates, 16 were vaccine-type (11 were 19F), despite 87% vaccine coverage.
Our results highlight the need for local surveillance of pneumococcal epidemiology.
Acknowledgments
We thank Phillip Tarr and David Hunstad for critical review of the manuscript. We thank the surgeons, anesthesiologists and surgical staff of the St. Louis Children’s Hospital Same Day Surgery Center for facilitating recruitment, enrollment and sampling of pediatric subjects. We thank the many primary care providers’ offices that provided vaccine records. We thank the subjects and families for their participation.
Funding
Funding for the study was provided by the St. Louis Children’s Hospital Foundation and the Children’s Discovery Institute (grant numbers PD-II-2013–295 and PD-II-2014–366). SCM is also supported by NIH grant AI104732. SAF is supported by AHRQ grants HS021736 and HS024269. Institutional support of REDCap software is provided through grants CTSA Grant [UL1 TR000448] and Siteman Comprehensive Cancer Center and NCI Cancer Center Support Grant P30 CA091842.
Footnotes
Conflicts of interest
The authors have no financial conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tan TQ. Pediatric invasive pneumococcal disease in the United States in the era of pneumococcal conjugate vaccines. Clin Microbiol Rev. 2012;25:409–19. doi: 10.1128/CMR.00018-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harboe ZB, Slotved HC, Konradsen HB, Kaltoft MS. A pneumococcal carriage study in Danish pre-school children before the introduction of pneumococcal conjugate vaccination. Open Microbiol J. 2012;6:40–4. doi: 10.2174/1874285801206010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slotved HC, Dalby T, Hoffmann S. The effect of pneumococcal conjugate vaccines on the incidence of invasive pneumococcal disease caused by ten non-vaccine serotypes in Denmark. Vaccine. 2016;34:769–74. doi: 10.1016/j.vaccine.2015.12.056. [DOI] [PubMed] [Google Scholar]
- 4.Lee GM, Kleinman K, Pelton SI, Hanage W, Huang SS, Lakoma M, et al. Impact of 13-valent pneumococcal conjugate vaccination on Streptococcus pneumoniae carriage in young children in Massachusetts. J Pediatr Infect Dis Soc. 2014;3:23–32. doi: 10.1093/jpids/pit057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Desai AP, Sharma D, Crispell EK, Baughman W, Thomas S, Tunali A, et al. Decline in pneumococcal nasopharyngeal carriage of vaccine serotypes after the introduction of the 13-valent pneumococcal conjugate vaccine in children in Atlanta, Georgia. Pediatr Infect Dis J. 2015;34:1168–74. doi: 10.1097/INF.0000000000000849. [DOI] [PubMed] [Google Scholar]
- 6.Garbutt J, Rosenbloom I, Wu J, Storch GA. Empiric first-line antibiotic treatment of acute otitis in the era of the heptavalent pneumococcal conjugate vaccine. Pediatrics. 2006;117:e1087–94. doi: 10.1542/peds.2005-2651. [DOI] [PubMed] [Google Scholar]
- 7.Colvin JM, Muenzer JT, Jaffe DM, Smason A, Deych E, Shannon WD, et al. Detection of viruses in young children with fever without an apparent source. Pediatrics. 2012;130:e1455–62. doi: 10.1542/peds.2012-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hsieh YC, Chiu CH, Chang KY, Huang YC, Chen CJ, Kuo CY, et al. The impact of the heptavalent pneumococcal conjugate vaccine on risk factors for Streptococcus pneumoniae carriage in children. Pediatr Infect Dis J. 2012;31:e163–8. doi: 10.1097/INF.0b013e31825cb9f9. [DOI] [PubMed] [Google Scholar]
- 9.Ueno M, Ishii Y, Tateda K, Anahara Y, Ebata A, Iida M, et al. Prevalence and risk factors of nasopharyngeal carriage of Streptococcus pneumoniae in healthy children in Japan. Jpn J Infect Dis. 2013;66:22–5. doi: 10.7883/yoken.66.22. [DOI] [PubMed] [Google Scholar]
- 10.Zuccotti G, Mameli C, Daprai L, Garlaschi ML, Dilillo D, Bedogni G, et al. Serotype distribution and antimicrobial susceptibilities of nasopharyngeal isolates of Streptococcus pneumoniae from healthy children in the 13-valent pneumococcal conjugate vaccine era. Vaccine. 2014;32:527–34. doi: 10.1016/j.vaccine.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Gounder PP, Bruce MG, Bruden DJ, Singleton RJ, Rudolph K, Hurlburt DA, et al. Effect of the 13-valent pneumococcal conjugate vaccine on nasopharyngeal colonization by Streptococcus pneumoniae--Alaska, 2008–2012. J Infect Dis. 2014;209:1251–8. doi: 10.1093/infdis/jit642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fritz SA, Garbutt J, Elward A, Shannon W, Storch GA. Prevalence of and risk factors for community-acquired methicillin-resistant and methicillin-sensitive staphylococcus aureus colonization in children seen in a practice-based research network. Pediatrics. 2008;121:1090–8. doi: 10.1542/peds.2007-2104. [DOI] [PubMed] [Google Scholar]
- 13.Centers for Disease Control, Prevention. Licensure of a 13-valent pneumococcal conjugate vaccine (PCV13) and recommendations for use among children -- Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59:258–61. [PubMed] [Google Scholar]
- 14.Satzke C, Turner P, Virolainen-Julkunen A, Adrian PV, Antonio M, Hare KM, et al. Standard method for detecting upper respiratory carriage of Streptococcus pneumoniae: updated recommendations from the World Health Organization Pneumococcal Carriage Working Group. Vaccine. 2013;32:165–79. doi: 10.1016/j.vaccine.2013.08.062. [DOI] [PubMed] [Google Scholar]
- 15.Zhou JY, Isaacson-Schmid M, Utterson EC, Todd EM, McFarland M, Sivapalan J, et al. Prevalence of nasopharyngeal pneumococcal colonization in children and antimicrobial susceptibility profiles of carriage isolates. Int J Infect Dis. 2015;39:50–2. doi: 10.1016/j.ijid.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pai R, Gertz RE, Beall B. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. J Clin Microbiol. 2006;44:124–31. doi: 10.1128/JCM.44.1.124-131.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruce MG, Singleton R, Bulkow L, Rudolph K, Zulz T, Gounder P, et al. Impact of the 13-valent pneumococcal conjugate vaccine (PCV13) on invasive pneumococcal disease and carriage in Alaska. Vaccine. 2015;33:4813–9. doi: 10.1016/j.vaccine.2015.07.080. [DOI] [PubMed] [Google Scholar]
- 19.Dagan R, Juergens C, Trammel J, Patterson S, Greenberg D, Givon-Lavi N, et al. Efficacy of 13-valent pneumococcal conjugate vaccine (PCV13) versus that of 7-valent PCV (PCV7) against nasopharyngeal colonization of antibiotic-nonsusceptible Streptococcus pneumoniae. J Infect Dis. 2015;211:1144–53. doi: 10.1093/infdis/jiu576. [DOI] [PubMed] [Google Scholar]
- 20.Active Bacterial Core Surveillance Report, Emerging Infections Program Network, Streptococcus pnueumoniae. Centers for Disease Control and Prevention, Department of Health and Human Services; Atlanta: 2014. [Accessed February 28, 2017]. https://wwwcdcgov/abcs/reports-findings/survreports/spneu14html. [Google Scholar]
- 21.Richter SS, Heilmann KP, Dohrn CL, Riahi F, Diekema DJ, Doern GV. Pneumococcal serotypes before and after introduction of conjugate vaccines, United States, 1999–2011(1.) Emerg Infect Dis. 2013;19:1074–83. doi: 10.3201/eid1907.121830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oligbu G, Hsia Y, Folgori L, Collins S, Ladhani S. Pneumococcal conjugate vaccine failure in children: A systematic review of the literature. Vaccine. 2016;34:6126–32. doi: 10.1016/j.vaccine.2016.10.050. [DOI] [PubMed] [Google Scholar]
- 23.Park SY, Van Beneden CA, Pilishvili T, Martin M, Facklam RR, Whitney CG, et al. Invasive pneumococcal infections among vaccinated children in the United States. J Pediatr. 2010;156:478–83. e2. doi: 10.1016/j.jpeds.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 24.Dagan R, Patterson S, Juergens C, Greenberg D, Givon-Lavi N, Porat N, et al. Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: a randomized double-blind trial. Clin Infect Dis. 2013;57:952–62. doi: 10.1093/cid/cit428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.